Abstract
Objective:
To assess variations in rheumatoid arthritis treatment and outcomes at the community level from 1998 through 2009.
Methods:
The study used computerized data from 16 Kaiser Permanente Northern California Medical Centers. Mixed modeling was used to assess patterns across time and clinic. The analysis accounted for patient demographics, clustering of patients within Medical Centers, and repeated measures of patients over time. The metric used to measure drug use, months of use per patient per year, included both users and nonusers in the denominator, to account for both prevalence and duration of use.
Results:
Assessment was performed of 28,601 patients with rheumatoid arthritis, with all levels of severity. From 1998 through 2009, methotrexate use doubled in the typical patient to include 23% of the time they were observed; sulfasalazine and hydrochloroquine use declined. By 2008 through 2009, leflunomide and antitumor necrosis factor agents were used by the typical patient 4% and 9% of the time, respectively. Between 1998 and 2009, disease-modifying antirheumatic drug use increased in the typical patient from 38% to 63% of the time, and oral prednisone use declined from 23% to 15% of the time, whereas opioid use initially rose but then fell to 23% of the time. No variations over time were observed for the rate of hospitalized pneumonia or opportunistic infection. Variation across clinics, measured by the difference in drug use between clinics at the 75th and 25th percentiles, was lowest for opioids (25% vs 20% of the time) and greatest for infliximab (< 1% to 3%).
Conclusion:
Increased use of disease-modifying antirheumatic drugs and declines in prednisone are encouraging. Opioid use may need intervention.
INTRODUCTION
In recent decades, the treatment of rheumatoid arthritis (RA) has changed substantially, with introductions in the 1980s of methotrexate and sulfasalazine and in the 1990s of leflunomide and antitumor necrosis factor (TNF) agents. In 2002, the American College of Rheumatology established a quality measure specifying that a patient with established RA be treated with a disease-modifying antirheumatic drug (DMARD) unless there was a contraindication, inactive disease, or patient refusal.1,2 In 2005, the National Committee for Quality Assurance adopted the percentage of adult patients with a diagnosis of RA who have documentation of DMARD as a Healthcare Effectiveness Data and Information Set (HEDIS) measure.3 In 2012, the American College of Rheumatology revised its treatment recommendations for RA, targeting remission or low disease activity.4,5 With these changes, one expects population-level increases in use of therapies. In addition, because clinical trials have demonstrated the benefits of these therapies for reducing inflammation and joint damage and improving functional ability and health-related quality of life,6,7 one further expects improved outcomes.
We used computerized data for more than 28,000 patients with RA who were members of an integrated health care delivery system to explore changes in RA practice patterns and outcomes over time and across Medical Centers. We sought to understand the diffusion of new treatments and their effects on outcomes at the population level.
METHODS
The study was approved by the institutional review boards of the Kaiser Foundation Research Institute and Kaiser Permanente (KP) Northern California (KPNC). The outcomes used in the study—prednisone and opioid use and rates of pneumonia and opportunistic infection—were selected because they are easily defined using the computerized clinical data that were available for the study. Although these outcomes are clinically important, they serve as surrogates for clinical disease activity (prednisone) and pain (opioid), or they are side effects of aggressive treatment (infection). We used these surrogates because clinical disease activity measures, such as the Health Assessment Questionnaire, were not available.8 Nonetheless, the information we present increases understanding of how changing therapeutic approaches has advanced outcomes in the community setting. Furthermore, where variation results in underuse, overuse, or inappropriate use of therapy, it may be possible to improve outcomes by further standardizing patient selection for therapy.
Setting
This study was conducted among 3.2 million members of KPNC, which provides prepaid, comprehensive, integrated health care. KP physicians are on staff, and their compensation is unrelated to their patients’ utilization of services. Members receive care at 1 or more of the Medical Centers of their choosing, generally one nearest home and/or work. Every Medical Center contains a Department of Rheumatology, and referral to a rheumatologist is through the primary care physician.
Etanercept has been the preferred first-line anti-TNF therapy at KPNC, and over time, adalimumab has replaced infliximab as the second-line anti-TNF therapy. Because it is infused and not injected, infliximab is sometimes prescribed preferentially for patients whose out-of-pocket drug costs are high, such as those receiving Medicare without supplemental coverage. Because care is prepaid, no financial incentive exists for using infliximab over other anti-TNF agents. KP enforces strict conflict-of-interest rules through contract provisions, so that representatives of pharmacy companies may not pay for food, gifts, or educational events for KP clinicians. According to the Health Plan’s rules, only specialists and not primary care physicians can prescribe anti-TNF agents. Primary care physicians may prescribe nonbiologic drugs used to treat RA and may modify these regimens. Specialty RA clinics do not exist, and no other treatment guidelines were in place; nor were systemwide process improvement activities implemented to shift clinical practices. Over time, the Health Plan has offered a wider variety of insurance products, but during most of the study period, patients paid a $5 to $20 copayment for drugs, including injected anti-TNF, with a similar-sized copayment for visits to the infusion clinic. Medicare patients were covered under a risk contract, and those without supplemental insurance paid the so-called “donut hole” (Medicare Part D coverage gap). We are not aware of any other pressures on treatment decision making that are relevant to this study.
Study Design
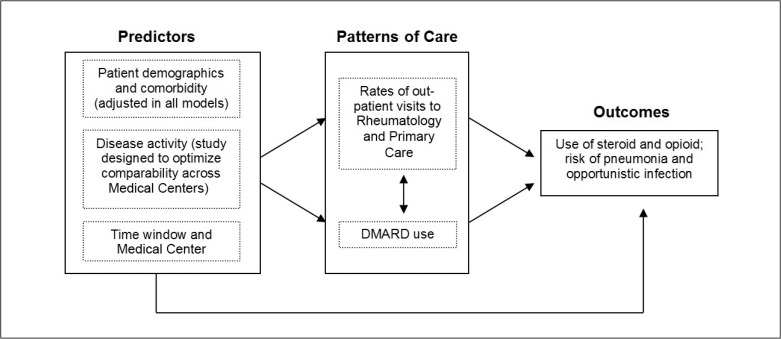
A conceptual model guided the study design and analysis (Figure 1). We sought to compare, at the population level, treatments and outcomes over time and across Medical Centers while accounting for confounding differences in patient-level characteristics, especially disease activity. Similar to our previous report, the study used a hierarchical mixed methods design,9 with time and Medical Center treated cross-sectionally at the higher level and patient characteristics treated longitudinally at the lower level.
Figure 1.
Conceptual model: predictors, patterns of care, and outcomes.
DMARD = disease-modifying antirheumatic drug.
To assess variations over time, we divided the study period into six 2-year windows and applied the same eligibility criteria to each window. To assess variation across Medical Centers, we grouped Medical Centers and medical offices into 12 categories on the basis of their geographic separation; we applied the same patient eligibility criteria to each of the 12 Medical Center groupings. For each patient and each 2-year time window, we assigned a Medical Center and computed the follow-up time, number of outpatient visits (visits per patient per year, known as “patient-year”), drug use (months of use per patient-year), and rates of hospitalized pneumonia and opportunistic infection (events per patient-year).
Although patient demographics and comorbidity were not the focus of analysis, we adjusted for these variables in all analyses. Disease activity was presumed to influence patterns of care and outcomes, but the information was not available for adjustment; thus, we designed the study to minimize bias from disease activity. This was done by including the entire census of RA-affected patients at each Medical Center, including those with mild disease, and by focusing the analysis at the level of the population, not the individual. Specifically, we assumed that the average baseline disease activity did not vary across time windows or Medical Centers except through differences in the use of therapy. To minimize any association of disease activity with a Medical Center resulting from migration across Medical Centers, we linked patients to their home Medical Center (primary care physician or address) to eliminate bias stemming from referral of patients with more severe disease to Medical Centers perceived to offer higher-quality care.
Study Population
Patients aged 18 to 89 years with 12 or more months of membership during the 1998 through 2009 study period were eligible. To identify patients with suspected RA, we identified those with 1 or more relevant assignments of International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of 714 in computerized clinical encounter data. In a random sample of 210 patients, we used manual chart review to confirm the diagnosis and identify the best-performing case-finding algorithm using computerized data alone. As described in the Results section, the best-performing algorithm was 2 or more physician-recorded diagnoses of RA, recorded at any time, without regard to use of DMARD. For the full study, patients were required to have 2 or more diagnoses of RA during the 48 months that started 2 years before and ended at the conclusion of each 2-year period under study. Data from 1996 through 1997 were used to identify RA in those patients who were included in the 1998 through 1999 period.
Data Collection
Patient-level information was obtained from computerized clinical databases to estimate proportions and rates across the six 2-year time windows and 12 Medical Centers. During 1998 through 2004, the data were recorded in information systems (eg, outpatient encounters, inpatient encounters, pharmacy, and laboratory) by health care physicians and administrators for measuring utilization and quality, not for submitting claims. Beginning in 2004, the Health Plan began implementing an electronic medical record that was fully established by 2006; during 2004–2006 both the legacy systems and the electronic medical record were in use. By 2006, all departments used the electronic medical record exclusively. Although few outside claims were processed, we also included these in the study. The pharmacy data were integral to this study, and a single pharmacy system was used throughout the study period. In addition, for 210 patients, we performed chart review using a trained medical record abstractor, a standardized instrument, and a procedure manual.
Independent Variables
Patient age, sex, and enrollment history were obtained from membership files. During the study period, the Health Plan did not ask members for their race or ethnicity; however, this information was available for 94% of the RA cohort. From the chart review of 108 patients, we also obtained affected joints and joint counts. We adjusted our analyses for comorbidity using the Charlson Comorbidity Index on the basis of diagnoses recorded during the 12-month period preceding the second recorded RA diagnosis that qualified the patient for the study.10 Age and comorbidity were recomputed for each 2-year time window.
The Health Plan assigns each patient to a home Medical Center on the basis of the location of his/her primary care visits or, if there is none, his/her residential address. However, patients face no barrier in using whichever Medical Center they choose. For this study, we linked the patient to his/her home Medical Center in each two-year window. This decision ensured comparability of RA severity across Medical Centers.
We ascertained all outpatient visits to rheumatology or primary care physicians for which the primary reason for the visit was RA (ICD-9 Code 714). The visit rate was computed as the number of visits in the 2-year window divided by the patients’ enrollment time during that window. The physician recorded the reason for the visit at the time of the visit using a diagnosis code for the primary purpose of tracking utilization and quality.
For each Medical Center and two-year time window, we estimated from computerized pharmacy data the average use of DMARD in months of use per patient per year; this was expressed in months per patient-year. In keeping with the study’s focus on measuring population-level utilization, the denominator included both users and nonusers of each drug; thus, the measure accounted for both prevalence of use and duration of use among users. To compute this measure, we ascertained the days supply of DMARD dispensed per patient-year. For dispensings extending before or after the two-year window, only the days supply available within the two-year window was counted. The calculation accounted for the recommended dosing of infliximab at two weeks initially and every eight weeks thereafter. We did not examine golimumab because the drug was not used appreciably in our Health Plan.
Outcome Measures
Four outcome measures were computed for each two-year time window and each Medical Center: 1) use of oral prednisone and 2) use of opioid, each in months of use per patient-year, and 3) rates of hospitalized pneumonia and 4) rates of opportunistic infection, each in events per patient-year. We focused on oral prednisone because of our interest in long-term glucocorticoid use; oral prednisone accounted for 98% of dispensings of oral glucocorticoids in the population. As described in the previous paragraph for DMARD, the denominator included both users and nonusers of each drug; thus, the measure accounted for both prevalence of use and duration of use among users. The four outcomes were evaluated in separate analytic models, with each patient counted no more than once per event.
Diagnoses of pneumonia were obtained from inpatient ICD-9 diagnosis Codes 481–483, 484.3, 484.5, 485, 486, and 513. Hospitalized opportunistic infections included Salmonella (ICD-9 Code 003), Mycobacterium tuberculosis (010–018), Listeriosis (027.0), other mycobacteria (031), Actinomycosis (039), progressive multifocal leukoencephalopathy (046.3), herpes zoster (053), Coccidiomycosis (114), Histoplasmosis (115), Blastomycosis (116), Aspergillosis (117.3), Cryptosporidiosis (117.5), Toxoplasmosis (130), pneumocystis pneumonia (136.3), Cryptosporidiosis meningitis (321.0), Legionnaire’s disease (482.84), and pneumonia in other mycoses (484.7).
Entry and Exit into Follow-up
For each 2-year time window, patients were included if they had at least 2 RA diagnoses during the current or immediately preceding 2-year time window (with inclusion in the 1998–1999 time window on the basis of a diagnosis recorded during 1996–1999). For each 2-year window, entry into follow-up was determined from the latest of the following dates: 1) the patient’s 18th birthday; 2) for RA identified in the current 2-year window, the date of the first primary or secondary RA diagnosis; 3) for RA identified in the preceding 2-year window, the starting date of the current 2-year window; or 4) for patients who had disenrolled in the Health Plan before the window’s start date, the start of their next enrollment period within the window. For each 2-year window, exit from follow-up was determined from the earliest of the following dates: 1) the end of the 2-year window; 2) the first date of disenrollment within the window; 3) the 90th birthday, and 4) the death date.
Analytic Methods
Data analyses used SAS Version 9 software (SAS Institute Inc, Cary, NC). We used mixed modeling, which provided a flexible approach for datasets that contained repeated measures over time and space. Correlations within subjects and Medical Centers could be modeled using random and fixed variables to elucidate the simultaneous effects of Medical Center and time window on outpatient visit rates, drug use, and infections.11 Mixed modeling is useful when repeated measures are available for clustered settings. An open-access article explaining mixed-methods design and analysis for the general reader was written by Minalu and colleagues.12 Every model included patient age (18 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 69, and 70 to 89 years), sex, race/ethnicity (Asian, black, Hispanic, white, unknown), and Charlson Comorbidity Index (none, 1, and 2 or more conditions).
To test whether the class variable “period” improved the model’s fit, we compared a less parsimonious model containing the variable “period” with a more parsimonious model that did not. Where the variable improved the model fit to a statistically significant degree (p < 0.05) as indicated by the likelihood ratio test, we inferred that “period” was important. Because the variable was coded as a class variable, it was not necessary that the fit be monotonic or linear; rather, the model tested for significant changes between periods as well as for overall trends, with the null hypothesis being no change.
To assess the role of the 2-year time window, both Medical Center and 2-year time window were coded as class variables and treated as fixed effects, with p values for each of these being obtained from Type 3 F-statistics. Each of these models was tested for an interaction between Medical Center and 2-year time window; the interaction term was retained when it was significant at p < 0.05.
We used a similar approach to test whether the variable “Medical Center” improved the model, but in this instance, we used a “shrinkage method” to pull estimates for smaller centers toward the mean to account for their lower stability.13 To assess the role of Medical Center and to estimate the interquartile ranges across the Medical Centers, we treated Medical Center as a random effect.
RESULTS
Validation of Rheumatoid Arthritis
The best-performing case-finding algorithm for detecting RA was 2 or more physician-recorded diagnoses of RA (ICD-9 Code 714) without regard to use of DMARD (Table 1). The algorithm had a sensitivity of 97% and a positive predictive value of 77%. In the full study population of 28,601 patients with RA, the length of enrollment was 1 to 3 years in 14% of the cohort, 4 to 6 years in 16%, 7 to 9 years in 18%, and 10 to 12 years in 52%.
Table 1.
Sensitivity and positive predictive value of diagnoses for confirming rheumatoid arthritis
| Diagnostic codes | DMARD dispensings | Using chart review as gold standard (N = 210) | ||||
|---|---|---|---|---|---|---|
| Stratified sample, no. | True-positives, no. | False-positives, no. | Sensitivity,a % | Positive predictive value,a % | ||
| ≥ 1 | 0 | 210 | 125 | 85 | 100b | 60 |
| ≥ 1 | ≥ 1 | 175 | 119 | 56 | > 99 | 74 |
| ≥ 2 | 0 | 140 | 108 | 32 | 97 | 77 |
| ≥ 2 | ≥ 1 | 105 | 91 | 14 | 81 | 87 |
The sensitivity, positive predictive value, is weighted to reflect the sampling fractions.
By definition.
DMARD = disease-modifying antirheumatic drug.
Table 2 shows patient characteristics for the 108 persons included in the chart review as well as the 28,601 patients included in the full study. The full cohort was older (p = 0.01) and included more women (p = 0.16) than the chart review. From the chart review, we ascertained the location of joint involvement, number of involved small joints, disease duration, and radiologic findings as of December 31, 2009 (Table 2).
Table 2.
Characteristics of patients with rheumatoid arthritis, 1998–2009
| Characteristic | Patients with chart review, % (n = 108) | Patients with computerized data, % (n = 28,601) |
|---|---|---|
| Sexa | ||
| Men | 19 | 26 |
| Women | 81 | 74 |
| Age group, yearsb | ||
| 18–39 | 18 | 7 |
| 40–49 | 12 | 11 |
| 50–59 | 24 | 19 |
| 60–69 | 23 | 22 |
| 70–89 | 23 | 41 |
| Race/ethnicity | ||
| White | 53 | 57 |
| Hispanic | 17 | 16 |
| Black | 10 | 8 |
| Asian | 12 | 8 |
| Unknown/other | 8 | 11 |
| Antibody positivity | ||
| Rheumatoid factor positive | 69 | — |
| Anticyclic citrullinated peptide antibody positive | 24 | — |
| Radiographic evidence | ||
| Juxta-articular osteopenia | 15 | — |
| Soft-tissue/fusiform swelling | 4 | — |
| Joint space narrow | 17 | — |
| Periarticular erosions | 14 | — |
| Subluxations | 7 | — |
| Comorbid gout | 6 | — |
| Joint involvement | ||
| Shoulder, elbows | 12 | — |
| Knees | 20 | — |
| Ankles | 18 | — |
| Metacarpophalangeal (MPT) joints | 27 | — |
| Proximal interphalangeal joints | 32 | — |
| Second through fifth MPT joints | 17 | — |
| Wrist | 37 | — |
| Hands | 15 | — |
| Feet | 6 | — |
| Not recorded | 27 | — |
| Number of involved small joints | ||
| 0–1 | 9 | — |
| 2 | 16 | — |
| 3–10 | 42 | — |
| Not recorded | 33 | — |
| Disease duration, years | ||
| 0–4 | 25 | — |
| 5–9 | 14 | — |
| 10–19 | 31 | — |
| ≥ 20 | 23 | — |
| Unknown | 6 | — |
p = 0.16.
p < 0.01.
— = not available.
Variations over Time
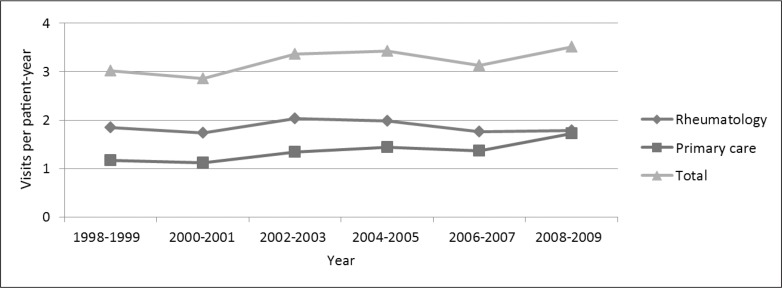
All variations over time were significant at p < 0.005 unless otherwise stated. Between 1998 and 2009, the adjusted annual visit rate to the Rheumatology Department decreased by 3%; visits to primary care physicians with the primary reason for visit being RA increased by 47% (Figure 2).
Figure 2.
Trends in rates of visits to rheumatology and primary care physicians.a,b
a All results for 28,601 patients with rheumatoid arthritis were adjusted for age, sex, race/ethnicity, comorbidity, and Medical Center.
b p values for change over time were < 0.01 for all visit types.
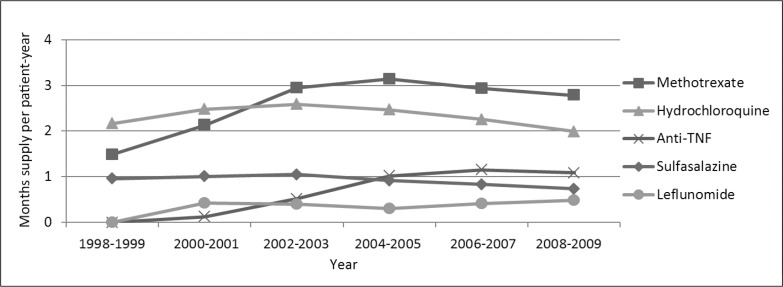
Drug use was measured in months of use per patient-year, including both users and nonusers. This measure accounted for both prevalence of drug use and duration of use among users. The use of anti-TNF agents increased from essentially zero to an average 3% of the follow-up time (including both users and nonusers; Figure 3). Etanercept use increased consistently from its introduction in 1998 through the 2006–2007 period, but then declined slightly. Adalimumab use increased consistently from the time of its introduction through the end of the study period. Infliximab use peaked in 2004–2005. Methotrexate use nearly doubled, from 13% to 23% of the time per patient-year in 2004–2005, but then declined slightly. Use of sulfasalazine and hydrochloroquine increased from 1998–1999 to 2002–2003, but then declined through 2008–2009. Leflunomide did not come into use until 2000; by 2008–2009, the average patient with RA used leflunomide 4% of the time. Across these agents, DMARD use increased for the average RA-affected patient, in months per year, from 38% in 1998–1999 to 65% in 2006–2007, but then declined to 63% in 2008–2009.
Figure 3.
Trends in average drug use (months per patient-year) of disease-modifying antirheumatic drugs among users and nonusers.a,b
a All results for 28,601 patients with rheumatoid arthritis were adjusted for age, sex, race/ethnicity, comorbidity, and Medical Center.
b All p values were < 0.0001 except for hydrochloroquine (p = 0.47) and sulfasalazine (p = 0.34). TNF = tumor necrosis factor.
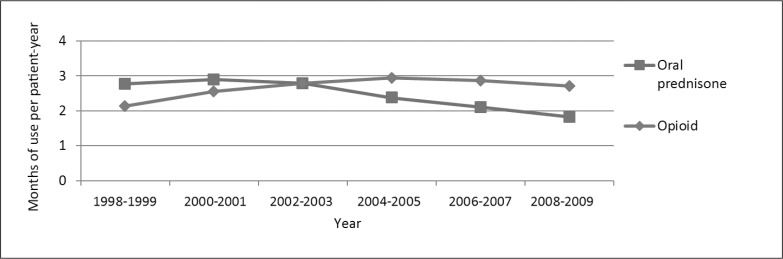
Between 1998 to 1999 and 2008 to 2009, use of oral prednisone decreased from 23% to 15% of the time (Figure 4). The dose of oral prednisone declined as well. Opioid use increased from 18% in 1998–1999 to 25% of the time in 2004–2005, but then declined to 23% of the time in 2008–2009. Across the study period, 60% of opioids were prescribed by rheumatologists and 23% by primary care physicians. The incidence rates of hospitalized pneumonia (average, 73.3 cases per 1000 patient-years) and opportunistic infection (2.9/1000 patient-years) did not vary over time (p values = 0.59 and 0.27, respectively).
Figure 4.
Trends in prednisone and opioid exposure among users and nonusers.a,b
a All results for 28,601 patients with rheumatoid arthritis were adjusted for age, sex, race/ethnicity, comorbidity, and Medical Center.
b p values were < 0.0001.
The primary metric used in the present study was months of drug use in relation to months of observation with RA, which captures adherence with DMARD. In contrast, past studies have focused on the percentage of patients who received at least 1 dispensing of DMARD in each year; in the present study, we observed 75% to 83% of patients to have received at least 1 dispensing (or infliximab infusion) in each year.
Differences across Medical Centers
Adjusted rates of outpatient visits to rheumatology (ratio of 75th to 25th percentile, 1.5) and primary care (ratio, 1.3) differed across the 12 Medical Centers, as did average months of use per patient-year (including both users and nonusers) of DMARDs, prednisone, and opioid per patient Population Variations in Rheumatoid Arthritis Treatment and Outcomes, Northern California, 1998–2009 (all p values < 0.0001; Table 3). The largest difference was in the use of infliximab, which differed by a factor of 13.9 across the interquartile range. However, the incidence rates of pneumonia (p = 0.40) and opportunistic infection (p = 0.61) did not differ significantly across Medical Centers. One center had a disproportionate number of African Americans, and another had a disproportionate number of Asian Americans; otherwise, the Medical Center populations were similar in age, sex, race, and comorbidity; adjustment for these variables did not change the estimates to an important degree.
Table 3.
Interquartile ranges across 12 Medical Centers of visits, drugs, and outcomes among patients with rheumatoid arthritis, 1998–2009a
| Characteristic | 25th percentile | Median | 75th percentile | Ratio of 75th percentile to 25th percentile |
|---|---|---|---|---|
| Outpatient visits per patient-year | ||||
| Rheumatology | 1.5 | 1.9 | 2.3 | 1.5b |
| Primary care | 1.2 | 1.4 | 1.6 | 1.3b |
| Average anti-TNF agent usec | ||||
| Any | 0.5 | 0.7 | 0.9 | 1.8d |
| Etanercept | 0.3 | 0.3 | 0.5 | 1.9d |
| Adalimumab | 0.06 | 0.12 | 0.15 | 2.6d |
| Infliximab | 0.03 | 0.08 | 0.37 | 13.9d |
| Average nonbiologic use | ||||
| Hydrochloroquine | 2.1 | 2.3 | 2.5 | 1.2b |
| Sulfasalazine | 0.5 | 0.7 | 0.8 | 1.7b |
| Methotrexate | 2.0 | 2.4 | 2.5 | 1.3b |
| Leflunomide | 0.3 | 0.3 | 0.6 | 2.5b |
| Glucocorticoid | 2.2 | 2.7 | 3.2 | 1.5b |
| Opioid | 2.5 | 2.8 | 3.0 | 1.2b |
| Infection events per patient-year | ||||
| Hospitalized pneumoniae | 0.55 | 0.70 | 0.78 | 1.4 |
| Opportunistic infectionse | 0.02 | 0.03 | 0.04 | 1.6 |
Adjusted for age, sex, race/ethnicity, comorbidity, and calendar year in all 28,601 patients.
p values < 0.0001.
In months of use per patient-year, including users and nonusers.
Per 1000 patient-years.
p value for hospitalized pneumonia was 0.40, and for opportunistic infections was 0.61.
TNF = tumor necrosis factor.
DISCUSSION
Practice variations can be used to identify targets for the development of guidelines, quality measures, and quality improvement. KP’s community-based setting and detailed computerized clinical data provide an excellent opportunity to assess practice variations. During a 12-year period, 28,601 adults with RA showed shifts in treatment patterns, including an increasing rate of primary care visits and increasing use of DMARDs. Total DMARD use increased for the average patient with RA to 63% of follow-up in 2008–2009. Similar to previous findings in the Veterans Affairs population,14 this increase was predominantly related to higher rates of continuation of methotrexate and increased uptake of anti-TNF agents. Nearly half of all visits coded for RA were to primary care physicians, with the rate of these visits increasing nearly 50% over the study period. A comanagement by generalists and subspecialists has not been comprehensively evaluated, although previous studies suggest lower use of DMARD among patients in primary care, likely reflecting lower disease activity.6 Comanagement and team-based care of RA are important topics for further research.
We further observed important variations in use of infliximab and adalimumab, with more modest variations in etanercept and nonbiologic DMARDs. We suspect that clinic-based variation in use of anti-TNF therapy may have been a consequence of the drugs’ relatively recent introduction, resulting in differences in rheumatologists’ knowledge, attitudes, and preferences toward use. In addition, because infliximab is infused and not injected, it was not subject to the Medicare “donut hole,” with some rheumatologists using it for that reason. The approach to addressing patients’ cost barriers may have varied from Medical Center to Medical Center. The study results suggest opportunities for standardizing DMARD use across settings.
This study was designed to assess variations at the population level. The increased use of methotrexate, leflunomide, and anti-TNF agents together with the declining use of hydrochloroquine and sulfasalazine suggest a more aggressive approach to therapy. However, increased adherence and continuation of therapy among patients receiving a prescription remains an important topic for future research. Furthermore, brief patient-reported outcomes, such as might be adapted from the Health Assessment Questionnaire, will become feasible to assess during routine clinical care, and these outcomes will be valuable for future research studies.
Past studies of variations in care for RA have been conducted in population-based studies, cohorts of insured patients (through administrative data), and rheumatology cohorts, with the latter having high levels of DMARD use as expected.6 We observed 75% to 83% of patients to have received at least 1 dispensing of a DMARD. The frequency of DMARD use (number of patients with at least 1 dispensing in each year) ranged from 16% to 87% in 245 Medicare managed care plans (2005–2008), compared with 75% to 83% in the present study.15 In the National Ambulatory Medical Care Survey, 1996–2007, DMARD was examined at the unit of the visit and not the patient, with 47% of 859 visits across the entire study period having documentation of DMARD use.16 In the TennCare population, the proportion of patients with RA (N = 23,342) who received at least 1 dispensing of anti-TNF or nonbiologic DMARD increased from 1995 through 2004, from 62% to 71%.17 The TennCare study was similar to ours in finding a reduction in glucocorticoid prescribing and an increase, through 2004, of opioid prescribing. In the West Virginia Medicaid data in 2003, the proportion of patients with RA aged 50 to 64 years (N = 143,211) with a narcotic dispensing was quite high at 68%; glucocorticoid use was 48%; DMARD use was 40%; and use of a biologic agent, 12%.18
In the years that opiate use was rising in the present study, KPNC was engaged in an aggressive program to limit the use of cyclooxygenase 2 inhibitors and other nonsteroidal anti-inflammatory drugs, particularly among older patients, who were deemed appropriate for low-level opioids such as hydrocodone-acetaminophen (Vicodin). Opioid use may be related to RA or comorbidities; increasing use reflects national trends.19 We cannot comment on whether this increase was appropriate because we did not analyze patient-level data on the indication for opioid use. Optimal use of opioids remains an area for intervention.
The use of prednisone for treatment of RA is controversial20; in the US, many physicians try to avoid using the drug, particularly at higher doses. Recent studies using administrative data have observed increased risk of serious infection, even with low doses of corticosteroid.21,22 We observed declining use of prednisone during the study period; however, this decline was not linked to a reduction in the rates of pneumonia and opportunistic infection. A difference between this and earlier studies was the design; we did not compare prednisone users with nonusers, which is subject to bias by disease severity, but rather examined changes in both prednisone use and infection risk over time in the population of users and nonusers.
A minor limitation of the study is the inclusion of persons without RA in the study population. The positive predictive value of the case-finding algorithm we used, 77%, was similar to the value of 76% reported in a Canadian study using criteria more appropriate to health care utilization in Canada.23 Inclusion of persons without RA would depress the overall prevalence of outcomes and use of treatment. A more important limitation was lack of information on disease severity. However, our design minimized this limitation. Also, the variation in practice within KP most likely is lower than outside KP. Based on data from the 2003 California Health Interview Survey, the KP adult membership is very similar to the non-KP population with a health insurance plan other than Medicaid with regard to education and health; it differs by having fewer non-Hispanic whites and fewer members with very low and very high household incomes.24 Finally, the study cohort of 28,601 persons was somewhat older than expected, possibly reflecting greater use of medical care among older persons and the greater opportunity to record diagnoses of RA.
CONCLUSION
This study demonstrated a trend of more aggressive treatment in RA, consistent with treatment recommendations from the American College of Rheumatology. It is encouraging that use of prednisone decreased. Opioid use remains an important topic for investigation and intervention. Variation across Medical Centers was high for infliximab and adalimumab, suggesting physician differences and opportunities to further standardize use of newer agents. Despite increased use of DMARDs and decreased use of oral prednisone, we did not find evidence for a change in the risk of infection. We recommend further research to better understand comanagement and team-based care of RA, and to better standardize use of anti-TNF agents and opioids in RA.
Acknowledgments
This research was funded by a grant (1RC1086107) from the National Institutes of Health (NIH), National Institute for Allergy and Infectious Diseases, Bethesda, MD. Dr Herrinton was supported by NIH Grant R01 HS19912 and Agency for Healthcare Research and Quality Grants U18 HS010391, U18 HS017919, and R01 HS0215900. Dr Curtis receives support from NIH Grant K23 AR053351 and Agency for Healthcare Research and Quality Grant R01 HS018517. Dr Harrold was supported by NIH Grant K23 AR053856. Dr Gelfand was supported by NIH Grant K24 AR064310. Dr Asgari was supported by NIH Grants R01 CA166672 and R03 AR064014.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
Dr Herrinton has received research grants from Genentech, South San Francisco, CA; Procter & Gamble Co, Cincinnati, Ohio; and MedImmune, Gaithersburg, MD. Dr Harrold has a research contract with the Consortium of Rheumatology Researchers of North America (CORRONA), Southborough, MA. Dr Asgari has research contracts with Valeant, Laval, Quebec, Canada; and Pfizer Inc, New York, NY. Dr Gelfand has served as a consultant for and received honoraria from AbbVie, North Chicago, IL; Amgen Inc, Thousand Oaks, CA; Celgene Corp, Summit, NJ; Coherus Biosciences, Camarillo, CA; Eli Lilly and Co, Indianapolis, IN; LEO Pharma, Ballerup, Denmark; Merck, Kenilworth, NJ; Janssen Biotech Inc (formerly Centocor), Horsham, PA; Novartis Corp, Basel, Switzerland; and Pfizer; he has had grants or has pending grants from Amgen; Janssen Pharmaceuticals Inc, Titusville, NJ; AbbVie; Novartis; Eli Lilly; and Pfizer. Dr Wu has received research grants from AbbVie; Amgen; Coherus Biosciences; Eli Lilly; Janssen Pharmaceuticals; Merck; Novartis; Pfizer; Regeneron, Tarrytown, NY; and Sandoz International, Holzkirchen, Germany; he is a consultant for AbbVie; Amgen; Celgene; DUSA Pharmaceuticals Inc, Wilmington, MA; Eli Lilly; and Pfizer. Dr Curtis has received research funding from Genentech; UCB, Brussels, Belgium; AbbVie; Janssen Pharmaceuticals; Bristol-Myers Squibb Co, New York, NY; and Amgen. Other authors have reported no competing interests.
The National Institute for Allergy and Infectious Diseases did not play a role in the design, analysis, or interpretation of the study.
Indiscriminate
The chronical [sic] differs from the acute rheumatism in being joined with little or no fever, in having a duller pain, and commonly no redness, but the swellings are more permanent, and the disease of much longer duration; for if the acute species have continued some months, the other has continued for many years …. Both kinds of the rheumatism attack indiscriminately males and females, rich and poor.
— Commentaries on the History and Cure of Diseases, William Heberden, 1710–1801, English physician
References
- 1.Khanna D, Arnold EL, Pencharz JN, et al. Measuring process of arthritis care: the Arthritis Foundation’s quality indicator set for rheumatoid arthritis. Semin Arthritis Rheum. 2006 Feb;35(4):211–37. doi: 10.1016/j.semarthrit.2005.08.004. DOI: http://dx.doi.org/10.1016/j.semarthrit.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.MacLean CH, Saag KG, Solomon DH, Morton SC, Sampsel S, Klippel JH. Measuring quality in arthritis care: methods for developing the Arthritis Foundation’s quality indicator set. Arthritis Rheum. 2004 Apr 15;51(2):193–202. doi: 10.1002/art.20248. DOI: http://dx.doi.org/10.1002/art.20248. [DOI] [PubMed] [Google Scholar]
- 3.Disease—modifying anti-rheumatic drug therapy in rheumatoid arthritis [Internet] Washington DC: National Committee for Quality Assurance; 2014. [cited 2015 July 20]. Available from: www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality/2014TableofContents/DMARDs.aspx. [Google Scholar]
- 4.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012 May;64(5):625–39. doi: 10.1002/acr.21641. DOI: http://dx.doi.org/10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Rheumatology . Rheumatoid arthritis quality indicators [Internet] Atlanta, GA: American College of Rheumatology; c2014. [cited 2013 Oct 10]. Available from: www.rheumatology.org/practice/clinical/quality/RA.asp. [Google Scholar]
- 6.Schmajuk G, Solomon DH, Yazdany J. Patterns of disease-modifying antirheumatic drug use in rheumatoid arthritis patients after 2002: a systematic review. Arthritis Care Res (Hoboken) 2013 Dec;65(12):1927–35. doi: 10.1002/acr.22084. DOI: http://dx.doi.org/10.1002/acr.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrold LR, Peterson D, Beard AJ, Gurwitz JH, Briesacher BA. Time trends in medication use and expenditures in older patients with rheumatoid arthritis. Am J Med. 2012 Sep;125(9):937.e9–15. doi: 10.1016/j.amjmed.2011.11.014. DOI: http://dx.doi.org/10.1016/j.amjmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillegraven S, Kvien TK. Measuring disability and quality of life in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007 Oct;21(5):827–40. doi: 10.1016/j.berh.2007.05.004. DOI: http://dx.doi.org/10.1016/j.berh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Herrinton LJ, Liu L, Fireman B, et al. Time trends in therapies and outcomes for adult inflammatory bowel disease, Northern California, 1998–2005. Gastroenterology. 2009 Aug;137(2):502–11. doi: 10.1053/j.gastro.2009.04.063. DOI: http://dx.doi.org/10.1053/j.gastro.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. DOI: http://dx.doi.org/10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.The MIXED procedure [Internet] Cary, NC: SAS Institute Inc; c2015. [cited 2013 Oct 10]. Available from: http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#mixed_toc.htm. [Google Scholar]
- 12.Minalu G, Aerts M, Coenen S, et al. Application of mixed-effects models to study the country-specific outpatient antibiotic use in Europe: a tutorial on longitudinal data analysis. J Antimicrob Chemother. 2011 Dec;66(Suppl 6):vi79–87. doi: 10.1093/jac/dkr460. DOI: http://dx.doi.org/10.1093/jac/dkr460. [DOI] [PubMed] [Google Scholar]
- 13.Copas JB. Using regression models for prediction: shrinkage and regression to the mean. Stat Methods Med Res. 1997 Apr;6(2):167–83. doi: 10.1177/096228029700600206. DOI: http://dx.doi.org/10.1177/096228029700600206. [DOI] [PubMed] [Google Scholar]
- 14.Ng B, Chu A, Khan MM. A retrospective cohort study: 10-year trend of disease-modifying antirheumatic drugs and biological agents use in patients with rheumatoid arthritis at Veteran Affairs Medical Centers. BMJ Open. 2013 Apr 5;3(4):e002468. doi: 10.1136/bmjopen-2012-002468. pii. DOI: http://dx.doi.org/10.1136/bmjopen-2012-002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmajuk G, Trivedi AN, Solomon DH, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011 Feb 2;305(5):480–6. doi: 10.1001/jama.2011.67. DOI: http://dx.doi.org/10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012 Feb;64(2):184–9. doi: 10.1002/acr.20674. DOI: http://dx.doi.org/10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grijalva CG, Chung CP, Stein CM, Mitchel EF, Jr, Griffin MR. Changing patterns of medication use in patients with rheumatoid arthritis in a Medicaid population. Rheumatology (Oxford) 2008 Jul;47(7):1061–4. doi: 10.1093/rheumatology/ken193. DOI: http://dx.doi.org/10.1093/rheumatology/ken193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna R, Smith MJ. Utilization and costs of medical services and prescription medications for rheumatoid arthritis among recipients covered by a state Medicaid program: a retrospective, cross-sectional, descriptive, database analysis. Clin Ther. 2007 Nov;29(11):2456–67. doi: 10.1016/j.clinthera.2007.11.009. DOI: http://dx.doi.org/10.1016/j.clinthera.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010 Dec;100(12):2541–7. doi: 10.2105/AJPH.2009.180646. DOI: http://dx.doi.org/10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirwan JR. Combination therapy including glucocorticoids: the new gold standard for early treatment in rheumatoid arthritis? Ann Intern Med. 2012 Mar 6;156(5):390–1. doi: 10.7326/0003-4819-156-5-201203060-00014. DOI: http://dx.doi.org/10.7326/0003-4819-156-5-201203060-00014. [DOI] [PubMed] [Google Scholar]
- 21.Widdifield J, Bernatsky S, Paterson JM, et al. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013 Mar;65(3):353–61. doi: 10.1002/acr.21812. DOI: http://dx.doi.org/10.1002/acr.21812. [DOI] [PubMed] [Google Scholar]
- 22.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011 Dec 7;306(21):2331–9. doi: 10.1001/jama.2011.1692. DOI: http://dx.doi.org/10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res (Hoboken) 2013 Oct;65(10):1582–91. doi: 10.1002/acr.22031. DOI: http://dx.doi.org/10.1002/acr.22031. [DOI] [PubMed] [Google Scholar]
- 24.2011 member health survey [Internet] Oakland, CA: Kaiser Permanente Division of Research; 2011. [cited 2015 Mar 24]. Available from: www.dor.kaiser.org/external/DORExternal/mhs/index.aspx. [Google Scholar]