Abstract
Context:
The dental setting represents an unrealized opportunity to increase adherence to preventive services and improve health outcomes.
Objective:
To compare adherence to a subset of Healthcare Effectiveness Data and Information Set (HEDIS) measures among a population that received dental care with a population that did not receive dental care.
Design:
Using a retrospective cohort design, we identified 5216 adults who received regular dental care and 5216 persons who did not. The groups were matched on propensity scores, were followed for 3 years, and retained medical and dental benefits. Receipt of dental care was defined as 1 or more dental visits in each 12-month period.
Main Outcome Measures:
Outcome measures were assessed in a subpopulation that qualified for 1 of 5 HEDIS denominator groups (dental = 4184 patients; nondental = 3871 patients). They included 3 preventive measures (cervical, colorectal, and breast cancer screening), 4 chronic disease management services (hemoglobin A1c and low-density lipoprotein cholesterol testing, and nephropathy and retinopathy screening among the diabetes mellitus [DM] population), and 4 health outcome measures (poor glycemic control, low-density lipoprotein cholesterol control, blood pressure control in the DM population, and blood pressure control in the hypertensive population).
Results:
Dental care was associated with higher adherence to all three cancer screening measures, one of four disease management services (higher retinopathy screening), and three of four health outcomes (better glycemic control in the DM population and better blood pressure control in the DM and hypertensive populations).
Conclusions:
Dental care was associated with improved adherence to 7 of 11 HEDIS measures.
INTRODUCTION
An extensive body of research has shown correlations between oral health and systemic disease such as diabetes mellitis (DM), obesity, and heart disease.1–9 Expert panels have issued consensus statements outlining the biologic plausibility that periodontal disease may have an impact on systemic health via dissemination of bacterial species and their virulence factors from the mouth into circulation.10
Evidence that the receipt of dental care can improve health outcomes for systemic conditions is mixed, with some studies showing improvements and others showing no difference. For example, previous research has found that regular receipt of dental care among patients with DM can reduce the effects of periodontal inflammation,11–17 thereby improving glycemic control. Furthermore, a study by Mosen and colleagues18 found DM-specific hospital admissions and Emergency Department (ED) visits were lower in a population with DM that received ongoing dental care compared with a population that did not receive dental care. However, a recent randomized control trial on the effect of nonsurgical treatment of periodontal disease in Type 2 diabetic patients did not demonstrate improved hemoglobin A1c (HbA1c) values in patients with chronic periodontitis.19
Dental care can facilitate linkage to preventive and chronic disease care-management services among populations in need of those services. A study by Greenberg and colleagues20 found that nearly 20% of patients receiving care in the University of Medicine and Dentistry of New Jersey Dental School were identified as having previously unidentified cardiovascular disease risk factors after testing blood pressure and cholesterol, and administering a screening questionnaire in the dental setting. Moreover, previous research has found that both patients and dentists view dental providers as an entry to the larger medical system—and as important members of a larger health care team.21,22
Despite the potential benefits of providing linkage to needed preventive medical services in dental settings, the topic has largely been understudied, possibly because few health systems share a common delivery system to facilitate such a study. The objectives of this study were to study the association of receipt of dental care with adherence to a subset of Healthcare Effectiveness Data and Information Set (HEDIS) metrics,23 as mandated by the National Committee on Quality Assurance for Health Plan accreditation. Our hypothesis was that adherence to HEDIS metrics would be higher among a population that received ongoing dental care compared with a similar population that did not receive dental care.
METHODS
The institutional review board of Kaiser Permanente Northwest (KPNW) in Portland, OR, approved the study protocol and waived the need for patient consent for data use.
Study Setting and Data Sources
The study was conducted in collaboration with Permanente Dental Associates and KPNW, a nonprofit group-model health care system in southern Washington and northern Oregon, consisting of both a dental and medical health system. The medical system comprises 15 medical clinics and more than 500,000 members, whereas the dental program has 17 offices and serves approximately 229,000 dental members, with many of the dental clinics located on a medical campus. Demographics of KPNW members (age, sex, and race/ethnicity) are similar to those of the area population. The KPNW regional electronic databases provided data on patient membership, demographics, clinical data including weight and height, laboratory results, medical health care utilization, dental utilization, and dental periodontal risk status. A common health record number links medical and dental utilization files.
Patient Support Tool Used in Dental Clinics
KPNW is the only Region in the larger Kaiser Permanente delivery system to have an integrated dental program. In the Dental Program, the KPNW Patient Support Tool (PST) is used by nearly 100% of dentists and hygienists to inform members of existing medical care gaps during their dental visits. The PST has been described previously24 and is supported by an informatics system that enables tracking of patient appointments, clinicians, care gaps, reminders given, and follow-up care. The PST is Internet-based and graphically displays care gaps for each patient in a primary care physician’s panel, on the basis of current clinical guidelines and evidence.25–27
Care gaps included in the PST align closely with HEDIS metrics and include overdue status for preventive screening measures, for example, breast cancer or colorectal cancer (CRC) screening. The gaps also include care management metrics for populations with chronic disease, such as being overdue for an HbA1c test or, for those with DM, not having good glycemic control. Last, care gaps include health outcome measures such as populations with hypertension (HTN) and poor blood pressure control.
Specific to the Dental Program, dentists or hygienists print and review a tailored report on existing care caps for the individual member. Included in the report is a list of recommended departments and/or phone numbers to schedule appointments to close existing care gaps.
Population Selection
This was a retrospective cohort study of KPNW members who met 3 inclusion criteria over a 36-month observation between September 1, 2011, and August 31, 2014:
Age 18 to 80 years as of September 1, 2011
Three years of continuous medical and dental health coverage between September 1, 2011, and August 31, 2014
One or more primary care medical visits in each 12-month period between September 1, 2011, and August 31, 2014.
Dental Population
We further identified patients who received regular dental care over the 36-month observation period. The dental population received 1 or more hygiene prophylactic treatments and/or periodontal care visits in each of the same three 12-month periods: September 1, 2011, to August 31, 2012; September 1, 2012, to August 31, 2013; and September 1, 2013, to August 31, 2014. We chose the frequency of this care algorithm primarily to ensure that patients had at least yearly contact with the KPNW dental care delivery system, consistent with practice guidelines used by Permanente Dental Associates dentists.
Nondental Population
The nondental population met the initial population inclusion criteria but had no dental utilization visits during the 36-month observational period.
Propensity Score Matching
To make the dental and nondental population comparable, we constructed propensity scores for likelihood of being in the dental population.
Propensity scores were constructed for both the dental and nondental populations, generally available between September 1, 2011, and August 31, 2012, the first year of observation. We used a 1:1 propensity matching method, and scores were based on the following 12 characteristics: 1) age (on September 1, 2011), 2) sex, 3) race/ethnicity (white/unknown, nonwhite), 4) low socioeconomic status measure (SES; yes, no), 5) body mass index (BMI; < 24.99 kg/m2, 25.00–29.99 kg/m2, ≥ 30 kg/m2), 6) Charlson Comorbidity Index score (0, 1, 2-plus), 7) baseline periodontal risk status (low [no DM and no smoking], moderate [DM or smoking], high [DM and smoking]), 8) baseline DM status [DM; yes, no], 9) baseline smoking status (yes, no), 10) baseline primary care utilization (1 visit, 2–4 visits, 5-plus visits), 11) baseline ED utilization (1-plus admission, none), and 12) baseline hospital admissions (1-plus admission, none). We selected these measures to make the two populations as comparable as possible, with respect to demographics, medical comorbidities, periodontal risk factors, and medical utilization measures.
The rationale for selection of these variables in the propensity models was threefold. First, we selected measures associated with dental use in the literature: age,28,29 sex,30,31 race/ethnicity,28,29 SES,30,32,33 smoking status,34 and DM.35–37 Second, we selected primary care utilization—ensuring that both populations had similar levels of primary care utilization—an important facilitator to complete HEDIS metrics. Last, we selected BMI, Charlson Comorbidity Index, and ED and hospital admission measures to ensure that physical health and comorbidities were comparable between the two populations, important factors also influencing adherence with HEDIS metrics.
Race was self-reported and derived from the electronic medical record (EMR). We assessed SES through census block (demographic data are available through the EMR). Low SES was defined as more than 20% of the individual’s census block with less than a 12th-grade education, or more than 20% of the individual’s census block below the federal family poverty level and was assessed as of September 1, 2011.38 For BMI, height and weight values were collected as close as possible to September 1, 2011, with a lookback period of 24 months (September 1, 2009). The Charlson Comorbidity Index is a well-established risk score in the medical literature.39 The index was calculated on the basis of inpatient and outpatient utilization information between September 1, 2011, and August 31, 2012, and it measured the presence of up to 19 comorbid conditions. The periodontal risk measures documented smoking and DM status between September 1, 2011, and August 31, 2012, in the EMR. Those with DM and no documentation of current smoking status were classified as moderate risk; those with current smoking were classified as high risk.18 Primary care utilization, ED utilization and hospital admissions all were calculated during the baseline year between September 1, 2011, and August 31, 2012.
Summary of Patient Population
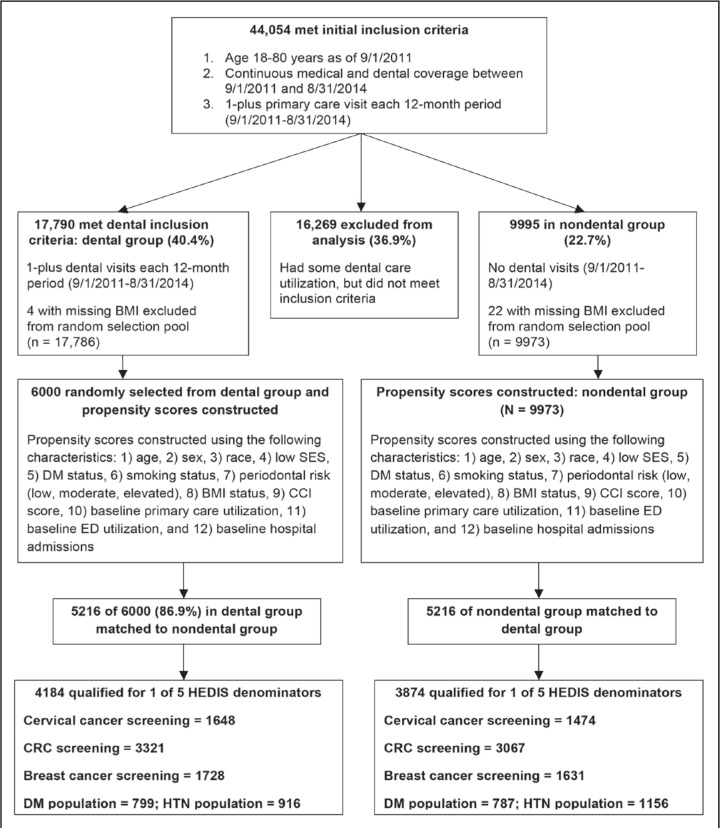
The total number of patients meeting the initial inclusion criteria of having continuous medical and dental coverage, and receiving regular primary care over the 36-month observation period, was 44,054. Of those patients, 17,790 (40.4%) met the criteria for continuous dental visits over time; 9995 (22.7%) had no dental visits; and 16,269 (36.9%) had some dental visits, but did not meet the inclusion criteria threshold. Of the 17,790 dental users, 4 were excluded from analysis because of missing BMI, leaving 17,786 for analysis, whereas 22 were excluded from the nondental group, leaving 9973 for analysis.
We used a 3-step process to select the final population for analysis. First, we selected a representative random sample of 6000 (of 17,786) from the dental population. The rationale was to select the largest sample size possible to match with the nondental population (n = 9973). Second, we constructed propensity scores for the random sample of the dental population (n = 6000; variables described earlier) and the eligible nondental population (n = 9973). Of the 6000 individuals in the dental population, we were able to match 5216 (86.9%) to the nondental population for a total of 10,432 available for analysis. The process flow to identify the patient population is described in Figure 1.
Figure 1.
Population process flow diagram.
BMI = body mass index; CCI = Charlson Comorbidity Index; CRC = colorectal cancer; DM = diabetes mellitus; ED = Emergency Department; HEDIS = Healthcare Effectiveness Data and Information Set; HTN = hypertension; SES = socioeconomic status.
Study Design
This study is a retrospective cohort analysis of 10,432 members: 5216 members with continuous dental utilization during a 36-month period were 1:1 propensity matched with 5216 similar members with no dental utilization. Among the 10,432 members, a further subpopulation that qualified for 1 of 5 HEDIS denominator groups was selected for outcomes analysis: 1) cervical cancer screening population (N = 3122; dental = 1648 nondental = 1474), 2) CRC screening population (N = 6388; dental = 3321, nondental = 3067), 3) breast cancer screening population (N = 3359; dental = 1728, non-dental = 1631), 4) DM population (N = 1586; dental = 799, nondental = 787) and 5) HTN population (N = 2072; dental = 916; nondental = 1156).
Study Variables
All outcome measures were HEDIS metrics routinely trended by KPNW on a monthly basis. They included 3 preventive screening measures, 4 chronic disease management measures, and 4 health outcome measures. Outcome measures were assessed in August 2014, the last month of the 36-month observation period, among those who qualified for each of the HEDIS denominator groups. Detailed definitions of each denominator population and lookback periods to assess numerator information are described in Table 1.40
Table 1.
HEDIS Measurement Specifications
| Measure | Denominator | Numerator |
|---|---|---|
| Preventive screening services | ||
| Cervical cancer screening | Women aged 24–64 years due for screening | At least 1 Papanicolaou test in previous 3 years (9/1/2011–8/31/2014) or 1 cervical cytology/human papillomavirus (HPV) co-test in the previous 5 years for women > 29 years old |
| Colorectal cancer (CRC) screening | Adults aged 50–75 years | One or more screenings for CRC. Eligible screenings are FOBT/FIT screening in previous 12 months (9/1/2013–8/31/2014), flexible sigmoidoscopy in last 5 years, (9/1/2009-8/31/2014), or colonoscopy in last 10 years (9/1/2005–8/31/2014) |
| Breast cancer screening | Women aged 52–74 years | One or more mammograms in previous 2 years (9/1/2012–8/31/2014) |
| Chronic disease management services | ||
| DM population: HbA1c screening | Adults with diabetes (aged 18–75 years) | HbA1c test performed in previous 12 months (9/1/2013–8/31/2014) |
| DM population: low-density lipoprotein cholesterol (LDL-C) screening | Adults with diabetes (aged 18–75 years) | LDL-C test performed in previous 12 months (9/1/2013–8/31/2014) |
| DM population: nephropathy screening | Adults with diabetes (aged 18–75 years) | Includes any of the following between 9/1/2013 and 8/31/2014: 1) claim/encounter with a code to indicate evidence of treatment of nephropathy, 2) nephrologist visit, 3) abnormal result of urine microalbumin test, 4) prescription for a drug in ACE inhibitor or ARB class, or 5) nephropathy screening examination |
| DM population: retinopathy screening | Adults with diabetes (aged 18–75 years) | A diabetic retinopathy screening test in measurement year (9/1/2013–8/31/2014) or normal results of an eye examination for diabetes retinopathy in year before measurement year (9/1/2012–8/31/2013) |
| Health outcome measures | ||
| DM population: HbA1c > 9% | Adults with diabetes (aged 18–75 years) | Most recent HbA1c level > 9.0% or test not done during measurement year (9/1/2013–8/31/2014) |
| DM population: low-density lipoprotein cholesterol (LDL-C) level < 100 mg/dL | Adults with diabetes (aged 18–75 years) | Most recent LDL-C level < 100 mg/dL during measurement year (9/1/2013–8/31/2014) |
| DM population: good blood pressure control | Adults with diabetes (aged 18–75 years) | Most recent blood pressure systolic value < 140 mmHg and diastolic value < 90 mmHg during measurement year (9/1/2013–8/31/2014). Members with missing blood pressure values are not in good control. |
| Hypterension (HTN) population: good blood pressure control | Adults with HTN (aged 18–85 years) diagnosed in first 6 months of measurement year (9/1/2013–2/28/2014) | Most recent blood pressure systolic value < 140 mmHg and diastolic value < 90 mmHg obtained after HTN diagnosis (9/1/2013–8/31/2014). Members with missing blood pressure values are not in good control. |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; DM = diabetes mellitus; FOBT/FIT = fecal occult blood test/fecal immunochemical test; HbA1c = hemoglobin A1c; HEDIS = Healthcare Effectiveness Data and Information Set.
We selected this approach to measure HEDIS outcomes in August 2014 to maximize the potential effectiveness of receipt of ongoing dental care over the 36-month observation period. We based the selection of outcome measures for this study on 2 factors: completion of measures that could be easily initiated by the patient without the need for a physician referral, for example, cancer screening tests, and measures specific to DM and HTN, given the previous association of oral health with these chronic conditions as established in the peer-reviewed literature.
Preventive Screening Measures
We included 3 cancer prevention screening measures for the following populations (see Table 1 for measurement specifications): 1) cervical cancer screening of women aged 24 to 64 years, 2) CRC screening of men and women aged 50 to 75 years, and 3) breast cancer screening of women aged 52 to 74 years.
Chronic Disease Management Measures
To gauge appropriate screening tests in the recommended time intervals, we included 4 screening measures for the DM population (in which adults were aged 18 to 75 years): 1) HbA1c screening of adults who received the HbA1c test, 2) low-density lipoprotein cholesterol (LDLC) screening of adults who received an LDL-C test, 3) retinopathy screening of adults who received retinopathy screening or had a normal result of an eye examination for retinopathy, and 4) diabetic nephropathy screening of adults who received retinopathy screening or had evidence of nephropathy documented.
Health Outcome Measures
We included 4 health outcome measures in the analysis in adults aged 18 to 75 years: 1) good blood pressure control in population with HTN, adults with a diagnosis of HTN and most recent blood pressure value in good control (diastolic < 140 mmHg; systolic < 90 mmHg), 2) good blood pressure control in population with DM, adults with the most recent blood pressure value in good control (diastolic < 140 mmHg; systolic < 90 mmHg), 3) poor glycemic control in population with DM, adults with the most recent blood HbA1c value above 9%, and 4) good LDL-C control in the DM population, adults with the most recent LDL-C value below 100 mg/dL.
Analysis
We first compared population characteristics among the propensity-matched samples using descriptive statistics. Next, we compared population characteristics between the dental and nondental population in each of the 5 eligible HEDIS denominator subgroups. Third, we examined the bivariate association of receipt of regular dental care with study outcome measures using χ2 analysis. Last, multivariate logistic regression was used to analyze the independent effect of regular receipt of dental care with outcome measures, adjusting for population characteristics that differed between the dental and nondental groups in each HEDIS-eligible denominator population.
RESULTS
Table 2 presents the baseline characteristics of the total population that received dental care compared with the population that received no dental care. As expected, no statistical differences were observed in the propensity-matched variables in the analysis. For both the dental and nondental groups, the mean age was about 57 years, most patients were white, 40% were men, and less than 20% were considered of low SES. Nearly 20% of both groups had DM, less than 10% were current smokers, about 25% had moderate/high periodontal risk, and about 45% were obese. Reflecting a population that regularly uses primary medical care, about two-thirds in both groups had 2 or more primary care visits in a 12-month baseline period (September 1, 2011, to August 31, 2012), whereas about 15% had 1 or more ED visits, and less than 10% had 1 or more hospital admissions in the same period.
Table 2.
Population characteristics: dental group versus nondental group
| Measure | Dental group (n = 5216) | Nondental group (n = 5216) | p value |
|---|---|---|---|
| Demographics | |||
| Age, years (mean ± SD)a | 57.0 ± 14.2 | 57.2 ± 14.5 | 0.31 |
| Male, no. (%) | 2131 (40.9) | 2118 (40.6) | 0.80 |
| White (vs nonwhite), no. (%) | 4485 (86.0) | 4473 (85.8) | 0.74 |
| Low socioeconomic status, no. (%)b | 949 (18.2) | 945 (18.1) | 0.90 |
| Health status/comorbidities, no. (%)c | |||
| Diabetes | 926 (17.8) | 949 (18.2) | 0.56 |
| Current smoker | 437 (8.4) | 425 (8.2) | 0.67 |
| Periodontal risk measured | |||
| Low | 3913 (75.0) | 3895 (74.7) | 0.68 |
| Moderate/high | 1303 (25.0) | 1268 (24.3) | |
| Obesity status | |||
| BMI > 30 kg/m2 (obese),e no. (%) | 2318 (44.4) | 2381 (45.7) | 0.17 |
| Charlson Comorbidity Index score, no. (%) | |||
| 0–1 | 4266 (81.8) | 4210 (80.7) | 0.16 |
| 2+ | 950 (18.2) | 1006 (19.3) | |
| Utilization measures, no. (%)c | |||
| 1 primary care visit | 1694 (32.5) | 1736 (33.3) | 0.38 |
| 2+ primary care visits | 3522 (67.5) | 3480 (66.7) | |
| ED utilization (1+ visits) | 786 (15.1) | 773 (14.8) | 0.72 |
| Hospital admissions (1+ admissions) | 378 (7.3) | 350 (6.7) | 0.28 |
Age calculated as of September 1, 2011.
Low socioeconomic status defined as more than 20% of member census bloc: with less than 12th grade education or below the federal poverty level.
Measures assessed between September 1, 2011, and August 31, 2012.
Low periodontal risk = nonsmoker and diabetes mellitus (DM); moderate/high periodontal risk = smoker and/or DM.
Body mass index (BMI) assessment was as close as possible to September 1, 2009, with 2-year lookback window.
ED = Emergency Department; SD = standard deviation.
Population characteristics (between dental and nondental) were also similar in each HEDIS denominator subgroup, with 1 or 2 exceptions for each group (results not shown). For the cervical cancer screening population, the dental group was less likely to have BMI 30 kg/m2 or greater (38.0% vs 43.2%, p < 0.01) compared with the nondental group. For the CRC screening population, the dental population was slightly older (mean age = 62.4 vs 62.0 years, p < 0.01) and slightly more likely to be white (87.4% vs 85.2%; p < 0.01). In the breast cancer screening population, the dental population was slightly more likely to be white (88.5% vs 84.6%, p < 0.001). In the DM population, the dental population was slightly older (mean age = 63.0 vs 62.0 years, p < 0.05). Last, in the HTN population, the dental population was more likely to have a BMI of 30 kg/m2 and above (62.2% vs 57.7%, p < 0.05) and to use 2-plus primary care visits (75.7% vs 71.6%, p < 0.05). No other differences in population characteristics were observed. Specific population differences in HEDIS subgroups (eg, BMI ≥ 30 kg/m2 for the cervical cancer screening population) were adjusted in multivariate logistic regression models.
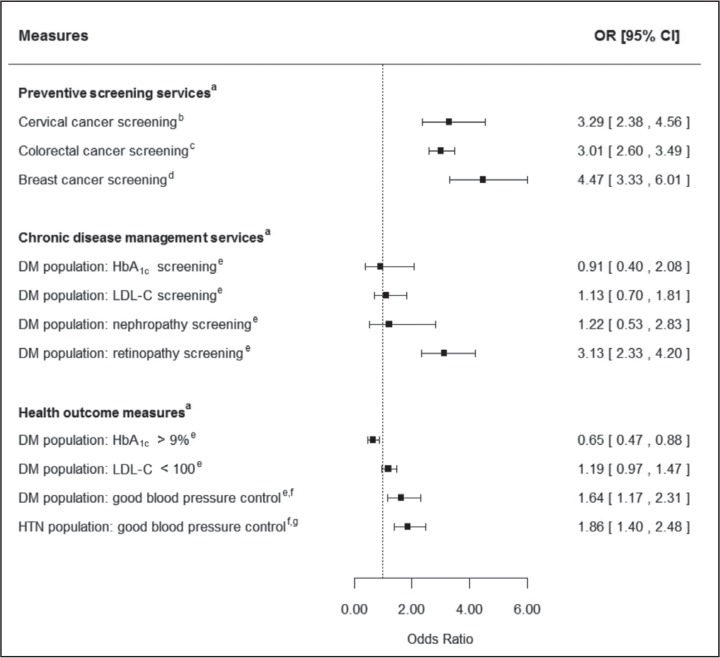
Table 3 and Figure 2 present bivariate and multivariate logistic regression results, respectively. We found significant relationships in 7 of 11 outcome measures. Those in the dental group were more likely to have completed all 3 preventive screening cancer measures compared with those in the nondental group: 1) cervical cancer screening (96.8% vs 90.2%; odds ratio [OR] = 3.29, 95% confidence interval [CI] = 2.38–4.56, p < 0.0001); 2) CRC screening (91.4% vs 77.7%; OR = 3.01, 95% CI = 2.60–3.49, p < 0.0001); and 3) breast cancer screening (96.6% vs 86.3%; OR = 4.47, 95% CI = 3.33–6.01, p < 0.0001).
Table 3.
Outcome measures by dental versus nondental groupa
| Measure | Dental group, no. (%) | Nondental group, no. (%) | p value |
|---|---|---|---|
| Preventive screening servicesa | |||
| Cervical cancer screeningb | 1596/1648 (96.8) | 1329/1474 (90.2) | < 0.0001 |
| Colorectal cancer screeningc | 3034/3321 (91.4) | 2382/3067 (77.7) | < 0.0001 |
| Breast cancer screeningd | 1669/1728 (96.6) | 1408/1631 (86.3) | < 0.0001 |
| Chronic disease management servicesa | |||
| DM population: HbA1c screeninge | 787/799 (98.5) | 776/787 (98.6) | 0.86 |
| DM population: LDL-C screeninge | 765/799 (95.7) | 747/787 (94.9) | 0.43 |
| DM population: nephropathy screeninge | 789/799 (98.8) | 774/787 (98.4) | 0.50 |
| DM population: retinopathy screeninge | 727/799 (91.0) | 597/787 (75.9) | < 0.0001 |
| Health outcome measuresa | |||
| DM population: HbA1c > 9%e | 76/799 (9.5) | 116/787 (14.7) | 0.001 |
| DM population: LDL-C < 100 mg/dLe | 549/799 (68.7) | 505/787 (64.2) | 0.06 |
| DM population: good BP controle,f | 739/799 (92.5) | 694/787 (88.2) | 0.004 |
| HTN population: good BP controlf,g | 841/916 (91.8) | 991/1156 (85.7) | < 0.0001 |
Healthcare Effectiveness Data and Information Set (HEDIS) metrics assessed in August 2014. Detailed definitions of each denominator population and lookback periods to assess numerator information are described in Table 1.
Includes 1648 members of dental population (n = 5216) and 1474 of nondental population (n = 5216) who qualified for HEDIS cervical cancer screening denominator.
Includes 3321 members of dental population and 3067 of nondental population who qualified for HEDIS colorectal cancer screening denominator.
Includes 1728 members of dental population and 1631 of nondental population who qualified for HEDIS breast cancer screening denominator.
Includes 799 members of dental population and 787 of nondental population who qualified for diabetes mellitus (DM) HEDIS denominator.
Good blood pressure control defined as systolic value < 140 mmHg and diastolic value < 90 mmHg.
Includes 916 of dental population and 1156 members of nondental population who qualified for hypertension (HTN) HEDIS denominator.
BP = blood pressure; HbA1c = hemoglobin A1c; LDL-C = low-density lipoprotein cholesterol.
Figure 2.
Multivariate logistic regression results: dental group vs nondental group.
aHealthcare Effectiveness Data and Information Set metrics assessed between September 1, 2013, and August 31, 2014.
bCervical cancer screening model adjusted for body mass index ≥ 30 kg/m2 vs < 30 kg/m2 (reference group).
cColorectal cancer screening model adjusted for age (continuous) and race (white vs nonwhite [reference group]).
dBreast cancer screening model adjusted for race (white vs nonwhite [reference group]).
eDiabetes mellitus (DM) population models adjusted for age.
fGood blood pressure control was defined as systolic value < 140 mmHg and diastolic value < 90 mmHg.
gHypertension (HTN) population model adjusted for body mass index ≥ 30 kg/m2 vs < 30 kg/m2 (reference group) and primary care utilization (2-plus visits vs 1 visit [reference group]).
CI = confidence interval; HbA1c = hemoglobin A1c; LDL-C = low-density lipoprotein cholesterol (mg/dL); OR = odds ratio.
Only 1 of 4 chronic disease management measures reached statistical significance: retinopathy screening (91.0% vs 75.9%; OR = 3.13, 95% CI = 2.33–4.20, p < 0.0001). Last, 3 of 4 health outcome measures reached statistical significance. Those in the dental care group were more likely to have good blood pressure control for HTN (91.8% vs 85.7%; OR = 1.86, 95% CI = 1.40–2.48, p < 0.0001) and DM (92.5% vs 88.2%; OR = 1.64, 95% CI = 1.17–2.31, p = 0.006) populations. In addition, the dental group was less likely to have poor glycemic control (9.5% vs 14.7%; OR = 0.61, 95% CI = 0.45–0.83, p = 0.002) than the nondental group.
DISCUSSION
We found that receipt of ongoing dental care was associated with better adherence to 7 of 11 HEDIS metrics compared with a similar population that did not receive dental care. Specifically, the dental group was more likely to complete 3 cancer screening tests (cervical, CRC, and breast) and retinopathy screening (among adults with DM) compared with the nondental group. Last, the dental group had better health outcomes, with both the DM and HTN populations more likely to have good blood pressure control, and adults with DM more likely to have good glycemic control.
This study is the first we are aware of to find that patients who receive ongoing dental care are more likely to complete needed cancer screenings compared with those not receiving dental care. The findings are intuitive in that populations receiving dental care in KPNW are notified of being overdue for cancer screenings during their dental visits and encouraged to follow-up with their primary care clinicians to complete testing. Thus, the availability of the PST in the dental setting may have encouraged service linkage to complete needed screening tests.
This study is also the first of which we are aware to find an association between dental care and improved blood pressure control. However, the results are consistent with previous research that found that measurement of blood pressure in a dental setting could identify HTN. For example, Friman and colleagues41 found that about 14% of patients receiving care in a dental setting had diastolic blood pressure values high enough to warrant further follow-up in a medical setting. Furthermore, Greenberg and colleagues22 found that nearly 20% of patients receiving care in the University of Medicine and Dentistry of New Jersey Dental School were identified as having previously unidentified cardiovascular disease risk factors when their blood pressure and cholesterol were tested and a screening questionnaire was administered in the dental setting.
The finding that receipt of dental care was associated with better glycemic control among the DM population is consistent with previous research findings that also found improved glycemic control. Kiran et al,13 Stewart and colleagues,15 and Yun et al17 found that receipt of periodontal therapy improved glycemic control for patients with Type 2 DM. Similarly, Mosen and colleagues18 found that receipt of dental care was associated with HbA1c below 7% among adults with DM; however, the finding did not remain significant in multivariate analysis.
Two factors may explain the lack of differences among the dental and nondental group for 3 DM screening measures: HbA1c, LDL-C, and nephropathy screening. First, both groups had similar levels of primary care utilization, ensuring similar levels of contact with the medical system. Second, KPNW consistently scores high on these 3 HEDIS measures overall, with 90% or more of patients completing screening.
The higher HEDIS scores in the dental group are consistent with ongoing operational reports provided by KPNW. For example, a February 2015 report measured two items by medical/dental department: 1) ranking of care gap closure rates and 2) total member contacts. A review of the information reveals that the KPNW Dental Program ranks third in closing care gaps, behind only Internal Medicine and Family Practice. In addition, the KPNW Dental Program has the second greatest number of patient contacts (behind only Family Practice) in the total dental and medical system, providing ample opportunity to close care gaps.
Our study findings are important from both clinical and policy perspectives. Although results are associative and not causal, they suggest that the regular receipt of dental care may have the potential to increase performance on HEDIS metrics. They also suggest that dental providers may play an important role in educating patients about and linking them to needed medical services, although more research is needed to understand what specific mechanisms in the dental setting are most effective to close care gaps. Although KPNW is only one of the few health delivery systems that share medical and dental records information, education and enhanced service linkage to complete needed medical services can occur in dental practices that are not linked by a common medical and dental health record.
Our study has several limitations. First, we do not know whether the better adherence to HEDIS metrics found in the study was causally related to receipt of regular dental care, or whether the population that received dental care was inherently different from the nondental population. Specifically, the population that received ongoing dental care may have been more compliant with health behaviors, resulting in improved outcomes. In response to this limitation, we created 2 similar populations using propensity score matching on critical factors available in the EMR that could influence HEDIS outcomes: age, sex, SES, DM status, smoking status, periodontal risk factors, obesity status, severity of illness/comorbidity characteristics, and prior utilization. Furthermore, both groups had 1 or more primary care visits every 12 months over the 36-month observation period, ensuring contact with the medical system in both populations, with the major difference being receipt of dental care. Therefore, although unmeasured differences may still exist between the dental and nondental populations, these differences were reduced with the rigorous propensity matching used in this analysis. The second limitation is that HEDIS measures were assessed at only 1 point in time; thus, we cannot assess change scores over time. Finally, our results cannot be generalized outside dental systems without a shared dental and medical EMR.
CONCLUSION AND FUTURE DIRECTIONS
We found receipt of dental care was associated with improved adherence to 7 of 11 HEDIS measures studied. These findings included: 3 of 3 cancer preventive screening services, 1 of 4 disease management services (higher retinopathy screening), and 3 of 4 health outcomes (better glycemic control in the DM population and better blood pressure control in the DM and HTN populations).
Future research is needed to understand which mechanisms, if any, in the dental setting are most strongly associated with higher HEDIS scores. For example, is higher use of the PST in KPNW dental clinics more strongly associated with higher HEDIS performance compared with lower use of the PST? Also, more research is needed to understand whether dental clinics with closer proximity to medical office buildings have higher performance on HEDIS metrics compared with dental clinics that are farther way from medical office buildings. Moreover, further research is needed assessing HEDIS scores for two time periods; specifically matching on baseline scores among a population with no prior receipt of dental care – to better assess the association of receipt of dental care with HEDIS outcomes.
Future prospective research is needed to better understand the association of receipt of dental care with improvement in health outcomes over time. For example, how can rigorous interventions in the dental setting centered on motivational interviewing and behavior change lead to sustained changes in weight loss, smoking cessation, and reduction in critical laboratory values (eg, cholesterol values) prospectively over time? Such research can add to the knowledge base in exploring how the dental setting can integrate with the larger medical system to improve not only links to needed services but also health outcomes.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The authors (s) have no conflicts of interest to disclose.
References
- 1.Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ. 1989 Mar 25;298(6676):779–81. doi: 10.1136/bmj.298.6676.779. DOI: http://dx.doi.org/10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullinan MP, Ford PJ, Seymour GJ. Periodontal disease and systemic health: current status. Aust Dent J. 2009 Sep;54(Suppl 1):S62–9. doi: 10.1111/j.1834-7819.2009.01144.x. DOI: http://dx.doi.org/10.1111/j.1834-7819.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008 Apr;122(4):417–33. doi: 10.1016/j.puhe.2007.07.004. DOI: http://dx.doi.org/10.1016/j.puhe.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Lamster IB, Lalla E, Borgnakke WS, Taylor GW. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008 Oct;139(Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. DOI: http://dx.doi.org/10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 5.Mealey BL, Oates TW, American Academy of Periodontology Diabetes mellitus and periodontal diseases. J Periodontol. 2006 Aug;77(8):1289–303. doi: 10.1902/jop.2006.050459. DOI: http://dx.doi.org/10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 6.Skamagas M, Breen TL, LeRoith D. Update on diabetes mellitus: prevention, treatment, and association with oral diseases. Oral Dis. 2008 Mar;14(2):105–14. doi: 10.1111/j.1601-0825.2007.01425.x. DOI: http://dx.doi.org/10.1111/j.1601-0825.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- 7.Ship JA. Diabetes and oral health: an overview. J Am Dent Assoc. 2003 Oct;134(Spec No):4S–10S. doi: 10.14219/jada.archive.2003.0367. DOI: http://dx.doi.org/10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 8.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993 Jan;16(1):329–34. DOI: http://dx.doi.org/10.2337/diacare.16.1.329. [PubMed] [Google Scholar]
- 9.Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008 Apr;14(3):191–203. doi: 10.1111/j.1601-0825.2008.01442.x. DOI: http://dx.doi.org/10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 10.Tonetti MS, Van Dyke TE, Working Group 1 of the Joint EFP/AAP Workshop Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013 Apr;84(4 Suppl):S24–9. doi: 10.1902/jop.2013.1340019. DOI: http://dx.doi.org/10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 11.Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):92–102. doi: 10.1034/j.1600-051x.29.s3.5.x. DOI: http://dx.doi.org/10.1034/j.1600-051X.29.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones JA, Miller DR, Wehler CJ, et al. Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol. 2007 Jan;34(1):46–52. doi: 10.1111/j.1600-051X.2006.01002.x. DOI: http://dx.doi.org/10.1111/j.1600-051X.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005 Mar;32(3):266–72. doi: 10.1111/j.1600-051X.2005.00658.x. DOI: http://dx.doi.org/10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 14.Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005 Sep;11(5):293–8. doi: 10.1111/j.1601-0825.2005.01119.x. DOI: http://dx.doi.org/10.1111/j.1601-0825.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 15.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001 Apr;28(4):306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. DOI: http://dx.doi.org/10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 16.Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab. 2008 Nov;34(5):497–506. doi: 10.1016/j.diabet.2008.03.006. DOI: http://dx.doi.org/10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Yun F, Firkova EI, Jun-Qi L, Xun H. Effect of non-surgical periodontal therapy on patients with type 2 diabetes mellitus. Folia Med (Plovdiv) 2007;49(1–2):32–6. [PubMed] [Google Scholar]
- 18.Mosen DM, Pihlstrom DJ, Snyder JJ, Shuster E. Assessing the association between receipt of dental care, diabetes control measures and health care utilization. J Am Dent Assoc. 2012 Jan;143(1):20–30. doi: 10.14219/jada.archive.2012.0014. DOI: http://dx.doi.org/10.14219/jada.archive.2012.0014. [DOI] [PubMed] [Google Scholar]
- 19.Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013 Dec 18;310(23):2523–32. doi: 10.1001/jama.2013.282431. DOI: http://dx.doi.org/10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg BL, Glick M, Goodchild J, Duda PW, Conte NR, Conte M. Screening for cardiovascular risk factors in a dental setting. J Am Dent Assoc. 2007 Jun;138(6):798–804. doi: 10.14219/jada.archive.2007.0268. DOI: http://dx.doi.org/10.14219/jada.archive.2007.0268. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML. Dentists’ attitudes toward chairside screening for medical conditions. J Am Dent Assoc. 2010 Jan;141(1):52–62. doi: 10.14219/jada.archive.2010.0021. DOI: http://dx.doi.org/10.14219/jada.archive.2010.0021. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg BL, Kantor ML, Jiang SS, Glick M. Patients’ attitudes toward screening for medical conditions in a dental setting. J Public Health Dent. 2012 Winter;72(1):28–35. doi: 10.1111/j.1752-7325.2011.00280.x. DOI: http://dx.doi.org/10.1111/j.1752-7325.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 23.Rectal cancer: NCCN guidelines 20th annual edition [Internet] [password protected] Fort Washington, PA: National Comprehensive Cancer Network; 2015. [cited 2015 Jul 8]. Available from: www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [Google Scholar]
- 24.Livaudais G, Unitan R, Post J. Total panel ownership and the panel support tool—”it’s all about the relationship”. Perm J. 2006 Summer;10(2):72–9. doi: 10.7812/tpp/06-002. DOI: http://dx.doi.org/10.7812/TPP/06-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010 Jan;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. DOI: http://dx.doi.org/10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC. National Heart, Lung, and Blood Institute AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006 May 16;113(19):2363–72. doi: 10.1161/CIRCULATIONAHA.106.174516. DOI: http://dx.doi.org/10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 27.Recommendations of the US Preventive Services task force Section 2 Recommendations for adults Guide to clinical preventive services, 2012 [Internet] Rockville, MD: Agency for Healthcare Research and Quality; 2012. [cited 2015 Jul 23]. Available from: http://archive.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide2012/section2.html. [Google Scholar]
- 28.Manski RJ, Moeller JF, Maas WR. Dental services. An analysis of utilization over 20 years. J Am Dent Assoc. 2001 May;132(5):655–64. doi: 10.14219/jada.archive.2001.0243. DOI: http://dx.doi.org/10.14219/jada.archive.2001.0243. [DOI] [PubMed] [Google Scholar]
- 29.Brown LJ, Lazar V. Dental care utilization: how saturated is the patient market? J Am Dent Assoc. 1999 Apr;130(4):573–80. doi: 10.14219/jada.archive.1999.0255. DOI: http://dx.doi.org/10.14219/jada.archive.1999.0255. Erratum in: J Am Dent Assoc 1999 Oct;130(10):1430. DOI: http://dx.doi.org/10.14219/jada.archive.1999.0049. [DOI] [PubMed] [Google Scholar]
- 30.Manski RJ, Magder LS. Demographic and socioeconomic predictors of dental care utilization. J Am Dent Assoc. 1998 Feb;129(2):195–200. doi: 10.14219/jada.archive.1998.0177. DOI: http://dx.doi.org/10.14219/jada.archive.1998.0177. [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Liang J, Plassman BL, Remie C, Luo X. Edentulism trends among middle-aged and older adults in the United States: comparison of five racial/ethnic groups. Community Dent Oral Epidemiol. 2012 Apr;40(2):145–53. doi: 10.1111/j.1600-0528.2011.00640.x. DOI: http://dx.doi.org/10.1111/j.1600-0528.2011.00640.x. Erratum in: Community Dent Oral Epidemiol 2012 Jun;40(3):288. DOI: http://dx.doi.org/10.1111/j.1600-0528.2012.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2006 Jan;60(1):7–12. doi: 10.1136/jech.2004.023531. DOI: http://dx.doi.org/10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolan TA, Atchison K, Huynh TN. Access to dental care among older adults in the United States. J Dent Educ. 2005 Sep;69(9):961–74. [PubMed] [Google Scholar]
- 34.Drilea SK, Reid BC, Li CH, Hyman JJ, Manski RJ. Dental visits among smoking and nonsmoking US adults in 2000. Am J Health Behav. 2005 Sep-Oct;29(5):462–71. doi: 10.5555/ajhb.2005.29.5.462. DOI: http://dx.doi.org/10.5993/AJHB.29.5.9. [DOI] [PubMed] [Google Scholar]
- 35.Beck JD, Offenbacher S. Oral health and systemic disease: periodontitis and cardiovascular disease. J Dent Educ. 1998 Oct;62(10):859–70. [PubMed] [Google Scholar]
- 36.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999 Feb;6(1):7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 37.Petersen PE, Ueda H. Oral health in ageing societies: integration of oral health and general health. Report of a meeting convened at the WHO Centre for Health Development in Kobe; Japan. 1–3 June 2005; Geneva, Switzerland: World Health Organization; 2006. [Internet] [cited 2015 Jul 8]. Available from: www.who.int/oral_health/events/Oral%20health%20report%202.pdf. [Google Scholar]
- 38.Kuntz JL, Johnson ES, Raebel MA, et al. Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infect Control Hosp Epidemiol. 2012 Oct;33(10):1031–8. doi: 10.1086/667733. DOI: http://dx.doi.org/10.1086/667733. [DOI] [PubMed] [Google Scholar]
- 39.Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999 Feb;52(2):137–42. doi: 10.1016/s0895-4356(98)00154-1. DOI: http://dx.doi.org/10.1016/S0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 40.HEDIS 2014. Volume 2: technical specifications. Washington DC: National Committee for Quality Assurance; 2014. [Google Scholar]
- 41.Friman G, Wårdh I, Nilsson G, Hultin M. Identifying patients in dental settings at risk of cardiovascular disease and diabetes. Cardiovascular System. 2013;1:1–9. DOI: http://dx.doi.org/10.7243/2052-4358-1-5. [Google Scholar]