Abstract
Context:
Osteoporosis is a major cause of morbidity and mortality in both men and women. The mortality rate in men within 1 year of hip fracture is 37.5%, which is 51% higher than in women. Although clear guidelines exist for osteoporosis screening in women, these are less clear for men. The available guidelines recommend screening high-risk men; however, screening does not appear to be a standard practice.
Objective:
To increase screening rates of osteoporosis in high-risk men in our primary care clinic by 50%.
Design:
The screening rate of osteoporosis was determined in high-risk male veterans more than 50 years of age enrolled in the resident physician- and nurse practitioner-staffed primary care clinics at a Veterans Affairs Medical Center in Cleveland, OH. High-risk factors included prolonged use of steroids; hypogonadism; and autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and systemic lupus erythematosus, which are known to be associated with osteoporosis. We surveyed health care professional trainees and nurses to explore their barriers to screening for osteoporosis in high-risk men.
Main Outcome Measures:
After creating awareness about the importance of this condition among the health care professionals, we analyzed whether this education had any impact on the screening rate.
Results:
The baseline screening rate in high-risk men was 11%. After phased surveys and awareness building, the screening rate increased to 20%.
Conclusion:
Osteoporosis in high-risk men is under-screened. Creating more awareness about the impact of this condition among health professional trainees and nurses can lead to improved screening rates.
INTRODUCTION
Osteoporosis is a major cause of morbidity and mortality in the form of fractures as a primary outcome, and later affecting quality of life. About 80,000 men sustain a hip fracture annually in the US.1 The rate of hip fractures in men living in residential care facilities is estimated to be 40%, and those who have broken a hip have a 20% chance of recurrence.2 The mortality rate in men in the first year after hip fracture is estimated to be up to 37.5%, which is 51% higher than in women.3,4 The annual burden of osteoporotic fractures in elderly individuals is estimated to cost between $12.2 and $17.9 billion, measured in 2002 US dollars, and this does not account for the loss of productivity of the patients and their caregivers.5 This financial burden is increasing as the US population ages.
Osteoporosis in men seems to be an underdiagnosed and undertreated problem. Much is discussed about osteoporosis in women but not in men. The number of men with osteopenia (now called low bone mass) or osteoporosis is less than in women but is still substantial. Furthermore, the morbidity and mortality associated with osteoporosis in men is higher than in women.2,6,7 In men older than age 50, the likelihood of an osteoporotic fracture is more than the likelihood of developing prostate cancer.1 The exact number of men with osteoporosis is unclear at this time. However, it is estimated that about 2 million American men have osteoporosis and about 12 million men have low bone mass.1
According to World Health Organization guidelines, osteoporosis is diagnosed when the T-score on bone densitometry is less than −2.5.8 There is an ongoing debate whether this criterion is applicable for men, because it is only an extrapolation from the data available for women.9 The causes of osteoporosis in men may differ from those in women. They are divided into primary and secondary causes. The primary causes are aging, relative estrogen and androgen deficiency, and bone loss. There are numerous causes of secondary osteoporosis as summarized in the Sidebar: Causes of Secondary Osteoporosis in Men.3 In some studies, these secondary causes have reportedly been shown to account for 40% of cases of osteoporosis in men.9 The leading secondary causes include heavy alcohol consumption, exogenous steroid use, and hypogonadism.9
Causes of secondary osteoporosis in men1.
| Cushing syndrome or prolonged steroid use |
| Excessive alcohol use: > 533 mL (18 fl oz) of beer, > 207 mL (7 fl oz) of wine, or > 59 mL (2 fl oz) of spirits per day Smoking |
| Primary or secondary hypogonadism |
| Family history of fracture with trivial trauma |
| Chronic liver or kidney disease |
| Rheumatoid arthritis/ankylosing spondylitis |
| Malabsorption syndromes |
| Type 1 or type 2 diabetes mellitus |
| Human immunodeficiency virus |
| Organ transplantation or immunosuppressive therapy |
| Antiepileptic drugs |
| Low body mass index |
Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med 2008 Apr 3;358(14):1474–82. DOI: http://dx.doi.org/10.1056/NEJMcp0707217.
The US Preventive Services Task Force does not recommend screening men routinely for osteoporosis but recognizes that there is an increasing burden of fractures in the aging male population.10 The Endocrine Society recommends testing higher-risk men, including men aged 70 years or older and men aged 50 to 69 years with secondary risk factors, using dual-energy x-ray absorptiometry (DXA).11 The International Society for Clinical Densitometry recommends bone mineral density screening in men age 70 years or older, and screening earlier if there is a fragility fracture or other known high-risk condition.12 In summary, high-risk men with a history of fracture or secondary causes of osteoporosis should be screened because benefits outweigh risks.10
After reviewing the guidelines for screening in high-risk men, we wanted to assess the existing standard of practice in a primary care setting at the Louis Stokes Cleveland Veterans Affairs (VA) Medical Center in Cleveland, OH. Anticipating low screening rates, we wanted to identify barriers to screening with the goal of improving standard of care.
METHODS
We conducted a quality-improvement project as a part of the Transforming Outpatient Care curriculum in the Center of Excellence in Primary Care Education (COE-PCE) at the Louis Stokes Cleveland VA Medical Center. The COE-PCE is an Office of Academic Affiliations nationally funded, innovative training program for future health care professionals at 5 primary care outpatient clinic sites in the VA Medical Centers. As part of this project, we extracted data for all male veterans older than age 50 years who were enrolled with the 23 resident physicians and resident nurse practitioners’ primary care clinics between January 2003 and September 2013, using the Computerized Patient Record System, an electronic record system.
We obtained the number of DXA scans performed in men older than age 50 years between January 2003 and November 2012. Subsequently, the charts were reviewed to identify the reasons why DXA scans were ordered in these men. It was found that men were screened because of a history of long-term steroid use, primary or secondary hypogonadism, liver cirrhosis, height loss, or a history of previous fracture or known low bone mass. Some uncommon indications were psoriatic arthropathy, primary or secondary hyperparathyroidism, and low body mass index (BMI). Because it was difficult to identify all the men who were appropriately screened and those who were not screened despite having risk factors, it was decided to use certain inclusion criteria, simply for the ease of data collection and analysis. These inclusion criteria were based on a brief review of the literature,13,14 which suggested that bone mineral density declines consistently in men age 50 years or older with any of the following risk factors: 1) steroid use of more than 5 mg/day for more than 3 months; 2) hypogonadism (testosterone level ≤ 2 ng/mL); and 3) systemic lupus, inflammatory bowel disease, rheumatoid arthritis, or sarcoidosis.
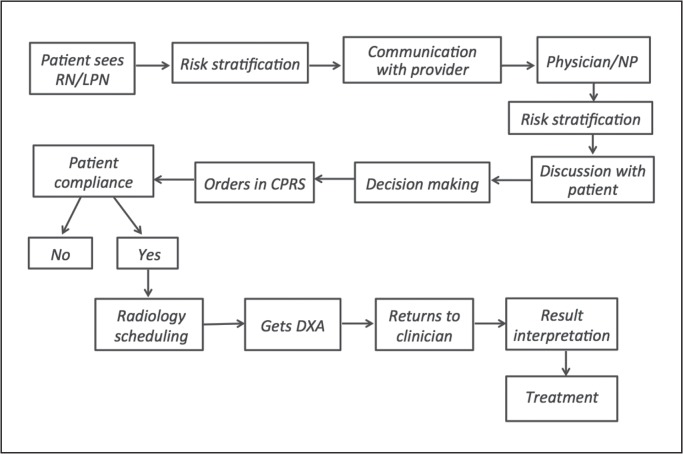
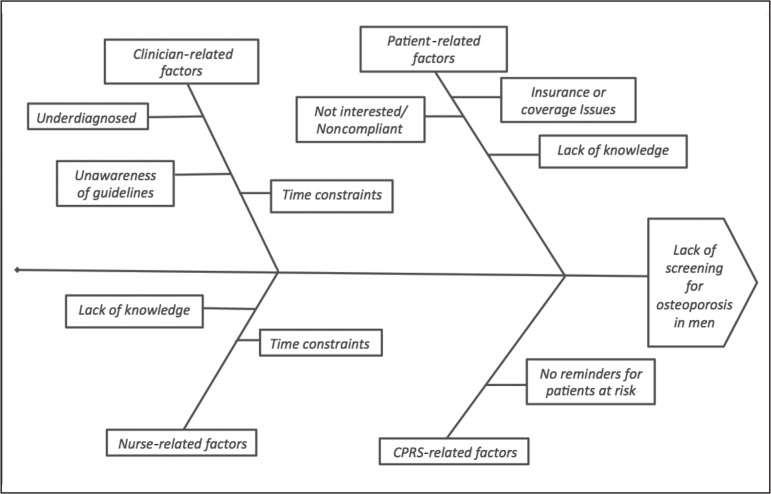
We wanted to explore the barriers causing low screening rates. First, we analyzed the steps involved in the process of ordering DXA in the clinic. The process map in Figure 1 shows these steps. We thought of possible barriers at each step, including clinician-related factors and patient-related factors, on the basis of which we made a fishbone diagram (Figure 2). This fishbone diagram helped us devise the survey questionnaire.
Figure 1.
Process map depicting steps involved in screening for osteoporosis in men in a primary care clinic.
CPRS = Computerized Patient Record System; DXA = dual-energy x-ray absorptiometry; LPN = licensed practical nurse; NP = nurse practitioner; RN = registered nurse.
Figure 2.
Fishbone diagram representing possible barriers to screening high-risk men.
CPRS = Computerized Patient Record System.
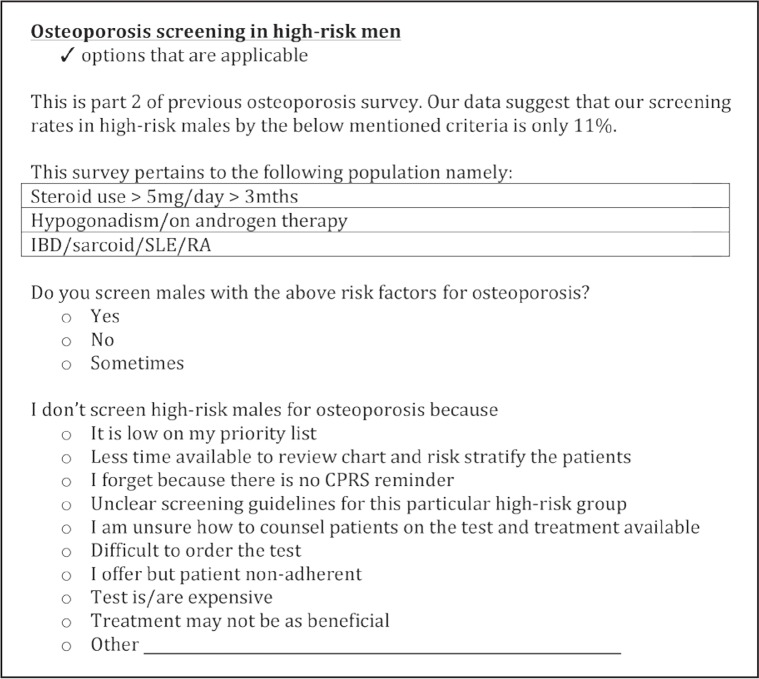
We then conducted online surveys to assess the level of existing awareness about osteoporosis in men and to assess the barriers for screening osteoporosis in high-risk men among nursing staff and resident physicians and nurse practitioners. We invited 23 residents and 10 nurses, who were enrolled with the COE-PCE curriculum and were involved with the care of these veterans, to participate in the surveys. Two surveys were performed about 5 months apart, in December 2012 and May 2013, respectively. We received 23 responses for the first survey and 16 for the second. Survey 1 included questions pertaining to whether screening for osteoporosis in men is important, who is at high risk, and so on (Figure 3). Survey 2 was devised to look for specific barriers in screening the high-risk men belonging to the 3 categories selected for this study (Figure 4). Survey 2 was analyzed separately for residents and nurses because the barriers for each of them were perceived to be different.
Figure 3.
Survey 1: Osteoporosis screening in men.
CAD = coronary artery disease; CPRS = Computerized Patient Record System; DXA = dual-energy x-ray absorptiometry; H/o = history of; IBD = inflammatory bowel disease; QI = quality improvement; SLE = systemic lupus erythematosus.
Figure 4.
Survey 2: Barriers to osteoporosis screening.
CPRS = Computerized Patient Record System; IBD = inflammatory bowel disease; mths = months; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus.
On the basis of the survey results, our intervention to improve screening rates by 50% was mainly focused on educating the residents and nurses regarding the guidelines for screening and the impact of osteoporosis in men. A review of the literature and the available data regarding osteoporosis in men were presented to the residents on two occasions. The surveys themselves also served as an intervention to create awareness among the residents and nurses and provided them an opportunity to learn the screening guidelines.
Data were then collected regarding the number of DXA scans performed, after creating awareness among the health care professionals. These data were collected at 2 different time points: the first set of data was collected after completion of 1 Plan-Do-Study-Act (PDSA) cycle (5 months from the first survey), referred to as Phase 1; and the second set, after another 5-month interval (Phase 2). The second set of data was to ensure continued improvement in the screening rate.
RESULTS
Chart Review
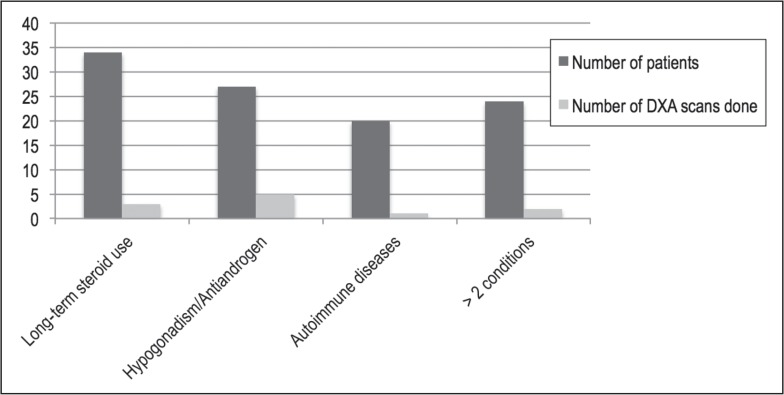
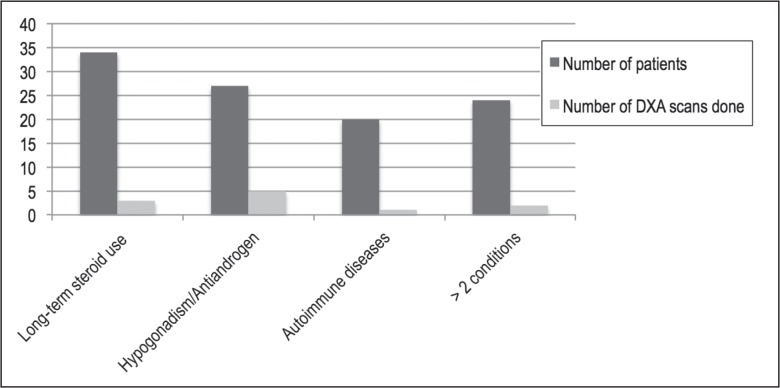
A total of 854 male veterans over 50 years of age were enrolled in the residents’ clinic between January 2003 and November 2012, and 34 DXA scans were performed in these men during this period. By the criteria described in the Methods section, there were 105 men in the high-risk category, of whom only 11 men had undergone DXA scans for screening for osteoporosis. These data have been graphically represented in Figure 5. The baseline screening rate was found to be 10.4%.
Figure 5.
Baseline data of high-risk men and number of dual-energy x-ray absorptiometry (DXA) scans obtained in these men between July 2003 and November 2012.a
a Long-term steroid use indicates doses above 5 mg/day for longer than 3 months; antiandrogens indicates patient received antiandrogen therapy; and autoimmune diseases refers to irritable bowel disease, sarcoidosis, systemic lupus erythematosus, or rheumatoid arthritis.
Survey
Survey 1 reflected that the residents and nurses were aware of the importance of screening high-risk men for osteoporosis with the appropriate screening tool. However, it was suggested that lack of clear guidelines posed a barrier, and the condition was perceived as low priority. Survey 2 obtained from physician and nurse practitioner residents revealed that the main reasons for not screening high-risk men for osteoporosis were its low priority (16%), insufficient time available for counseling patients (15%), absence of a reminder in the electronic medical record (15%), unclear guidelines (14%), and lack of comfort in counseling the patients (13%). The survey from nurses revealed absence of a reminder in electronic medical records (20%), unclear guidelines (16%), and lack of comfort in counseling the patients (10%) as the major limiting factors.
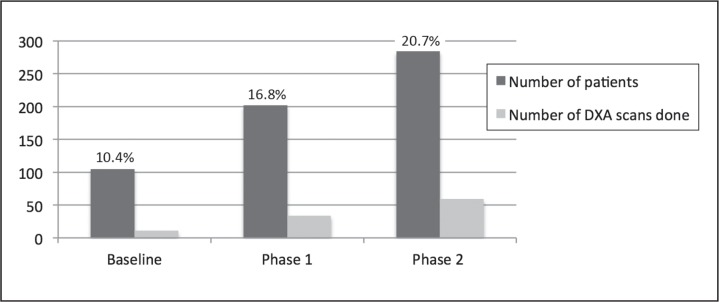
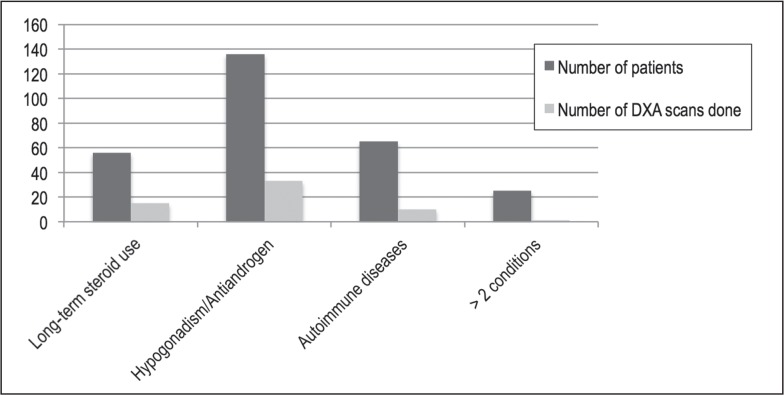
The total number of DXA at the end of Phase 1 had increased compared with the baseline screening data. In April 2013, a total of 32 DXA scans were performed for a high-risk population of 202 (16.8% compared with 11% at baseline). This number further increased over the next 5 months: 59 DXA scans in 284 high-risk men, resulting in a screening rate of 20.7%. These results are graphically depicted in Figures 6 to 8. Thus, there was a positive trend toward screening high-risk men. Hence, we met our goal to improve osteoporosis screening rates in high-risk men by at least 50% (from 10.4% to 20.7%) in a 10-month period.
Figure 6.
Graph depicting Phase 1 data: number of high-risk men and number of dual-energy x-ray absorptiometry (DXA) scans obtained in these men between July 2003 and April 2013.a
aLong-term steroid use indicates doses above 5 mg/day for longer than 3 months; antiandrogens, receipt of antiandrogen therapy; and autoimmune diseases, irritable bowel disease, sarcoidosis, systemic lupus erythematosus, or rheumatoid arthritis.
Figure 8.
Comparison of baseline data (July 2003 - November 2012), phase 1 data (July 2003 - April 2013) and phase 2 data (July 2003 - September 2013). Graph depicts a trend toward increased number of dual-energy x-ray absorptiometry (DXA) scans after intervention.
Interestingly, we found that an increasing number of men were tested for hypogonadism with testosterone levels. It is unclear what factors led to this. The increased general awareness of tests available for erectile dysfunction, andropause, and fatigue in elderly men could have contributed. In fact, the number of DXA scans ordered in men with hypogonadism increased from 9% in Phase 1 to 24% in Phase 2.
DISCUSSION
No clear guidelines are available for screening men for osteoporosis, but it is clear that fractures in men lead to substantial mortality and morbidity, increased number of hospitalizations, and thereby increasing health care costs. Our data analysis suggests osteoporosis screening rates in men are low at our VA primary care resident trainee clinics mainly because of lack of awareness. We successfully improved the screening rate by creating awareness among residents and nurses. Readily available data through the electronic medical record system and a multidisciplinary team approach helped the project.
Our project has a few limitations. The number of subjects in the data analysis is small. Also, the follow-up period was short; therefore, it cannot be determined whether the effects of the intervention are sustainable over a longer time. We suspect that for continued benefits of this intervention, we would have to conduct educational sessions regularly. This will not only educate the new residents and nurses but will also keep reminding the existing health care professionals of the importance of screening.
The challenging aspects of increased screening would be an increased workload for the clinicians to counsel patients regarding the need for screening, to interpret the results, and to diagnose, to treat, and to monitor the patients, as well as increased burden on the Radiology Department to scan these men and scheduling issues. From the patients’ perspective, this may be anxiety provoking and may add to the burden of medications with calcium supplements or bisphosphonates. Bisphosphonates have been recommended for treatment of osteoporosis in men with hypogonadism, along with testosterone replacement.15
Not many studies have been done to look at the cost-effectiveness of DXA to detect osteoporosis in men, followed by its treatment. Schousboe et al16 found that bone densitometry followed by bisphosphonate therapy may be cost-effective for osteoporotic men older than age 65 years with a history of fracture, and for men over 80 years with no history of fracture. The cost-effectiveness of this strategy in younger high-risk men has not been evaluated, although Schousboe et al16 have suggested that it may be beneficial for men as young as 70 years of age.
CONCLUSION
In the future, more studies are required to look at the cost-effectiveness of screening younger high-risk men with DXA, followed by therapy with bisphosphonates and/or androgen replacement. It would be important to include risk stratification questions in the history and physical examination during the office interview to screen high-risk men. It would possibly be beneficial to use a tool such as the FRAX (Fracture Risk Assessment) calculator to detect men at higher risk of fragility fractures.17
Figure 7.
Graph depicting Phase 2 data: number of high-risk men and number of dual-energy x-ray absorptiometry (DXA) scans obtained in these men between July 2003 and September 2013.a
aLong-term steroid use indicates doses above 5 mg/day for longer than 3 months; antiandrogens, receipt of antiandrogen therapy; and autoimmune diseases, irritable bowel disease, sarcoidosis, systemic lupus erythematosus, or rheumatoid arthritis
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
The Bones of a Dove
Bones of male doves are osteoporotic as compared with those of female doves. Estrin therapy in male doves produces a marked increase in the density of the bone by stimulating the osteoblasts.
— Albright F, Bloomberg E, Smith PH. Postmenopausal osteoporosis. Trans Assoc Am Physicians 1940;55:298–305.
References
- 1.Just for men [Internet] Washington, DC: National Osteoporosis Foundation; c2015. [cited 2015 Jun 22]. Available from: www.nof.org/articles/236. [Google Scholar]
- 2.Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10(1):73–8. doi: 10.1007/s001980050197. DOI: http://dx.doi.org/10.1007/BF02499971. [DOI] [PubMed] [Google Scholar]
- 3.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008 Apr 3;358(14):1474–82. doi: 10.1056/NEJMcp0707217. DOI: http://dx.doi.org/10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 4.Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009 Nov 15;170(10):1290–9. doi: 10.1093/aje/kwp266. DOI: http://dx.doi.org/10.1093/aje/kwp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. [PubMed] [Google Scholar]
- 6.Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007 Jul;17(7):514–9. doi: 10.1016/j.annepidem.2006.12.004. DOI: http://dx.doi.org/10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999 Mar 13;353(9156):878–82. doi: 10.1016/S0140-6736(98)09075-8. DOI: http://dx.doi.org/10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 8.Bone mass measurement: what the numbers mean [Internet] Bethesda, MD: NIH Osteoporosis and Related Bone Diseases—National Resource Center; 2012. Jan, [cited 2015 Jul 21]. Available from: www.niams.nih.gov/health_info/bone/bone_health/bone_mass_measure.asp#c. [Google Scholar]
- 9.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008 Jun;29(4):441–64. doi: 10.1210/er.2008-0002. DOI: http://dx.doi.org/10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockville, MD: US Preventive Services Task Force; 2011. Jan, Screening for osteoporosis: clinical summary of US Preventive Services Task Force recommendation. AHRQ publication no. 10-05145-EF-3 [Internet] [cited 2015 Jul 21]. Available from: www.uspreventiveservicestaskforce.org/Home/GetFileByID/269. [Google Scholar]
- 11.Watts NB, Adler RA, Bilezikian JP, et al. Endocrine Society Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012 Jun;97(6):1802–22. doi: 10.1210/jc.2011-3045. DOI: http://dx.doi.org/10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008 Dec;43(6):1115–21. doi: 10.1016/j.bone.2008.08.106. DOI: http://dx.doi.org/10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 13.Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut. 2000 Jan;46(Suppl 1):i1–8. doi: 10.1136/gut.46.suppl_1.I1. DOI: http://dx.doi.org/10.1136/gut.46.suppl_1.I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adinoff AD, Hollister JR. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983 Aug 4;309(5):265–8. doi: 10.1056/NEJM198308043090502. DOI: http://dx.doi.org/10.1056/NEJM198308043090502. [DOI] [PubMed] [Google Scholar]
- 15.Shimon I, Eshed V, Doolman R, Sela BA, Karasik A, Vered I. Alendronate for osteoporosis in men with androgen-repleted hypogonadism. Osteoporos Int. 2005 Dec;16(12):1591–6. doi: 10.1007/s00198-005-1879-3. DOI: http://dx.doi.org/10.1007/s00198-005-1879-3. [DOI] [PubMed] [Google Scholar]
- 16.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007 Aug 8;298(6):629–37. doi: 10.1001/jama.298.6.629. DOI: http://dx.doi.org/10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 17.WHO fracture risk assessment tool: calculation tool [Internet] Sheffield, United Kingdom: World Health Organization Collaborating Centre for Metabolic Bone Disease, University of Sheffield; [cited 2015 Sep 2]. Available from: www.shef.ac.uk/FRAX/tool.aspx?country=9. [Google Scholar]