Abstract
Background
Vietnam is a lower middle-income country with no national surveillance system for hospital-acquired infections (HAIs). We assessed the prevalence of hospital-acquired infections and antimicrobial use in adult intensive care units (ICUs) across Vietnam.
Methods
Monthly repeated point prevalence surveys were systematically conducted to assess HAI prevalence and antimicrobial use in 15 adult ICUs across Vietnam. Adults admitted to participating ICUs before 08:00 a.m. on the survey day were included.
Results
Among 3287 patients enrolled, the HAI prevalence was 29.5% (965/3266 patients, 21 missing). Pneumonia accounted for 79.4% (804/1012) of HAIs Most HAIs (84.5% [855/1012]) were acquired in the survey hospital with 42.5% (363/855) acquired prior to ICU admission and 57.5% (492/855) developed during ICU admission. In multivariate analysis, the strongest risk factors for HAI acquired in ICU were: intubation (OR 2.76), urinary catheter (OR 2.12), no involvement of a family member in patient care (OR 1.94), and surgery after admission (OR 1.66). 726 bacterial isolates were cultured from 622/1012 HAIs, most frequently Acinetobacter baumannii (177/726 [24.4%]), Pseudomonas aeruginosa (100/726 [13.8%]), and Klebsiella pneumoniae (84/726 [11.6%]), with carbapenem resistance rates of 89.2%, 55.7%, and 14.9% respectively. Antimicrobials were prescribed for 84.8% (2787/3287) patients, with 73.7% of patients receiving two or more. The most common antimicrobial groups were third generation cephalosporins, fluoroquinolones, and carbapenems (20.1%, 19.4%, and 14.1% of total antimicrobials, respectively).
Conclusion
A high prevalence of HAIs was observed, mainly caused by Gram-negative bacteria with high carbapenem resistance rates. This in combination with a high rate of antimicrobial use illustrates the urgent need to improve rational antimicrobial use and infection control efforts.
Introduction
Hospital-acquired infections (HAIs) and antimicrobial resistance are growing global public health problems [1,2]. The incidence of HAIs is substantially higher in Low and Middle Income Countries (LMICs), with an average prevalence of 15.5%, compared to prevalence of 7.1% and 4.5% in Europe and USA, respectively [3]. This problem is more serious in intensive care units (ICUs). The HAI prevalence in ICUs ranges from 9.1% in the United States to about 23.0%-23.5% in Europe and England [4–7], and even higher in LMICs with a pooled prevalence of 35.2% [1]. A recent report of the International Nosocomial Infection Control Consortium 2007–2012 from 503 ICUs shows that ventilator-associated pneumonia is fifteen times and catheter-associated urinary tract infection four times higher in LMICs than in better resourced settings [8]. Due to economic development in LMICs, the healthcare systems are changing rapidly, with increasing ICU capacities. However resource constraints often result in high occupancy rates, crowding, a lack of isolation facilities, and insufficient resources for adequate infection control all of which may contribute to the reported high incidence of HAIs and drug-resistant infections at ICU’s in these settings [1,9,10].
Vietnam is a LMIC with a population of 90.796 million [11] and an increasingly sophisticated health care system, typical of countries in the region. Health expenditure per capita in Vietnam was around 100$ per annum in 2012, approximately a seventh of the regional average [11]. Up to now, there is no national surveillance system for HAIs and limited data about HAIs in ICUs. The few studies performed are small and only some include ICUs, but reported that the HAI prevalence in those ICUs ranged from 19.3% to 31.3% [12–17]. Only one of these studies is from the international peer reviewed literature [12], the others are published in the Vietnamese medical literature. Antimicrobial resistance levels are high in Vietnam; up to 70% of Enterobacteriaceae were resistant to 3rd generation cephalosporins and > 40.0% of Acinetobacter spp. resistant to carbapenems in 2009 [9,18].
In order to provide up-to-date, systematic data and to demonstrate the feasibility of developing a national surveillance network for ICUs in a LMIC, we performed a prospective study on the prevalence of HAI in ICUs across Vietnam, exploring risk factors, antimicrobial use, and antimicrobial resistance [19].
Materials and Methods
Study design, hospital and patient selection
We conducted a repeated point prevalence survey (PPS) to determine the prevalence of HAIs, and to assess antimicrobial use and antimicrobial resistance using the methodology developed by the European Center for Disease Prevention and Control (ECDC) [20]. The survey was conducted on one day each month from October 2012 through September 2013 in 15 adult ICUs in 14 acute care hospitals, of which 7 were tertiary hospitals and 7 provincial hospitals, throughout Vietnam (Fig 1). Patients aged ≥ 18 years, admitted to participating ICUs before 8 a.m. on the survey day, and remaining there at the survey time were included regardless after that time patient was discharged or remain in that ICU.
Fig 1. The Study Site Locations.
Data collection
The following patient data were collected: reason for admission, location of patient at admission to ICU, comorbidity, current interventions, involvement of patient’s family in patient care (participating in bathing, cleaning, changing position, and feeding patients), antimicrobial agent use for any indication, presence of HAI according to ECDC definitions [20], and results of routine microbiological investigations.
All participating hospitals provided data on basic infrastructure and infection control indicators at the beginning of the study, including total number of beds, rooms, single bed rooms, number of doctors and nurses at the ICUs, admissions per year, patient days per year, alcohol hand rub consumption, and availability of alcohol hand rub at ICU bed.
All participating hospital laboratories were trained to follow the Clinical and Laboratory Standards Institute guidelines (CLSI) for antimicrobial susceptibility testing and were enrolled in an external quality assurance program (The United Kingdom National External Quality Assessment Service (UK NEQAS) for Microbiology) [19]. All participating doctors received training on the protocol, HAI definitions, how to complete case record forms, and data entry before the study. A database adapted from HELICSWin.Net developed by the ECDC was used for the study [21]. Each ICU received a laptop with the software installed and Vietnamese instructions. The anonymised data were submitted to the project coordinators electronically once a month during the implementation period. Data were then checked for missing or inconsistent data, with regular queries sent to the hospitals and visits by study investigators to assist in data reconciliation.
Statistical analysis
For descriptive statistics we calculated percentage, frequency, mean, and median values and 95% confidence interval (95% CI) or interquartile ranges (IQR), as appropriate. Where patients were included in more than one PPS, we only used the data from the first survey and had no HAI at ICU admission to calculate the odds ratios of risk factors for developing HAIs in ICU. After univariate analysis, we included all HAI risk factors in the multivariate logistic regression models. We used IBM SPSS Statistics software (version 22 IBM, California, USA) for data analysis. P-values < 0.05 (two-sided) were considered statistically significant.
Ethical considerations
The Ethical Committee of the National Hospital for Tropical Diseases (27/HDDD-NHTD) had approved of the study and confirmed that the need for informed consent was waived due to data were anonymous and collected by surveillance and no intervention was conducted; and the study was also approved by the Vietnamese Ministry of Health (4921/QD-BYT).
Results
Hospital and ICU resources
Hospital sizes ranged from 280 to 2362 beds (median 950; IQR 750–1650) and the participating ICU sizes ranged from 10 to 60 beds (median 20; IQR 18–31). Mean length of ICU stays per individual ICU ranged from 3.8 to 16.0 days with a median mean length of stay across the ICUs of 6.4 days (IQR: 4.8–9.3). There were no national antimicrobial therapy guidelines, but locally derived guidelines were used at 7 of 15 ICUs. In each ICU one nurse took care of 1.9 to 5.9 beds (median 3.6, IQR 3.1–4.4 beds) per 8 hours shift. During working hours, there was a median of 3.5 doctors (IQR: 2.5–4.8) for 10 ICU beds. Alcohol hand rub was available at the bedside in all participating ICUs. Median alcohol hand rub consumption per patient day was 66.4 ml (IQR: 23.7–100.5). More details are presented in Table A in S1 File.
Patient characteristics
In total, 3,401 patients were screened, of whom 3287 (114 were excluded due to age < 18 years) were enrolled from 15 ICUs of 14 participating hospitals between October 2012 and October 2013. Due to prolonged ICU stay, 162 patients were enrolled in more than one survey leaving 3125 unique patients.
The median age was 61.0 years (IQR 45.0–77.0) and 63.9% (2101/3287) of patients were male. Most patients (46.2%; 1427/3088; 199 missing) were admitted directly from the community, while 30.1% (930/3088) of the patients entered the ICU from another department in the same hospital and 20.7% (638/3088) were referred from other hospitals. 52.3% (1719/3287) patients were intubated, 28.0% (921/3287) had a central vascular catheter, 49.2% (1616/3287) had a urinary catheter, and 8.2% (270/3287) were on renal replacement therapy. In 63.0% (2072/3287) of patients, family members were involved in patient care. Details are presented in Table B in S1 File.
Comorbidity was present in 39.6% of patients (1249/3151, 136 missing), of which 190 patients had two or more comorbidities. Common comorbidities were: stroke sequelae 27.5% (343/1249), diabetes mellitus 24.8% (310/1249), chronic obstructive pulmonary disease 20.7% (259/1249), renal failure 11.5% (144/1249), harmful alcohol use 11.3% (141/1249), active malignancy 9.5% (119/1249), and induced immunosuppression 6.1% (76/1249).
HAI prevalence
Overall, 29.5% (965/3266 patients, 21 missing) had at least one HAI; 922 patients had one HAI, 39 patients had 2 HAIs, and 4 patients had 3 HAIs. The HAI prevalence ranged widely between ICUs from 5.6% to 60.9% with a median prevalence of 30.5% (Table B in S1 File). Prevalence of HAI per month in year fluctuated from 23.8% in April to 34.8% in November, prominent in March (34.1%), July (33.4%), November (34.8%), and December (34.7%) (Table C in S1 File). Pneumonia was the most common HAI (79.4% [804/1012]), followed by blood stream infection (4.4% [44/1012]), and surgical site infections (4.2% [42/1012]). Most HAIs (84.5% [855/1012]) were acquired in the survey hospital: 42.5% (363/855) acquired prior to ICU admission and 57.5% (492/855) developed after ICU admission. The median time from hospital admission to diagnosis of HAI was 7 days (IQR: 3–15 days). Device-associated HAIs accounted for 643/1012 (63.5%) of HAIs, mainly pneumonia (589/643 [91.6% of device-associated HAIs]) (Table 1).
Table 1. Location of Acquired HAI and Association with Invasive Devices.
| Type of infections | Location Acquired HAI | Related device | Total No. of HAI, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Current hospital | Other hospital | UNK | Yes | No | UNK | |||
| In ICU | Out ICU | |||||||
| Pneumonia & LRTIa, n (%) | 389 (48.4) | 301 (37.4) | 60 (7.5) | 54 (6.7) | 589 (73.3) | 120 (14.9) | 95 (11.8) | 804 (79.4) |
| Bloodstream infection, n (%) | 34 (77.3) | 8 (18.2) | 2 (4.5) | 0 | 27 (61.4) | 11 (25.0) | 6 (13.6) | 44 (4.4) |
| Surgical site infection, n (%) | 23 (54.8) | 11 (26.2) | 6 (14.3) | 2 (4.8) | NA | NA | NA | 42 (4.2) |
| Gastrointestinal infection, n (%) | 13 (32.5) | 19 (47.5) | 7 (17.5) | 1 (2.5) | NA | NA | NA | 40 (4.0) |
| Urinary tract infection, n (%) | 18 (62.1) | 8 (27.6) | 3 (10.3) | 0 | 27 (93.1) | 2 (6.9) | 0 | 29 (2.9) |
| Central nervous system infection, n (%) | 5 (26.3) | 4 (21.1) | 8 (42.1) | 2 (10.5) | NA | NA | NA | 19 (1.9) |
| Skin and soft tissue infection, n (%) | 2 (13.3) | 6 (40.0) | 1 (6.7) | 6 (40.0) | NA | NA | NA | 15 (1.5) |
| Other infectionsb, n (%) | 8 (42.1) | 6 (31.6) | 2 (10.5) | 3 (15.8) | NA | NA | NA | 19 (1.9) |
| Total, n (%) | 492 (48.6) | 363 (35.9) | 89 (8.8) | 68 (6.7) | 643 (63.5) | 133 (13.1) | 101 (10.0) | 1012 (100) |
UNK: unknown or missing data
NA: Not applicable
LRTI: low respiratory tract infection
a Only one case of LRTI
b Including: nine cases of systemic infection, four catheter related infections, four reproductive tract infections, one endocarditis and one eyes ear nose throats infection not classified.
Note: Twenty-one patients whose infection could not be differentiated between community-acquired and hospital-acquired infection were excluded from calculating HAI prevalence.
Risk factors for HAIs Acquired in ICU
We assessed risk factors for ICU-acquired HAIs in 2,618 patients who were enrolled in the study for the first time and had no HAI at ICU admission. The prevalence of HAI among these patients at survey time was 16.2% (424/2618 patients). In the univariate analysis, exception for gender, age group, and comorbidity, all other factors had risk for HAI with statistically significant. The highest risk factors were intubation (OR 6.31 [95% CI 4.86–8.18]), having surgery after admission (minor surgery OR 4.78 [95% CI 3.57–6.40], major surgery OR 3.78 [95% CI 2.90–4.93]), and urinary catheter (OR 3.90 [95% CI 3.09–4.92]) (Table 2).
Table 2. Risk Factors for Acquiring HAI in ICU.
| Risk factors | No. of patients (total = 2618), n | Patients with HAI (total = 424), n (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Gender | ||||||
| Male | 1656 | 271 (16.4) | 1.03 (0.83–1.28) | 0.758 | 1.22 (0.94–1.59) | 0.137 |
| Female | 962 | 153 (15.9) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Age group | ||||||
| 18–60 years | 1344 | 202 (15.0) | Reference | Reference | ||
| > 60 years | 1274 | 222 (17.4) | 1.19 (0.97–1.47) | 0.097 | 1.17 (0.90–1.52) | 0.233 |
| Missing | 0 | 141 | ||||
| Location of patients at admission to ICU | ||||||
| Community and others | 1352 | 144 (10.7) | Reference | Reference | ||
| Same hospital | 655 | 171 (26.1) | 2.96 (2.32–3.79) | < 0.001 | 1.49 (1.09–2.04) | 0.013 |
| Other hospital | 507 | 109 (21.5) | 2.30 (1.75–3.02) | < 0.001 | 1.23 (0.87–1.72) | 0.239 |
| Missing | 104 | 141 | ||||
| Reason for admission | ||||||
| Medical disease | 1029 | 120 (11.7) | Reference | Reference | ||
| Infectious disease | 1106 | 214 (19.3) | 1.82 (1.43–2.31) | < 0.001 | 0.91 (0.67–1.23) | 0.536 |
| Surgery | 368 | 90 (24.5) | 2.45 (1.81–3.32) | < 0.001 | 0.73 (0.45–1.19) | 0.214 |
| Missing | 115 | 141 | ||||
| Comorbidity | ||||||
| No comorbidity | 1560 | 250 (16.0) | Reference | Reference | ||
| Have comorbidity | 972 | 171 (17.6) | 1.12 (0.90–1.38) | 0.303 | 0.90 (0.70–1.17) | 0.445 |
| Missing | 86 | 141 | ||||
| Surgery after admission | ||||||
| Non surgery | 2009 | 219 (10.9) | Reference | Reference | ||
| Minor surgery | 252 | 93 (36.9) | 4.78 (3.57–6.40) | < 0.001 | 1.67 (1.16–2.41) | 0.005 |
| Major surgery | 354 | 112 (31.6) | 3.78 (2.90–4.93) | < 0.001 | 1.66 (1.06–2.58) | 0.025 |
| Missing | 3 | 141 | ||||
| Intubation | ||||||
| Yes | 1252 | 346 (27.6) | 6.31 (4.86–8.18) | < 0.001 | 2.76 (2.03–3.75) | < 0.001 |
| No | 1366 | 78 (5.7) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Central vascular catheter | ||||||
| Yes | 693 | 197 (28.4) | 2.97 (2.39–3.68) | < 0.001 | 1.47 (1.05–2.06) | 0.026 |
| No | 1925 | 227 (11.8) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Urinary catheter | ||||||
| Yes | 1226 | 312 (25.4) | 3.90 (3.09–4.92) | < 0.001 | 2.12 (1.57–2.87) | < 0.001 |
| No | 1392 | 112 (8.0) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Hemodialysis | ||||||
| Yes | 203 | 51 (25.1) | 1.84 (1.31–2.57) | < 0.001 | 1.34 (0.87–2.08) | 0.188 |
| No | 2415 | 373 (15.4) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Peripheral vascular catheter | ||||||
| Yes | 2202 | 321 (14.6) | 0.52 (0.40–0.67) | < 0.001 | 1.47 (1.02–2.12) | 0.037 |
| No | 416 | 103 (24.8) | Reference | Reference | ||
| Missing | 0 | 141 | ||||
| Family involved patient care | ||||||
| Yes | 1717 | 190 (11.1) | Reference | Reference | ||
| No | 901 | 234 (26.0) | 2.82 (2.28–3.48) | < 0.001 | 1.94 (1.49–2.51) | < 0.001 |
| Missing | 0 | 141 | ||||
| Each day longer from ICU admission to survey time | ||||||
| In analysis | 2513 | 424 (16.9) | 1.08 (1.07–1.10) | < 0.001 | 1.08 (1.07–1.10) | < 0.001 |
| Missing | 105 | 141 | ||||
- Omnibus test of model coefficients: -2 Log likelihood 1720.120, Chi-square (538.055, df 16, p < 0.001)
- Nagelkerke R-Square: 0.326
- Hosmer & Lemeshow test (Chi-square 10.592, df 8, p = 0.226)
- Classification accuracy: 85.3% (97.0% for no HAI, 28.0% for HAI, with cut value is 0.50).
Multivariate logistic regression identified eight risk factors independently associated with HAIs, which included: intubation (OR 2.76 [95% CI 2.03–3.75]), urinary catheter (OR 2.12 [95% CI 1.57–2.87]), no involvement of a family member in patient care (OR 1.94 [95% CI 1.49–2.51]), surgery after admission (minor surgery OR 1.67 [95% CI 1.16–2.41], major surgery OR 1.66 [95% CI 1.06–2.58]), admission to ICU from the same hospital OR 1.49 [95% CI 1.09–2.04]), central vascular catheter (OR 1.47 [95% CI 1.05–2.06]), peripheral vascular catheter showed a protective effect in univariate analysis but was associated with a significant increased risk for HAIs in multivariate analysis (OR 1.47 [95% CI 1.02–2.12]), and every one day longer of ICU stay (OR 1.08 [95% CI 1.07–1.10]) (Table 2).
Microbiological Aetiology of HAIs
From 593/965 (61.5%) patients with HAI, 726 microorganisms were reported in association with 622 HAIs. For 390 HAIs in 372 patients no pathogens were isolated. All reported pathogens are summarized in Table 3. Antimicrobial resistance to frequently used antimicrobials was common. Carbapenem resistance was most common in Acinetobacter baumannii (89.2% [149/167]) and Pseudomonas aeruginosa (55.7% [49/88]). Slightly over 5% of the Enterobacteriaceae isolated were carbapenem resistant, while 14.9% (11/74) Klebsiella pneumonia were carbapenem resistant. More than 75% of the Staphylococcus aureus isolates were methicillin resistant and more than 57% of the Enterococci were resistant to glycopeptides.
Table 3. Microorganisms Causing HAIs.
| Name of Microorganisms | All pathogen isolated (total = 726) n (%) | Pathogen for pneumonia (total = 587) n (%) | Pathogen for blood stream infections (total = 44) n (%) | Pathogen for surgical site infections (total = 34) n (%) | Pathogen for urinary tract infections (total = 25) n (%) |
|---|---|---|---|---|---|
| Gram-negative bacteria | 611 (84.2) | 516 (87.9) | 28 (63.6) | 25 (73.5) | 15 (60.0) |
| Acinetobacter baumannii | 177 (24.4) | 151 (25.7) | 10 (22.7) | 2 (5.9) | 5 (20.0) |
| Pseudomonas aeruginosa | 100 (13.8) | 92 (15.7) | 2 (4.5) | 3 (8.8) | 0 |
| Klebsiella pneumoniae | 84 (11.6) | 68 (11.6) | 5 (11.4) | 8 (23.5) | 1 (4.0) |
| Acinetobacter spp. | 46 (6.3) | 46 (7.8) | 0 | 0 | 0 |
| Escherichia coli | 39 (5.4) | 20 (3.4) | 6 (13.6) | 4 (11.8) | 3 (12.0) |
| Enterobacteriaceae | 36 (5.0) | 26 (4.4) | 1 (2.3) | 2 (5.9) | 5 (20.0) |
| Klebsiella spp. | 35 (4.8) | 26 (4.4) | 2 (4.5) | 2 (5.9) | 1 (4.0) |
| Providencia spp. | 28 (3.9) | 25 (4.3) | 0 | 2 (5.9) | 0 |
| Klebsiella oxytoca | 19 (2.6) | 18 (3.1) | 0 | 1 (2.9) | 0 |
| Achromobacter spp. | 18 (2.5) | 17 (2.9) | 0 | 1 (2.9) | 0 |
| Stenotrophomonas maltophilia | 6 (0.8) | 6 (1.0) | 0 | 0 | 0 |
| Gram-negative bacilli others | 18 (2.5) | 17 (2.9) | 1 (2.3) | 0 | 0 |
| Gram-negative cocci other | 4 (0.6) | 4 (0.7) | 0 | 0 | 0 |
| Anaerobic bacilli | 1 (0.1) | 0 | 1 (2.3) | 0 | 0 |
| Gram-positive bacteria | 104 (14.3) | 65 (11.1) | 15 (34.1) | 9 (26.5) | 7 (28.0) |
| Staphylococcus aureus | 39 (5.4) | 28 (4.8) | 6 (13.6) | 3 (8.8) | 0 |
| Staphylococcus spp. | 19 (2.6) | 11 (1.9) | 6 (13.6) | 0 | 1 (4.0) |
| Streptococcus spp. | 11 (1.5) | 9 (1.5) | 0 | 1 (2.9) | 0 |
| Enterococcus spp. | 16 (2.2) | 9 (1.5) | 2 (4.5) | 1 (2.9) | 2 (8.0) |
| Enterococcus faecalis | 8 (1.1) | 2 (0.3) | 1 (2.3) | 3 (8.8) | 2 (8.0) |
| Enterococcus faecium | 5 (0.7) | 0 | 0 | 1 (2.9) | 2 (8.0) |
| Gram-positive bacilli other | 6 (0.8) | 6 (1.0) | 0 | 0 | 0 |
| Fungi | 11 (1.5) | 6 (1.0) | 1 (2.3) | 0 | 3 (12.0) |
| Candida spp. | 10 (1.4) | 5 (0.8) | 1 (2.3) | 0 | 3 (12.0) |
| Other fungi | 1 (0.1) | 1 (0.2) | 0 | 0 | 0 |
Antimicrobial use
Antimicrobials use was evaluated in all enrolled patients. The proportion of patients receiving antimicrobials at survey time ranged from 50.0% to 99.8% per ICU, with a pooled proportion of 84.8% (2787/3287). 733 (22.3%) patients were prescribed one, 1343 (40.8%) two, 552 (16.8%) three, and 159 (4.8%) four antimicrobial agents. The main indications for antimicrobial use were community-acquired infections 41.8% (2386/5711) and HAIs 33.9% (1937/5711). As infectious diseases are common in Vietnam, having a severe community-acquired infection was a common reason for admission to an ICU. Prophylaxis and other use were the indications for antimicrobial use in 9.4% (536/5711) and the indication was unknown in 14.9% (852/5711).
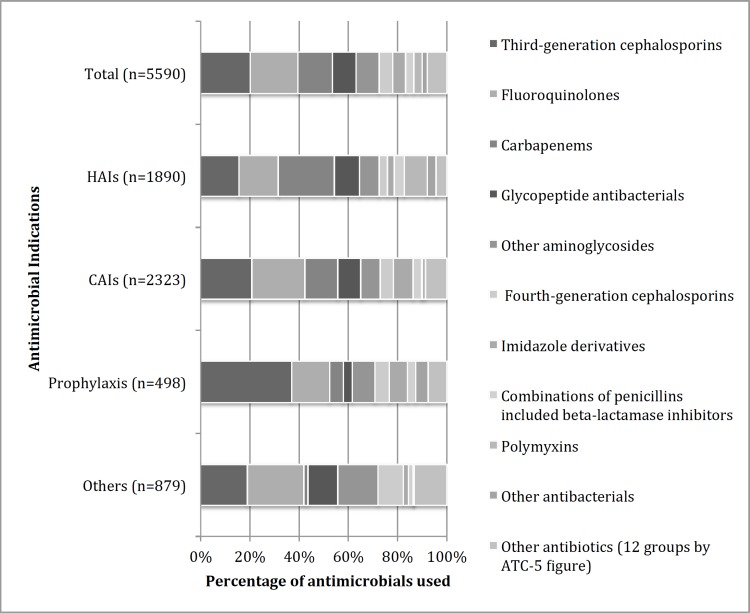
Antimicrobials for systemic use (ATC group J01) accounted for 97.9% (5590/5711) of the total antimicrobials used. Of which, third generation cephalosporins, fluoroquinolones, and carbapenems were used most common—accounting for 20.1% (1126/5590), 19.4% (1082/5590), and 14.1% (786/5590), respectively. For the treatment of HAIs, the most frequently used agents were carbapenems at 22.9% (432/1890), fluoroquinolones 16.0% (302/1890), and 3rd generation cephalosporins 15.5% (293/1890). Polymyxins (parenteral colistin) accounted for 3.3% (186/5590) of general use but were the fifth most frequently used agent for HAIs; (Fig 2). For HAIs associated with carbapenem resistant A. baumannii and P. aeruginosa, colistin was given to 65.1% (97/149) and 30.6% (15/49), respectively. More details in Tables D and E in S1 File.
Fig 2. Antimicrobials for Systemic Use by Indication.
CAIs: Community acquired infections; HAIs: Hospital-acquired infections; Prophylaxis: Medical and surgical prophylaxis; others: other indications (e.g. erythromycin use for prokinetic) and unknown indication.
Discussion
The HAI prevalence in this study (29.5%, 965/3266 patients) is higher than that reported from adult ICUs in European hospitals using the same protocol (23.0%, 1750/7613 patients) [5] and in ICUs in Southern Europe, Turkey and Iran (23.5%, 176/749 patients) [7] and much higher than rate of 9.1% (156/1707) in ICUs in the United States in 2011[4]. These differences can be explained by several factors: There were few single rooms in ICUs and common rooms usually contain 4–5 beds and even up to 30 beds; In addition with low healthcare staff ratios [11] and high bed occupancy rates [22], often over 110%, makes successful infection control very challenging. The lack of nursing staff for patient care allows little time for proper infection control measures, which may lead to an increased HAI rate [23–25]. This is supported by the smaller amount of alcohol hand rub used: a median of 66.4 ml (IQR: 23.7–100.5 ml)/patient day, compared with a median of 83 ml (IQR: 64–105)/patient day in ICUs of Germany in 2010 [26]. However, the HAI prevalence in Vietnamese ICUs was lower than the pooled prevalence of 35.2% in other LMICs [3].
Hospital-acquired pneumonia (HAP) was the most common type of HAI as in other studies [1,3,7]. HAP accounted for 79.4% of all HAIs in our study, nearly double that reported (40.0% - 45.3%) in ICUs of developed countries [5,6]. This is due to a large proportion of HAP (37.4%, 301/804) was acquired in the same hospital before admission to ICU. This figure raises the need for effective infection control program outside ICU for HAP prevention. Blood stream infections (BSI) accounted for 4.4% of all HAI, considerable lower than the reported 18.0% in European ICUs [5]. Potentially this is due to the high HAP prevalence, but there might also be an underestimation of BSI due to underutilization of blood cultures; as an indication there were a total of only 243 blood culture samples taken from the 3287 patients in the 24 hour preceding the survey day, we are not aware of any comparable data from other settings.
Multivariate analysis identified intubation as an important risk factor, which combined with the high prevalence of HAP suggests that future interventions should target this risk factor. Potential interventions to assess include ventilation and sedation strategies, cuff and tracheal tube design, cuff pressure management [27] Strategies like selective gut decontamination using polymyxins may not be appropriate in this setting with high antimicrobial resistance background rates with colistin as last resort drug [28]. Multivariate analysis revealed that the involvement of the patient’s family in patient care was a protective factor from HAI. As ICUs had department-wide policies to either allow or forbid family from taking care of their admitted family member this practice was highly clustered at the ICU level, the result needs to be interpreted with caution. Further research is needed to understand the risks for HAI regarding family members involved in patient care. National surveillance systems for HAI are scarce in LMIC settings yet ongoing surveillance is crucial to informing policymakers of the needs of the population and high quality data is critical to the development of potential interventions applicable at the national level.
The proportion of HAIs related to medical devices were higher than that reported by a European survey in 2011–2012, where device related HAIs were 59.5% for UTI, 57.3% for primary BSI, and 33.2% for HAP [5]. This may be partly explained by less compliance with hand hygiene [25] and low alcohol hand rub consumption, but probably also sub-optimal routines for insertion and care of urinary and blood catheters.
Nearly 85% of the ICU patients were on antimicrobials in this study, higher than the 56.5% and 77.3% reported in European and American ICUs, respectively [5,29]. Broad-spectrum beta-lactam antibiotics (3rd, 4th generation cephalosporins and carbapenems) and fluoroquinolones accounted for 39.7% and 19.4% of total antimicrobials use in Vietnamese ICUs, respectively. These proportions are higher than those reported by European ICUs of around 30% and 10% respectively [5]. Also combination therapy in our study is more common than in European ICUs: 62.5% versus 47.7% [5].
Gram-negative bacteria were the most common cause of HAI, similar to studies in mainly low and middle income countries [3,7,23]. Furthermore, the proportion of infection caused by Acinetobacter in ICUs was highest in Asia (19.2%), which was more than five times in compared with North America (3.7%) [30]. The two most frequently isolated bacteria were A. baumannii and P. aeruginosa (accounted for 38.2% of total isolates), similar to a study in Turkey in 2007–2008 (34.1% of isolates) [31]. Carbapenem resistance in A. baumannii, P. aeruginosa, and K. pneumoniae in this study was higher than rates of 69.1%, 44.4% and 2.9%, respectively, in Vietnam in 2007 and 2008 [25]. High prevalence of carbapenem resistance leads to an increased colistin use [9] and emergence and spread of colistin resistance will follow. Antimicrobial stewardship programmes and the deployment of alternatives to carbapenems may help to slow the development of further resistance to the remaining active antibiotics [32] and need to be evaluated in this context. The high rates of MRSA and glycopeptide resistant enterococci are also a major concern. However, the burden of Gram-positive infections as a cause of HAI is relatively low as compared to Gram-negative infections.
A potential limitation of this study is the paucity of microbiology data for many HAIs due to the few cultures taken in routine clinical care. However the microbiology results available represent valuable data for assessing the microorganisms causing HAIs and its susceptibility to antimicrobials in our setting. As HAIs had not previously been routinely surveyed in Vietnamese hospitals, criteria for the diagnosis of specific HAI may not have been fully understood, potentially leading to misclassification of specific HAIs. To limit this problem, we organized several workshops and site visits to train doctors. The enrolment of patients who were just admitted to ICU before 8.00 A.M. then were discharged from ICU soon after survey time could lead to underestimate the HAI prevalence. However, the mean ICU stays were much longer (4.8 to 16 days, Table A in S1 File) means that number of these cases was not many and its impact on the HAI prevalence was not significant. HAIs also are a sensitive issue in Vietnam; therefore ICU doctors may not have reported all HAIs, leading to an underestimation. To minimize this effect data, hospitals were anonymized using codes. When data were uploaded we ran data checks and returned queries to doctors that needed to be resolved. We therefore think that the hands-on supervision and input from the study team ensured that data quality was optimal.
Conclusions
High prevalence of HAIs in Vietnamese ICUs, mainly caused by Gram-negative bacteria with high rates of carbapenem resistance, and high levels of antimicrobial use illustrate the urgent need for capacity strengthening in both rational antimicrobial use and infection control efforts at national, regional and local levels.
Supporting Information
Hospital and ICU Characteristics (Table A), Patient Characteristics (Table B), HAI Prevalence per Month (Table C), Antimicrobials Combinations Used (Table D), The Common Antimicrobial Agents Used (Table E)
(DOCX)
Acknowledgments
We would like to thank all the doctors, pharmacists, microbiologists, infection control staff, nurses and administrators from the participating hospitals for their contribution in this survey. We would also like to thank the Ministry of Health in Vietnam for their support.
We would like to particularly thank the following people for their contribution in implementing this surveillance project, data collection and providing feedback to the findings:
Dong Phu Khiem–Intensive Care Unit, National Hospital for Tropical Diseases, Ha Noi, Vietnam.
Vu Tien Viet Dung, Ana Bonell, Do Thi Thuy Nga—Wellcome Trust Major Overseas Programme, Oxford University Clinical Research Unit, Hanoi, Vietnam.
Mai Van Cuong, Giang Thuc Anh–Intensive Care Unit, Bach Mai Hospital, Ha Noi, Vietnam.
Bui Trung Nghia, Vu Hoang Phuong, Nguyen Thi Thuy Ngan—Viet Duc Hospital, Ha Noi, Viet Duc Hospital, Ha Noi, Vietnam.
Bui Thi Mai Suong, Nguyen Van Hai—Saint-Paul Hospital, Ha Noi, Saint-Paul Hospital, Ha Noiietnam.
Bui Van Tam, Nguyen Van Lu—Viet Tiep Hospital, Hai Phong, Vietnam.
Hoang Thang Van—Vietnam-Sweden Uong Bi Hospital, Quang Ninh, Vietnam-Sweden Uong Bi Hospital, Quang Ninhietnam.
Ho Dac Hanh, Nguyen Tuan Long—Da Nang Hospital, Da Nang City, Vietnam.
Le Ba Hung, Tran Duy Hoa, Nguyen Xuan Khoi, Nguyen Xuan Tai—Hue Central General Hospital, Hue City, Vietnam.
Dang Dinh Vi, Nguyen Thi Ngoc Hue—Binh Dinh Hospital, Binh Dinh Province, Vietnam.
Nguyen Thi Thuy Ai—Khanh Hoa Hospital, Nha Trang City, Vietnam.
Vo Phi Binh—Dak Lak Hospital, Buon Ma Thuot City, Vietnam.
Vu Quynh Nga, Nguyen Manh Tuan—Cho Ray Hospital, Ho Chi Minh City, Vietnam.
Huynh Thi Loan, Nguyen Thi Ngoc Bich—Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
Ha Tan Duc, Le Thi Kim Dai—Can Tho Central General Hosptial, Can Tho City, Vietnam.
Carl Suetens—European Center for Disease Prevention and Control
Data Availability
Due to ethical restrictions regarding participant identifying information, data are available upon request. For further analyses or inclusion in larger datasets, please contact Dr Vu Dinh Phu at vudinhphu07@gmail.com.
Funding Statement
Swedish International Development Agency, The Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO, Report on the Burden of Endemic Health Care-Associated Infection Worldwide: a system review of the literature http://apps.who.int/iris/bitstream/10665/80135/1/9789241501507_eng.pdf. 2011.
- 2.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. , Antibiotic resistance-the need for global solutions. Lancet Infect Dis, 2013. 13(12): p. 1057–1098. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 3.Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. , Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet, 2011. 377(9761): p. 228–241. 10.1016/S0140-6736(10)61458-4 [DOI] [PubMed] [Google Scholar]
- 4.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. , Multistate point-prevalence survey of health care-associated infections. N Engl J Med, 2014. 370(13): p. 1198–1208. 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ECDC, Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012 Stockholm: European Centre for Disease Prevention and Control, 2013. [Google Scholar]
- 6.Susan Hopkins KS, Lisa Simpson, English National Point Prevalence Survey on Healthcare Associated Infections and Antimicrobial Use, 2011: Preliminary data. Health Protection Agency: London., 2012. [Google Scholar]
- 7.Erdem H, Inan A, Altindis S, Carevic B, Askarian M, Cottle L, et al. , Surveillance, control and management of infections in intensive care units in Southern Europe, Turkey and Iran—a prospective multicenter point prevalence study. J Infect, 2014. 68(2): p. 131–140. 10.1016/j.jinf.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal VD, Maki DG, Mehta Y, Leblebicioglu H, Memish ZA, Al-Mousa HH, et al. , International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007–2012. Device-associated module. Am J Infect Control, 2014. 42(9): p. 942–956. 10.1016/j.ajic.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KV, Thi Do NT, Chandna A, Nguyen TV, Pham CV, Doan PM, et al. , Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health, 2013. 13: p. 1158 10.1186/1471-2458-13-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. , International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control, 2012. 40(5): p. 396–407. 10.1016/j.ajic.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 11.WHO, Viet Nam: health profile World Health Organization, 2014. [Google Scholar]
- 12.Thu TA, Hung NV, Quang NN, Archibald LK, Thuy le TT, Harun Or R, et al. , A point-prevalence study on healthcare-associated infections in Vietnam: public health implications. Infect Control Hosp Epidemiol, 2011. 32(10): p. 1039–1041. 10.1086/661915 [DOI] [PubMed] [Google Scholar]
- 13.Vu VG, Nguyen VH, and Pham LT, Prevalence and risk factors of nosocomial infections in some hospitals of Hanoi, 2006. Journal of Clinical Medicine, 2008(6): p. 39–45 (Vietnamese). [Google Scholar]
- 14.Vu VG, Truong AT, and Nguyen VH, Prevalence of nosocomial infections and its relative factors in the general Dien Bien, Hoa Binh and Quang Ninh hospitals, 2005. Journal of Clinical Medicine, 2008(6): p. 46–50 (Vietnamese). [Google Scholar]
- 15.Truong AT and Nguyen VH, Prevalence of nosocomial infections and its relative factors in Bach Mai hospital 2006. Journal of Clinical Medicine, 2008(6): p. 51–56 (Vietnamese). [Google Scholar]
- 16.Truong AT, Nguyen VH, Nguyen DA, Nguyen TD, and Vu VD, Incidence of nosocomial infections in the intensive care units in Bach Mai hospital (2002–2003). Journal of Clinical Medicine, 2008(6): p. 57–62 (Vietnamese). [Google Scholar]
- 17.Nguyen HS and Tran QV, Incidence rate of nosocomial infections at the intensive care unit of No. 175 military hospital, 2006. Journal of Clinical Medicine, 2008(6): p. 63–66 (Vietnamese). [Google Scholar]
- 18.Van TD, Dinh Q- D, Vu PD, Nguyen TV, Pham CV, Dao TT, et al. , Antibiotic susceptibility and molecular epidemiology of Acinetobacter calcoaceticus–baumannii complex strains isolated from a referral hospital in northern Vietnam. Journal of Global Antimicrobial Resistance, 2014. 2(4): p. 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheim HF, Chandna A, Vu PD, Pham CV, Nguyen PD, Lam YM, et al. , Providing impetus, tools, and guidance to strengthen national capacity for antimicrobial stewardship in Viet Nam. PLoS Med, 2013. 10(5): p. e1001429 10.1371/journal.pmed.1001429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ECDC, Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals–protocol version 4.3 Stockholm, European Centre of Disease Prevention and Control, 2012. [Google Scholar]
- 21.ECDC, HELICSWinNet (HWN) 2012: p. http://www.ecdc.europa.eu/en/activities/surveillance/HAI/about_HAI-Net/Pages/HELICSWinNet-download-page-HWN.aspx (accessed Sept 30 2012).
- 22.Vietnam Ministry of Health, Project to decrease overload of hospitals, http://www.kcb.vn/ShowNews.aspx?lang=vn&cat=015&nid=97. 2012 (Vietnamese).
- 23.Barrera L, Zingg W, Mendez F, and Pittet D, Effectiveness of a hand hygiene promotion strategy using alcohol-based handrub in 6 intensive care units in Colombia. Am J Infect Control, 2011. 39(8): p. 633–639. 10.1016/j.ajic.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Hugonnet S, Villaveces A, and Pittet D, Nurse staffing level and nosocomial infections: empirical evaluation of the case-crossover and case-time-control designs. Am J Epidemiol, 2007. 165(11): p. 1321–1327. [DOI] [PubMed] [Google Scholar]
- 25.Johansson M, Phuong DM, Walther SM, and Hanberger H, Need for improved antimicrobial and infection control stewardship in Vietnamese intensive care units. Trop Med Int Health, 2011. 16(6): p. 737–743. 10.1111/j.1365-3156.2011.02753.x [DOI] [PubMed] [Google Scholar]
- 26.Behnke M, Gastmeier P, Geffers C, Monch N, and Reichardt C, Establishment of a national surveillance system for alcohol-based hand rub consumption and change in consumption over 4 years. Infect Control Hosp Epidemiol, 2012. 33(6): p. 618–620. 10.1086/665729 [DOI] [PubMed] [Google Scholar]
- 27.Bouadma L, Wolff M, and Lucet JC, Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis, 2012. 25(4): p. 395–404. 10.1097/QCO.0b013e328355a835 [DOI] [PubMed] [Google Scholar]
- 28.Oostdijk EA, de Smet AM, Kesecioglu J, Bonten MJ, and Dutch SODSDDTG, Decontamination of cephalosporin-resistant Enterobacteriaceae during selective digestive tract decontamination in intensive care units. J Antimicrob Chemother, 2012. 67(9): p. 2250–2253. [DOI] [PubMed] [Google Scholar]
- 29.Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, et al. , Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA, 2014. 312(14): p. 1438–1446. 10.1001/jama.2014.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. , International study of the prevalence and outcomes of infection in intensive care units. JAMA, 2009. 302(21): p. 2323–2329. 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 31.Dogru A, Sargin F, Celik M, Sagiroglu AE, Goksel MM, and Sayhan H, The rate of device-associated nosocomial infections in a medical surgical intensive care unit of a training and research hospital in Turkey: one-year outcomes. Jpn J Infect Dis, 2010. 63(2): p. 95–98. [PubMed] [Google Scholar]
- 32.Livermore DM and Tulkens PM, Temocillin revived. J Antimicrob Chemother, 2009. 63(2): p. 243–245. 10.1093/jac/dkn511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hospital and ICU Characteristics (Table A), Patient Characteristics (Table B), HAI Prevalence per Month (Table C), Antimicrobials Combinations Used (Table D), The Common Antimicrobial Agents Used (Table E)
(DOCX)
Data Availability Statement
Due to ethical restrictions regarding participant identifying information, data are available upon request. For further analyses or inclusion in larger datasets, please contact Dr Vu Dinh Phu at vudinhphu07@gmail.com.