Abstract
The aim of the present study was to investigate the neuroprotective mechanism of the miR-9-mediated activation of the nuclear factor (NF)-κB signaling pathway by electroacupuncture (EA) stimulation of the Quchi (LI11) and Zusanli (ST36) acupoints in a rat model of middle cerebral artery occlusion (MCAO). The present study demonstrated that EA alleviated the symptoms of neurological deficits and reduced the infarct volume in the rat brains. The expression of miR-9 in the peri-infarct cortex was increased in the EA group compared with the MCAO group, and the expression of NF-κB signaling pathway-associated factors, NF-κB p65, tumor necrosis factor (TNF)-α and interleukin (IL)-1β were reduced. Notably, miR-9 inhibitors were revealed to have the ability to suppress EA-alleviated cerebral inflammation and the expression of NF-κB downstream-related factors, NF-κB p65, TNF-α and IL-1β, and caused no alteration on the level of NF-κB upstream-related protein inhibitor of κBα, suggesting that the cerebral protective efficacy of EA targets miR-9-mediated NF-κB downstream pathway following ischemic stroke.
Keywords: electroacupuncture, ischemic stoke, miR-9, NF-κB signaling pathway
Introduction
Micro (mi)RNA is a small, non-coding RNA regulatory molecule of 18–24 nucleotides in length, which post-transcriptionally silences genes by base-pairing with the mRNA for degradation and/or translational repression (1). miRNA has been shown to be involved in cell proliferation, differentiation and apoptosis, and is therefore closely associated with the occurrence and progression of a variety of human diseases (2). It has been reported that miRNAs are involved in inflammatory reactions and immune responses to various neurological pathologies underlying central nervous system injuries (3).
It was previously revealed that the highly conserved miR-9 (homologous to miR-79) is involved in microglial inflammatory responses following cerebral injuries (4,5). miR-9 is encoded by three distinct genomic loci (miR-9-1, -2 and -3), which give rise to mature miR-9 species with identical sequences. It has also been found that miR-9 directly acts on the 3′-untranslated region (UTR) of the inflammatory regulator of nuclear factor (NF)-κB, an integral part of the inflammatory reaction of tumor cells (6,7). Similarly, miR-9 has been shown to be involved in the NF-κB signaling pathway in which microglial cells are activated by lipopolysaccharide stimuli, and a lack of miR-9 results in the upregulation of proinflammatory cytokines/chemokines [interleukin (IL)-1β, tumor-necrosis factor (TNF)-α, IL-6 and monocyte chemoattractant protein (MCP)-1] (5). NF-κB may be singularly important in regulating genetic responses to brain stresses through the innate immune response, since it belongs to the category of 'pre-formed' primary transcription factors, which are already present in cells in an 'inactive' state. In unstimulated cells, NF-κB is sequestered in the cytosol via interacting with inhibitor of NF-κB (IκB) proteins; however, when cells receive pathological stimuli, IκB proteins can be phosphorylated by IκB kinase (IKK), which is activated, leading to its translocation to the nucleus, where it induces the expression of various proinflammatory cytokines, including TNF-α, IL-1β and IL-6. NF-κB-regulated miR-9 has the potential to contribute to the regulation of neuroinflammation and innate immune signaling in stressed primary neural cells of the human brain (8). In addition, stroke downregulated the expression of miR-9 in neural progenitor cells (9). A study reported that the serum levels of miR-9 were suppressed to facilitate neuroinflammation and brain injury, and miR-9 was negatively correlated with the infarct volume and plasma high-sensitivity C-reactive protein levels in patients with acute ischemic stroke (10).
Stroke, also termed cerebrovascular disease, is characterized by high mortality, morbidity and recurrence rates. As an important medical methodology, electroacupuncture (EA) is a treatment modality of disease by inserting needles along specific pathways or meridians attaching a micro-electro-stimulation in oriental medicine, and has been widely used in clinical practice for motor impairment in patients with stroke (11). It has been previous demonstrated that EA stimulation of Zusanli (ST36) and Quchi (LI11) acupoints attenuates inflammatory injury and infarct volume in a rat model of middle cerebral artery occlusion (MCAO) (12). EA has been shown to significantly reduce the expression of proinflammatory cytokines, IL-1β and TNF-α, via the NF-κB signaling pathway in ischemic cerebral tissues (12,13); however, the affect of EA on NF-κB mediated regulators in ischemic stroke remains to be elucidated.
The present study hypothesized that EA regulates the expression of miR-9 to suppress the activation of the NF-κB signaling pathway and contribute to the release of proinflammatory cytokines, IL-1β, IL-6 and TNF-α, in MCAO injury.
Materials and methods
Statement of ethics
All experimental procedures were approved by the Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (FUTCM) and followed Chinese Specifications for the Production, Care and Use of the Laboratory Animals. All animal experiments were permitted, according to the License no. SYXK (Min) 2012-007. Throughout the experiments, the utmost effort was made to minimize animal suffering.
MCAO rat model for focal cerebral ischemia
Adult male Sprague-Dawley rats (n=90; weight 230–250 g) were housed in an environmentally controlled room at FUTCM (22±2°C, with a 12 h light/dark cycle). The rats were provided ad libitum access to standard rat chow and water. Focal cerebral ischemia was induced by MCAO, as previously described (14), with minor modifications. Briefly, left MCAO was performed using an occluding suture (diameter, 0.26 mm) for 2 h, and following 2 h of MCAO-evoked ischemia, the suture was slowly drawn back to allow reperfusion. The ipsilateral cerebral blood flow was measured using laser Doppler flowmetry (MoorDRT4; Biopac Systems, Inc. Goleta, CA, USA). The MCAO model was considered successful only when the drop in cerebral blood flow was ≥80% of baseline during occlusion. This blood flow rate was maintained for at least 1 h, with the exception of the 0 h time-point.
Rat groups and EA treatments
The rats were randomly divided into the following five groups (n=18/group): i) Sham operation group (Sham); ii) MCAO model group (MCAO); iii) EA group (MCAO + EA); iv) EA combined with dimethyl sulfoxide (DMSO) group (EA + DMSO); v) EA combined with miR-9 inhibitors using a random number table method (EA + miR-9 inhibitors) (15).
The MCAO + EA, EA + DMSO and EA + miR-9 inhibitors groups were stimulated with an electric potential difference of 4 V, and dense disperse wave of 1- or 20-Hz (adjusted to the muscle twitch threshold) using an EA apparatus (G6805; SMIF, Shanghai, China) for 30 min once daily. The rats were immobilized using a surgical fixation device (Beijing Science and Technology Development Co., Ltd., Beijing, China) (12). EA was performed by stimulating the right paralyzed limb at Zusanli (ST36) and Quchi (LI11) acupoints with a depth of 2–3 mm (12,14). The treatment was performed 24 h following the surgery. All animals were sacrificed by cervical dislocation following anesthesia with 10% chloral hydrate (Fujian Academy of Integrative Medicine, Fuzhou, China) at 72 h following the surgery.
Intraventricular injections
Intraventricular injections were performed in the EA + DMSO and EA + miR-9 inhibitors groups 30 min prior to MCAO. The animals were anesthetized with 10% chloral hydrate and were subsequently placed in a stereotaxic apparatus (68001; RWD Life Science Co., Ltd., Shenzhen China). A volume of 7 μl miR-9 inhibitors (10 mM/l in DMSO) or DMSO were injected into the left lateral ventricle in rats (16). Stereotactic coordinates were as follows: Anteroposterior, 0.8 mm; mediolateral, 1.5 mm; depth, 3.5 mm.
Scoring of neurological deficits
Neurological deficits in the MCAO rat model were scored using the Longa et al (17) method following resuscitation. The scores for the neurological behavioral were as follows: 0, Absence of neurological injury symptoms; 1, flexion of the right front paw; 2, body turning towards the right side while walking; 3, falling down on the right side; 4, complete loss of consciousness with the inability to walk. Rats scoring 0 and 4 were excluded from the present study.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
Brain tissues were dissected using surgical apparatus (Fujian Academy of Integrative Medicine). The brain tissues were frozen at −20°C for 20 min and cut into five 2 mm sections in the coronal plane at the level of the optic chiasm. The sections were preserved in 2% TTC (Sigma-Aldrich, St. Louis, MO, USA) phosphoric acid buffer [0.2 mol/l (pH 7.4)], as previously described (12) and placed in an incubation box at 37°C in the dark. The sections were alternately overturned twice and were removed following two 15 min long alternations. The normal tissues were stained red using TTC; the infarct loci were not stained and were white in color. Images of the sections were captured using a digital camera (Canon SX20; Canon, Inc., Tokyo, Japan). The percentage of the infarct volume in the total brain volume was calculated using a Motic Med 6.0 image analysis system (Motic Group Co., Ltd., Xiamen, China).
Hematoxylin and eosin (HE) staining
The tissue sections were embedded with paraffin and subjected to conventional gradient dewaxing, followed by HE staining for 3 min at 25°C. Following rinsing with flowing water for 1–2 min, the tissue sections were placed in a 75% hydrochloric acid/alcohol mixture to highlight hematoxylin for 30–45 sec. The tissue sections were subsequently rinsed with flowing water for 10–20 min until the blue color returned. Successive treatment with 95% ethanol and acidified eosin-ethanol staining were performed for 1 min at 25°C. Finally, the tissue sections were dehydrated with gradient ethanol, rendered transparent with xylene and sealed with neutral balsam. The morphological changes were observed under a light microscope (DM500; Leica Microsystems, Wetzlar, Germany).
Immunofluorescence
The paraffin-embedded brain-tissue sections were treated with microwave heat-induced epitope retrieval. Following three washes in phosphate-buffered saline (PBS; pH 7.4), the specimens were incubated for 1 h at 37°C in a 1:120 dilution of fluorescein isothiocyanate-labeled rabbit anti-rat NF-κB p65 antibody (cat. no. ab16502; Abcam, Cambridge, UK). Washing was repeated, as above. The nuclei of all cells were counterstained with 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) and incubated for 5 min at 25°C. Following a further three washes in PBS, the tissues were mounted in Prolong Gold Antifade reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Images were captured using a fluorescent microscope (Olympus BX61; Olympus Corp., Tokyo, Japan) at a magnification of ×200.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the ischemic cortex using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A SYBR Green® microRNA Reverse Transcription kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China) was used to reverse transcribe the RNA into cDNA prior to qPCR. The PCR primer kit used for miR-9 was rno-miR-9 (miRQP0825; GeneCopoeia, Guangzhou, China) and the PCR primer kit used for U6 was rno-miR-U6 (RQP047936; GeneCopoeia). The GeneAmp PCR System 9600 (Thermo Fisher Scientific, Inc.) was used. The PCR cycling conditions were 95°C for 10 sec and 38 cycles at 56.5°C for 20 min. The relative expression of miR-9 was quantified using the 2−ΔΔCq method. The reference gene was rno-miR-U6 (RQP047936; GeneCopoeia, Guangzhou, China).
Western blotting
Cell lysis buffer (1 ml; Invitrogen; Thermo Fisher Scientific, Inc.) and PMSF (10 μl) were added to 100 mg brain tissue at 25°C for 15 min for protein extraction. The protein concentration was determined using Coomassie brilliant blue staining (Invitrogen; Thermo Fisher Scientific, Inc.). The proteins were denatured by heating and 50 μg protein was used for 12% sodium dodecyl sulfate-polyacrylamide gel (Promega Corp., Madison, WI, USA) electrophoresis. The proteins were transferred onto nitrocellulose or polyvinylidene difluoride membranes (Promega Corp.) and were sealed with 5% non-fat milk at room temperature for 2 h. The membranes were incubated with NF-κB p65 (dilution, 1:800; cat. no. ab16502; Abcam), IκBα (dilution, 1:800; cat. no. ab7217; Abcam), TNF-α (1:800; cat. no. ab6671; Abcam), IL-1β (1:800; cat. no. ab200478; Abcam) and β-actin (1:1,000; cat. no. ab189073; Abcam) primary antibodies at 4°C overnight. The membranes were washed and horseradish peroxide-labeled secondary antibodies were added. The cells were incubated in an oscillation incubator at 37°C for 1 h. The color was developed by the addition of electrochemiluminescence solution (Invitrogen; Thermo Fisher Scientific, Inc.), and the images were visualized using a Bio-Image system (Bio-Rad Laboratories, Inc., Hercules, USA). Gray scale analysis was performed for the target bands using Image-Pro Plus software (version 7.0; UVP, LLC, Upland, CA, USA).
Luciferase reporter assay
The psiCHECK2-NF-κB-luc and psiCHECK2-NF-κB-Mut-luc firefly luciferase reporter vectors (GeneCopoeia Inc.), which contain the intact or mutated putative miR-9 recognition sequence from the 3′-UTR of NF-κB, respectively, cloned downstream of the firefly luciferase gene were constructed. The following primers were designed (Shanghai Sangon Biological Engineering Co., Ltd., Shanghai, China) to PCR the 3′-UTR of human NF-κB from the total RNA extracted from primary cortical neuronal cells: NF-κB, forward: 5′-gcuuuaaaaaaaggagaaaa-3′ and reverse: 5′-acccatctcacccattcttg-3′; nf-κb mutant, forward: 5′-cuggcuuuaaaaaauccucuuaa-3′ and reverse: 5′-tgacacagcaactcctttgg-3′.
Each PCR product was ~1 kb and covers the putative miR-9 recognition sequence or mutated sequence from the 3′-UTR of NF-κB. Clones were selected following screening by restriction digestion with XhoI and NotI. Primary cortical neuronal cells were seeded into 96-well white assay plates (Corning Inc., Corning, NY, USA) at a density of 30,000 cells/well 1 day prior to transfection. A total of 10 ng of each reporter construct was co-transfected with the miR-9 mimics or a mimic negative control (GeneCopoeia Inc.) at a final concentration of 100 nM into primary cortical neuronal cells using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Following incubation for 48 h, firefly and Renilla luciferase activities were measured using the Dual-Glo luciferase assay system, according to the manufacturer's protocol (Promega Corp.). Renilla luciferase activity was normalized against firefly luciferase activity for each sample. All experiments were performed in triplicate and three wells were used for each condition in each experiment.
Statistical analysis
All quantitative data are expressed as the mean ± standard error and were analyzed using SPSS 17.0 statistical analysis software (IBM, Corp., Armonk, NY, USA). The data were subjected to a one-way analysis of variance between different groups, followed by the least significant difference t-test. P<0.05 was considered to indicate a statistically significant difference. All final results were analyzed in a blinded manner.
Results
Effects of EA on neurological deficit scores
In the present study, the neuroprotective effect of EA treatment was examined using neurological deficit scores. Table I shows that all MCAO rats demonstrated obvious neurological signs, as compared with the rats in the Sham group (P<0.05), suggesting successful model construction. No significant differences were observed in the neurological signs among the MCAO, EA + DMSO and EA + miR-9 inhibitors groups at 24 h following MCAO and reperfusion. However, 72 h after MCAO and reperfusion, the neurological deficits of all rats in the EA + DMSO group exhibited a significant improvement compared with the MCAO and EA + inhibitors groups (P<0.05). These results suggested that EA treatment effectively alleviated the symptoms of neurological deficit of rats with cerebral ischemia. Furthermore, it was shown that miR-9 inhibitors suppressed the EA-triggered recovery of neurological function, while DMSO did not.
Table I.
Neurological deficit scores.
| Group (n=18) | Deficit score at different durations following MCAO
|
||
|---|---|---|---|
| 2 h | 24 h | 72 h | |
| Sham | 0 | 0 | 0 |
| MCAO | 1.87±0.23a | 2.25±0.18 | 2.08±0.20 |
| MCAO + EA | 1.85±0.24a | 2.07±0.28 | 1.14±0.25b |
| EA + DMSO | 1.83±0.24a | 2.17±0.28 | 1.08±0.23b |
| EA + miR-9 inhibitors | 1.87±0.18a | 2.21±0.30 | 1.84±0.20 |
Data are expressed as the mean ± standard error from 16 rats/group.
P<0.05, vs. Sham;
P<0.05, vs. MCAO and EA + miR-9 inhibitors. MCAO, middle cerebral artery occlusion; EA, electroacupuncture; miR-9, microRNA-9; DMSO, dimethyl sulfoxide.
Effects of EA on infarct volume
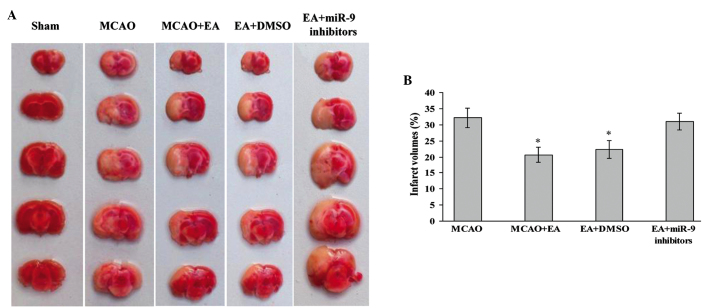
TTC-stained brain sections were evaluated 72 h after MCAO and reperfusion. The results showed that the sections from the Sham group were stained red, whereas the unstained white infarct area was visible on the left side of the brain in the other groups. Compared with the Sham group, the infarct volume in the MCAO+EA group was significantly decreased (P<0.05; Fig. 1). Furthermore, the infarct volume of the EA + DMSO group was lower compared with that of the MCAO and EA + miR-9 inhibitors groups (P<0.05; Fig. 1), suggesting that the miR-9 inhibitors suppressed the cerebral protective efficacy of EA treatment.
Figure 1.
Effect of EA on infarct volume and morphological structure of rat brains. (A) 2,3,5-Triphenyltetrazolium chloride staining indicating cerebral infarct volumes of Sham, MCAO, MCAO + EA, EA + DMSO and EA + miR-9 inhibitors groups. (B) Bar graph showing the percentage of total brain volume in each group (n=4; *P<0.05, vs. the MCAO and the EA + miR-9 inhibitors groups). MCAO, middle cerebral artery occlusion; EA, electroacupuncture; miR-9, microRNA-9; DMSO, dimethyl sulfoxide.
Effects of EA on inflammation
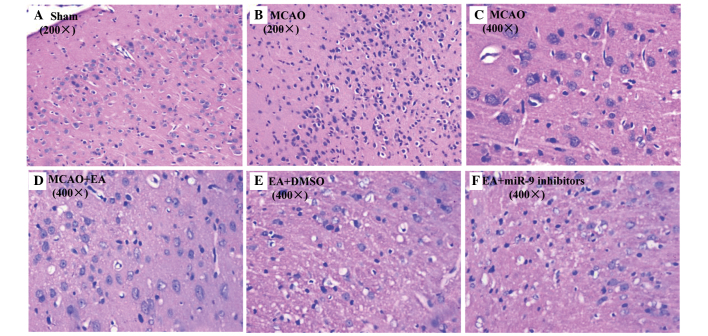
To further investigate the neuroprotective efficacy of EA treatment, its anti-inflammatory effect was examined using HE staining. As expected, no histopathological abnormalities and inflammatory cells were observed in the Sham group (Fig. 2A). By contrast, in the infarct core and bounding region of the cerebral cortical area of the MCAO group, the glial and neuron cells exhibited interstitial edema, were shrunken and showed condensed nuclei (Fig. 2B and C), which was ameliorated by EA treatment (Fig. 2D). Furthermore, compared with the MCAO group, considerably fewer inflammatory cells were infiltrated into the cerebral infarct areas in the EA + DMSO group (Fig. 2E), whereas inflammatory cells were infiltrated in the EA + miR-9 inhibitors group (Fig. 2F), suggesting that miR-9 inhibitors suppressed EA-alleviated cerebral inflammation in MCAO rats.
Figure 2.
HE staining. At the end of the experiment, cerebral tissues from the (A) Sham, (B and C) MCAO, (D) MCAO + EA, (E) EA + DMSO and (F) EA + miR-9 inhibitor groups were processed for HE staining (n=6). The cerebral cortical histopathological changes and degree of inflammatory cell infiltration in the infarct areas of each group were observed under an optical microscope at magnifications of (A and B) ×200 and (C–F) ×400. HE, hematoxylin and eosin; MCAO, middle cerebral artery occlusion; EA, electroacupuncture; DMSO, dimethyl sulfoxide; miR-9, microRNA-9.
Effects of EA on the expression of miR-9 in the cerebral cortex
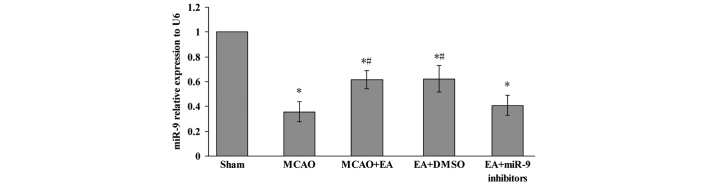
In order to investigate the effect of EA on the miR-9, the expression of miR-9 in the peri-infarct cerebral cortex was assessed using qPCR. Fig. 3 shows that the relative expression of the miR-9 in MCAO, MCAO + EA, EA + DMSO and EA + miR-9 inhibitors groups were significantly decreased compared with the Sham group 72 h after MCAO and reperfusion (P<0.05). In the MCAO + EA and EA + DMSO groups, however, a significant increase was observed compared with the MCAO group (P<0.05). In addition, the expression of miR-9 in the EA + miR-9 inhibitors group was found to be lower compared with that in the MCAO + EA and EA + DMSO groups (P<0.05).
Figure 3.
Effects of EA on the expression of miR-9 in the cerebral cortex. Reverse transcription-quantitative polymerase chain reaction of the miR-9 expression level in the peri-infarct cerebral cortex of the Sham, MCAO, MCAO + EA, EA + DMSO and EA + miR-9 inhibitor groups 72 h following MCAO and reperfusion (*P<0.05, vs. Sham group; #P<0.05, vs. MCAO and EA + miR-9 inhibitor groups). MCAO, middle cerebral artery occlusion; EA, electroacupuncture; miR-9, microRNA-9; DMSO, dimethyl sulfoxide.
Effect of EA on the NF-κB signaling pathway
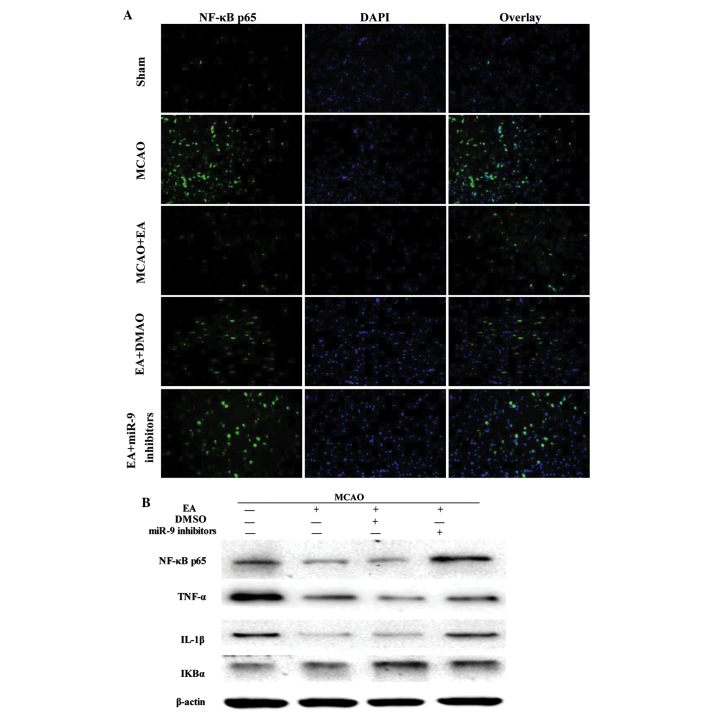
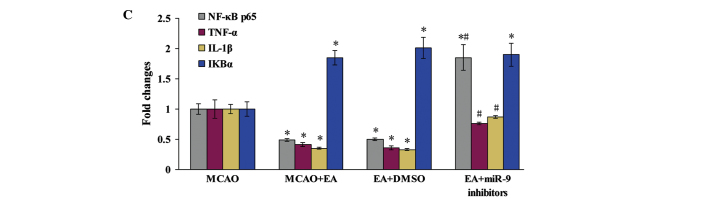
To investigate the mechanism of the anti-inflammatory effect of EA, the localization and expression of NF-κB signaling pathway-associated factors in the peri-infarct cortex was evaluated. The NF-κB p65 subunit was visualized using immunofluorescent staining, and the cells were counterstained with DAPI. NF-κB nuclear translocation was recognized by the colocalization of the p65 subunit with DAPI. The cerebral ischemic injury resulted in the nuclear translocation of the NF-κB p65 subunit in the MCAO group, which was not observed in the sham operation group. However, EA inhibited the NF-κB nuclear translocation and reduced the number of NF-κB p65-positive cells, whereas of NF-κB p65-positive cells decreased in the EA + miR-9 inhibitors group, as compared with the EA and EA + DMSO groups (Fig. 4A). The levels of expression of NF-κB p65, TNF-α and IL-1β in the EA group were significantly decreased compared with the Sham group (P<0.05). By contrast, the IκBα expression levels were increased in the EA group compared with those in the MCAO group (P<0.05). In the EA + miR-9 inhibitors group, the expression levels of NF-κB p65 not only increased, but also exceeded those of the MCAO group, suggesting that the administration of miR-9 inhibitors significantly promoted the expression of NF-κB p65. Furthermore, the differences in IκBα expression among the EA, EA + DMSO and EA + miR-9 inhibitors groups were found to be statistically significant (P>0.05), suggesting that miR-9 inhibitors did not alter the expression of NF-κB upstream-related protein IκBα.
Figure 4.
Effect of EA on NF-κB signaling pathway-associated factors. (A) Evaluation of NF-κB p65 (green), DAPI (blue) and the colocalization positive cells in peri-infarct cortical tissue of the Sham, MCAO, MCAO + EA, EA + DMSO and EA + miR-9 inhibitor groups (n=4). (B) Western blot analysis of the protein expression levels of NF-κB p65, TNF-α, IL-1β and IκBα in the peri-infarct cortical tissue of the MCAO, MCAO + EA, EA + DMSO and EA + miR-9 inhibitors groups. (C) Bar graph showing the fold change of NF-κB p65, TNF-α, IL-1β and IκBα in each group (n=4) (*P<0.05, vs. MCAO group; #P<0.05, vs. MCAO + EA and EA + DMSO groups). EA, electroacupuncture; DAPI, 4′,6-diamidino-2-phenylindole; MCAO, middle cerebral artery occlusion; DMSO, dimethyl sulfoxide; miR-9, microRNA-9; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IκBα, inhibitor of κBα.
Interaction of miR-9 and NF-κB
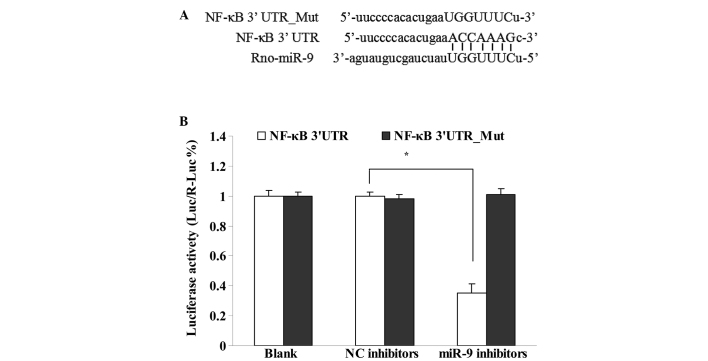
The target genes of miR-9 were predicted with an online algorithm using miRWalk database. Fig. 5A shows that the site where miR-9 binds to NF-κB was predicted and identified as ACCAAAG. The interaction of miR-9 and NF-κB was observed using a dual-luciferase reporter vector assay (Fig. 5B). The results showed that miR-9 bound to 3′UTR of the NK-κB gene (P<0.05), however, not to the mutant 3′UTR (P>0.05).
Figure 5.
Interaction between miR-9 and NF-κB. (A) The site of miR-9 binding to NF-κB. (B) Luciferase activity of psiCHECK2- NF-κB-luc and psiCHECK2-NF-κB-Mut-luc were detected in primary cortical neuronal cells transfected with miR-9 inhibitors (n=3; *P<0.05, vs. NC inhibitors group). Blank, blank control group; NC inhibitors, cells transfected with negative miR-9 inhibitors group; miR-9 inhibitors, cells transfected with miR-9 inhibitors group. miR-9, microRNA-9; NF-κB, nuclear factor-κB.
Discussion
For >3,000 years, practitioners in China have used acupuncture to treat various diseases, including stroke. Acupuncture is widely used for the improvement of motor, sensation, speech and other neurological functions in patients with stroke. Compared with other conventional interventions, acupuncture is relatively simple, inexpensive and safe. For those reasons, acupuncture is not only widely accepted by Chinese patients, but also increasingly practiced in certain Western countries (18). Electrical stimulation was added to traditional acupuncture and termed electro-acupuncture (EA), which quantified the inserting dose and enhanced treatment efficiency (19). A number of indices that evaluate the quality of life of stroke patients have suggested that EA treatment may improve patient self care (20,21). Studies have reported that the application of EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints results in a decrease in the neurological deficit scores of patients with hemiplegia (22). Our previous animal studies on the underlying mechanism of EA treatment on Quchi (LI11) and Zusanli (ST36) demonstrated that EA stimulation of those particular acupoints considerably improved the neurological deficit scores of rats, as well as reduce ischemic infarct volume-mediated anti-inflammatory pathway (12,14). The present study demonstrated that EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints clearly decreased the infarct volume of MCAO rats and reduced alleviated cerebral neurocyte interstitial edema and inflammatory invasion at 72 h after MCAO and reperfusion.
It has been reported that NF-κB is involved in regulating ischemic stroke injury (23). The classical pathway of NF-κB activation is through a heterodimer of p50 and p65 of two subunits of NF-κB. NF-κB dimers are maintained in the inactive state by a family of inhibitors called IκB. Receptor signaling leads to the activation of a multisubunit IKK complex, which phosphorylates IκB on two key serine residues. Phosphorylation of IκB marks it for degradation by the ubiquitin pathway, so that the NF-κB dimer is liberated to translocate to the nucleus, bind DNA and activate transcription. It has been shown that EA causes the downregulation of NF-κB signaling pathway-assocaited factors, NF-κB p65, TNF-α, IL-1β and MCP-1, and the upregulation of IκBα in the cortex, hippocampus and corpus striatum of MCAO rats (12,24,25). Consistent with previous reports, the results of the present study demonstrated that EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints reduced the expression of NF-κB p65 and inhibited nuclear translocation of NF-κB p65 in the cerebral peri-infarct cortex of rats with ischemia-reperfusion injuries.
miRNA is a small, non-coding RNA molecule involved in the growth and development of various diseases, including stroke (26). By recognizing the 3′UTR of target mRNA, mature miRNA degrades the target gene or inhibits its translation, therefore, regulating gene expression and protein synthesis (27). The miRNA-mediated immune response is achieved by a highly complex regulatory network in the presence of stimuli and pathogens (3). For example, miR-146a, miR-21, miR-221, miR-579, miR-125b and miR-155 are involved in the negative regulation of inflammatory reactions (28–30). Few studies have focused on the involvement of miRNA in the acute or sub-acute phase of inflammatory reactions induced by ischemic strokes. It has been reported that the level of miR-155 was significantly downregulated in the brain and blood following inflammatory damage (31,32). A previous study revealed that miR-9 is crucial in the NF-κB signaling pathway, and the activation of microglia by targeting MCPIP1, the latter of which is associated with inflammatory reactions following cerebral ischemia (5). miR-9 has been shown to regulate the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response (33). miR-9 is also induced by the proinflammatory cytokines, TNF-α and IL-1β (34).
miR-9 is a tissue-specific miRNA, which is predominantly expressed in the region close to the midbrain-hindbrain boundary and is involved in normal brain development (35,36). The present study found that miR-9 was associated with inflammatory response and a significant reduction of miR-9 expression in the peri-infarct cortex following ischemia-reperfusion injury; however, the expression of miR-9 was found to be upregulated following EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints. It has been shown that NF-κB is a direct target of miR-9 in tumors (6,37). The present study demonstrated that miR-9 negatively regulated the expression of NF-κB in primary cortical neuronal cells. Furthermore, miR-9 inhibitors suppressed EA-alleviated cerebral inflammation and the expression of NF-κB signaling pathway downstream-associated factors, NF-κB p65, TNF-α, IL-1β, without altering the level of NF-κB upstream-associated protein, IκBα. These results demonstrated that EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints targeted the miR-9-NF-κB downstream pathway following ischemic stroke, therefore suggesting a cerebral protective effect of EA.
In conclusion, miR-9 has been shown to be involved in the inflammatory reactions induced by cerebral ischemia-reperfusion injuries by binding to NF-κB. miR-9 may serve as a potential therapeutic target for the treatment of cerebral ischemic injuries. EA treatment on Quchi (LI11) and Zusanli (ST36) acupoints regulated the miR-9-mediated NF-κB signaling pathway and reduced the secretion of the proinflammatory cytokines, TNF-α and IL-1β. It is possible, however, that miR-9 is involved in multiple signaling pathways, which are associated with inflammatory injury following cerebral ischemia, however, this requires further elucidation.
Acknowledgments
The present study was supported by the Natural Science Foundation of China (nos. 81403450, 81373778 and 81273835), and by the Key Subjects of Fujian university of Traditional Chinese Medicine (no. X2014069-xueke).
References
- 1.Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connell RM, Rao DS, Baltimore D. MicroRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 4.Akerblom M, Sachdeva R, Quintino L, Wettergren EE, Chapman KZ, Manfre G, Lindvall O, Lundberg C, Jakobsson J. Visualization and genetic modification of resident brain microglia using lentiviral vectors regulated by microRNA-9. Nat Commun. 2013;4:1770. doi: 10.1038/ncomms2801. [DOI] [PubMed] [Google Scholar]
- 5.Yao H, Ma R, Yang L, Hu G, Chen X, Duan M, Kook Y, Niu F, Liao K, Fu M, et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun. 2014;5:4386. doi: 10.1038/ncomms5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M, Li X, Tang H. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappaB1. FEBS J. 2009;276:5537–5546. doi: 10.1111/j.1742-4658.2009.07237.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Gu Z, Ni P, Qiao Y, Chen C, Liu X, Lin J, Chen N, Fan Q. NF-kappaB P50/P65 hetero-dimer mediates differential regulation of CD166/ALCAM expression via interaction with micoRNA-9 after serum deprivation, providing evidence for a novel negative auto-regulatory loop. Nucleic Acids Res. 2011;39:6440–6455. doi: 10.1093/nar/gkr302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukiw WJ. NF-κB-regulated micro RNAs (miRNAs) in primary human brain cells. Exp Neurol. 2012;235:484–490. doi: 10.1016/j.expneurol.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Zhang J, Han R, Liu H, Sun D, Liu X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J Clin Neurosci. 2015;22:291–295. doi: 10.1016/j.jocn.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Gu HW, Ma WP, Li QS, Yu Q, Liu XQ, Liu SH, Li WH, Liu HL, Dai MT. Multicentral randomized controlled study on effects of acupuncture at Zusanli (ST 36) and Xuanzhong (GB 39) on cerebrovascular function in the patient of ischemic stroke. Zhongguo Zhen Jiu. 2006;26:851–853. In Chinese. [PubMed] [Google Scholar]
- 12.Lan L, Tao J, Chen A, Xie G, Huang J, Lin J, Peng J, Chen L. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med. 2013;31:75–80. doi: 10.3892/ijmm.2012.1184. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z, Liang J, Wang J, Kolattukudy PE. Delayed brain ischemia tolerance induced by electroacupuncture pretreatment is mediated via MCP-induced protein 1. J Neuroinflammation. 2013;10:63. doi: 10.1186/1742-2094-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue X, You Y, Tao J, Ye X, Huang J, Yang S, Lin Z, Hong Z, Peng J, Chen L. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14–19. doi: 10.1016/j.neulet.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Lu T, Song QH, Xu RM, Guo YH, Wang F, Hu JP, Wang Y, Zhang LY. Dance combined with magnetic pulse stimulates the ability of walk and balance in elder people. Int J Clin Exp Med. 2015;8:4381–4386. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44:1706–1713. doi: 10.1161/STROKEAHA.111.000504. [DOI] [PubMed] [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang L, He J, Zhuang X, Lu L. Quality of reporting on randomized controlled trials of acupuncture for stroke rehabilitation. BMC Complement Altern Med. 2014;14:151. doi: 10.1186/1472-6882-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L, He PL, Zhou ZZ, Li YH. Efficacy observation of dysphagia after acute stroke treated with acupuncture and functional electric stimulation. Zhongguo Zhen Jiu. 2014;34:737–740. In Chinese. [PubMed] [Google Scholar]
- 20.Sze FK, Wong E, Or KK, Lau J, Woo J. Does acupuncture improve motor recovery after stroke? A meta-analysis of randomized controlled trials. Stroke. 2002;33:2604–2619. doi: 10.1161/01.STR.0000035908.74261.C9. [DOI] [PubMed] [Google Scholar]
- 21.Shen PF, Kong L, Ni LW, Guo HL, Yang S, Zhang LL, Zhang ZL, Guo JK, Xiong J, Zhen Z, et al. Acupuncture intervention in ischemic stroke: A randomized controlled prospective study. Am J Chin Med. 2012;40:685–693. doi: 10.1142/S0192415X12500516. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Xie Y, Zhang Q, Xu N, Zhong H, Dong H, Liu L, Jiang T, Wang Q, Xiong L. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: A prospective, randomized, placebo-controlled trial. Br J Anaesth. 2014;112:1075–1082. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- 23.Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZK, Ni GX, Liu K, Xiao ZX, Yang BW, Wang J, Wang S. Research on the changes of IL-1 receptor and TNF-alpha receptor in rats with cerebral ischemia reperfusion and the chronergy of acupuncture intervention. Zhongguo Zhen Jiu. 2012;32:1012–1018. In Chinese. [PubMed] [Google Scholar]
- 26.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 27.Ambros V. MicroRNA pathways in flies and worms: Growth, death, fat, stress and timing. Cell. 2003;113:673–676. doi: 10.1016/S0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 28.Quinn SR, O'Neill LA. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 29.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 30.El Gazzar M, McCall CE. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J Biol Chem. 2010;285:20940–20951. doi: 10.1074/jbc.M110.115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 32.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thulin P, Wei T, Werngren O, Cheung L, Fisher RM, Grandér D, Corcoran M, Ehrenborg E. MicroRNA-9 regulates the expression of peroxisome proliferator-activated receptor δ in human monocytes during the inflammatory response. Int J Mol Med. 2013;31:1003–1010. doi: 10.3892/ijmm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaloy C, Gao FB. MicroRNA-9 multitasking near organizing centers. Nat Neurosci. 2008;11:625–626. doi: 10.1038/nn0608-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coolen M, Bally-Cuif L. MicroRNAs in brain development and physiology. Curr Opin Neurobiol. 2009;19:461–470. doi: 10.1016/j.conb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Wan HY, Guo LM, Liu T, Liu M, Li X, Tang H. Regulation of the transcription factor NF-kappaB1 by microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 2010;9:16. doi: 10.1186/1476-4598-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]