Abstract
The present study aimed to investigate whether the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway is involved in the transforming growth factor β2 (TGF-β2)-induced epithelial-mesenchymal transition (EMT) in human lens epithelial (HLE) cells. HLEB-3 cells were cultured and stimulated with 10 ng/ml TGF-β2 for 24 h. Western blotting was then performed to analyze the expression levels of connexin 43 and fibronectin, and the activities of Akt and mTOR. Confocal cell immunofluorescence was used to observe the expression of phosphorylated (p)-Akt. The toxicity of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) was assessed using a Cell Counting Kit-8 assay, and inhibition investigations were performed using a PI3K inhibitor. The expression of connexin 43 was suppressed and the expression of fibronectin was increased when the cells were stimulated with 10 ng/ml TGF-β2 for 24 h. In addition, Akt and mTOR were activated during TGF-β2-induced EMT. Treatment of with LY294002 (20 µM) inhibited the activation of Akt and mTOR and effectively prevented TGF-β2-induced EMT in the HLECs. Therefore, the results of the present study indicated that TGF-β2 induces EMT by activating the PI3K/Akt/mTOR signaling pathway in cultured HLECs.
Keywords: human lens, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathway, epithelial cells, transforming growth factor β2, LY294002
Introduction
Posterior capsule opacification (PCO) is the most common postoperative complication of cataract surgery (1) and results from the proliferation and migration of postoperative remnants of lens epithelial cells (LECs) in the posterior lens capsule (2,3). It has been reported that the residual postoperative LECs undergo the epithelial-mesenchymal transition (EMT) process, enabling pronounced migration and leading to PCO (4,5). EMT is a transdifferentiation process, in which an epithelial cell changes to exhibit a fibroblastic phenotype (4). EMT is activated during various cellular processes, and is triggered by different signaling molecules, including transforming growth factor β (TGF-β), fibroblast growth factor and Notch (6). Among these, TGF-β is a major inducer of EMT (7). TGF-β2 is the predominant isoform of TGF-β in the aqueous humor of the eye, the expression of which is elevated following cataract surgery (8,9).
In our previous study, it was found that mammalian target of rapamycin (mTOR) was activated during TGF-β2-induced EMT in human lens epithelial cells (HLECs), and inhibitors of mTOR impaired the EMT and reduced cell motility (10). These results suggest that mTOR is involved in the regulation of TGF-β2-induced EMT. The large, serine/threonine protein kinase mTOR is activated during various cellular processes, including EMT. The mTOR signaling pathway forms a complex signaling network, which integrates intracellular and extracellular signals (10,11). The upstream regulators of the mTOR signaling pathway include phosphatidylinositol 3-kinase (PI3K)/protein kinase B(Akt), Ras and AMP-activated protein kinase (11). One of the most important sensors involved in the regulation of mTOR activity is PI3K, which enhances the phosphorylation of Akt and subsequently activates mTOR (12). Therefore, the present study hypothesized that TGF-β2 induces the EMT of HLECs through the PI3K/Akt/mTOR signaling pathway.
In the present study, whether the PI3K/Akt/mTOR signaling pathway is involved in TGF-β2-induced EMT in HLECs was investigated. Activation of the PI3K/Akt/mTOR signaling pathway was investigated during the EMT process, and the PI3K inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002), was used to determine its effect on TGF-β2-induced EMT in HLECs.
Materials and methods
Cell culture and treatment
Immortalized HLEB-3 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), placed in a humidified atmosphere containing 5% CO2 at 37°C. The cells were washed with phosphate-buffered saline (PBS), and dissociated with 0.05% trypsin and 0.02% EDTA. As in our previous experiments (7), when the cell cultures reached a confluence of 80%, the cells were divided into three groups (1×106 cells/ml each). In the first group, the cells were stimulated with 10 ng/ml recombinant human TGF-β2 (Peprotech, Inc., Rocky Hill, NJ, USA) for 24 h in serum-free medium to induce EMT. This was termed the TGF-β2 group. To determine the effect of PI3K inhibition on EMT, the cells were pretreated with LY294002 (Cell Signaling Technology, Inc., Danvers, MA, USA) at an appropriate concentration for 1 h prior to being co-treated with 10 ng/ml TGF-β2 for 24 h. This was termed the LY294002+TGF-β2 group. The control group consisted of cells, which were incubated under conventional conditions without the presence of either TGF-β2 or LY294002 in the medium. Following treatment, the cells were collected for western blot analysis and confocal immunofluorescence assays.
Analysis of cytotoxicity
A Cell Counting Kit 8 (CCK8) assay was used to assess the cytotoxicity of LY294002 in the HLEB-3 cells. According to the manufacturer's protocol, the cells were cultured in a 100 µl DMEM in 96-well plates at a density of 1×104 cells/well for 24 h, and were subsequently treated with LY294002 at concentrations ranging between 0 and 80 µM (0, 10, 20, 30, 40, 50, 60, 70 and 80 µM) for another 24 h. Finally, 10 µl CCK8 solution (Sigma-Aldrich, St. Louis, MO, USA) was added to each well for 2 h. A soluble orange formazan product was produced, which was then quantified by light absorbance at 450 nm using a Synergy™ HT Multi-Mode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The quantity of dye generated by the activity of mitochondrial dehydrogenases in the cells is directly proportional to the number of living cells. This assay was performed in triplicate. In addition, the morphological changes of the HLEB-3 cells were observed and images were captured under an inverted microscope (ECLIPSE TE2000-S; Nikon, Tokyo, Japan).
Western blotting
The cells were lysed in ice-cold radioimmunoprecipitation buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1% Na3VO4, 0.5 µg/ml leupeptin, 1 mM phenylmethanesulfonylfluoride (Beyotime Institute of Biotechnology, Shanghai, China) with phosphatase inhibitors (10 ml Phosstop phosphatase inhibitor cocktail tablet; Roche Diagnostics, Basel, Switzerland) at 4°C for 30 min. The cell lysates were then centrifuged at 14,000 × g for 10 min to remove the insoluble material. The proteins were quantified using a Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Following being heated at 95°C for 5 min, 20 µg of each protein sample was separated by 8% SDS-PAGE (Beyotime Institute of Biotechnology), followed by transfer onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% non-fat milk for 3 h at room temperature and were washed with Tris-buffered saline with 0.1% Tween 20 (TBST) for three times for 5 mins. The membranes were subsequently incubated with the following primary antibodies overnight at 4°C: Rabbit anti-human monoclonal Akt (1:1,000; cat. no. 4691; Cell Signaling Technology, Inc.), rabbit anti-human monoclonal phosphorylated (p)-Akt (Ser473; 1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.), rabbit anti-human polyclonal mTOR (1:1,000; cat. no. 2972; Cell Signaling Technology, Inc.), rabbit anti-human polyclonal p-mTOR (Ser2448; 1:1,000; cat. no. 2971; Cell Signaling Technology, Inc.), rabbit anti-human polyclonal connexin 43 (1:8,000; cat. no. Ab11370; Abcam, Cambridge, UK), rabbit anti-human polyclonal fibronectin (1:1,000; cat. no. Ab23750; Abcam) and mouse anti-human monoclonal GAPDH (1:5,000; cat. no. M20006; Abmart, Shanghai, China). The membranes were then incubated for 1 h in goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. BA1054; Boster Systems, Inc., Pleasanton, CA, USA) or goat anti-mouse IgG-HRP (cat. no. BA1050; Boster Systems, Inc.) diluted at 1:5,000 in TBST. The membranes were washed three times with TBST for 5 min and the immunoreactive protein bands were detected using enhanced chemiluminescence (ECL) detection reagent (Applygen Technologies, Inc., Beijing, China) and BioMax film (Kodak, Rochester, NY, USA). The film was scanned and analyzed using GEL-PRO Analyzer software 4.0 (Media Cybernetics, Inc. Rockville, MD, USA).
Confocal cell immunofluorescence
The cells were placed on slides and fixed with 4% paraformaldehyde for 5 min, followed by washing with PBS. To inhibit the activity of endogenous peroxidase, the slides were incubated in 3% hydrogen peroxide (Guangzhou Chemical Reagent Factory, Guangzhou, China) at 37°C for 30 min, and were subsequently blocked for another 30 min at 37°C with 5% bovine serum albumin (Roche Diagnostics). Subsequently, the slides were immunostained overnight with p-Akt (Ser473; 1:200; Cell Signaling Technology, Inc.) at 4°C. Following washing with PBS, Alexa Fluor 555 goat anti-rabbit IgG (1:500; Invitrogen; Thermo Fisher Scientific, Inc.) was added, and the slides were incubated in the dark at 37°C for 30 min. Finally, the slides were embedded in glycerine (Beyotime Institute of Biotechnology) and observed under a confocal laser scanning microscope (Leica SP5-FCS; Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Values are presented as the mean ± standard deviation from at least three independently performed experiments, to account for possible variation in the cell cultures. For statistical evaluation, one way analysis of variance was used. Statistical analyses were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
TGF-β2 induces EMT in HLECs via the PI3K/Akt/mTOR signaling pathway
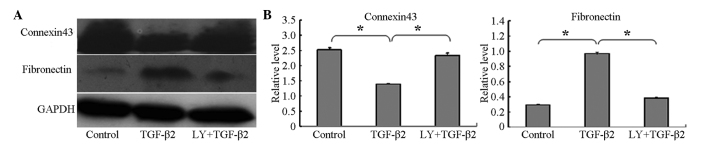
To confirm whether TGF-β2 induces EMT in HLECs, the present study investigated the expression levels of the epithelial marker, connexin 43, and the mesenchymal marker, fibronectin, using western blotting. The connexin 43 protein was expressed at high levels in the HLEB-3 cells in the absence of TGF-β2, and its level was significantly decreased following treatment of the cells with 10 ng/ml TGF-β2 for 24 h. By contrast, compared with the control group, the expression of fibronectin was significantly increased in the HLEB-3 cells following stimulation by TGF-β2, as shown in Fig. 1A and B.
Figure 1.
LY294002 inhibits TGF-β2-induced EMT. (A) Western blot analyses showed that the expression of connexin 43 decreased, whereas the expression of fibronectin increased following stimulation of the cells by TGF-β2. LY294002 reversed this expression pattern. Control group, cells incubated without TGF-β2 for 24 h; TGF-β2 group, cells incubated with 10 ng/ml TGF-β2 for 24 h; LY+TGF-β2 group, cells pretreated with 20 µM LY294002 for 1 h, prior to incubation with TGF-β2 for 24 h. Representative blots of three independent experiments are shown. (B) Data in the densitometric analyses of western blotting are presented as the mean ± standard deviation of three independent experiments (*P<0.05). LY294002/LY, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; TFG-β2, transforming growth factor β2; EMT, epithelial-mesenchymal transition.
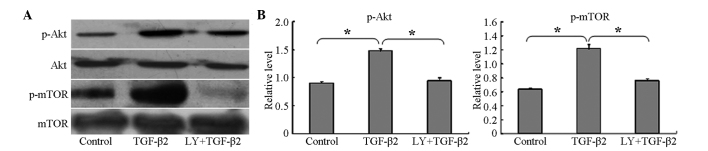
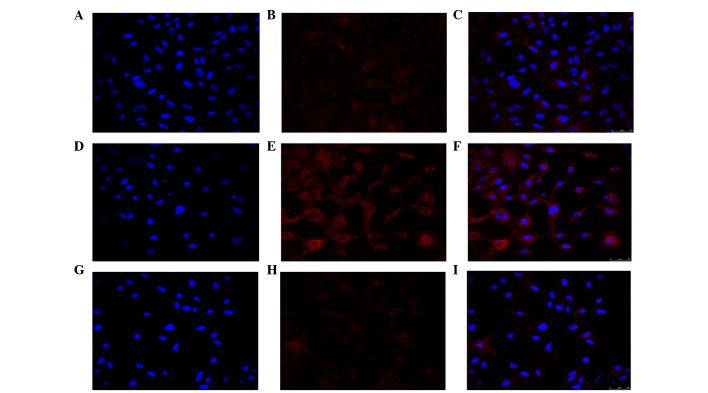
To determine whether the PI3K/Akt/mTOR signaling pathway was activated during the TGF-β2-induced EMT, the HLEB-3 cells were cultured with 10 ng/ml TGF-β2 for 24 h, and the phosphorylation levels of Akt and mTOR were then examined using western blotting. The results showed that the phosphorylation levels of Akt and mTOR were significantly increased, compared with the untreated control cells (Fig. 2A and B). In addition, the confocal cell immunofluorescence revealed that, the expression of p-Akt was higher following treatment with TGF-β2, compared with the untreated cells (Fig. 3). The expression of p-Akt was shown as red fluorescence, which was observed at low levels in the control cells (Fig. 3A–C). Following induction by TGF-β2, the levels of fluorescence were markedly higher (Fig. 3D–F). The confocal cell immunofluorescence also demonstrated that the expression of p-Akt was lower following co-treatment with LY294002 and TGF-β2 (Fig. 3G–I).
Figure 2.
LY294002 inhibits the phosphorylation of mTOR and Akt during TGF-β2-induced EMT. (A) Western blot analyses revealed that TGF-β2 increased the phosphorylation of Akt and mTOR, but did not alter the total expression levels of Akt or mTOR. LY294002 inhibited the phosphorylation of Akt and mTOR. Control group, cells were incubated without TGF-β2 for 24 h; TGF-β2, group, cells incubated with 10 ng/ml TGF-β2 for 24 h; LY+TGF-β2 group, cells pretreated with 20 µM LY294002 for 1 h, prior to incubation with TGF-β2 for 24 h. Representative blots of three independent experiments are shown. (B) Data in the densitometric analyses of western blotting are presented as the mean ± standard deviation of three independent experiments (*P<0.05). LY294002/LY, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Akt, protein kinase B; mTOR, mammalian target of rapamycin; TFG-β2, transforming growth factor β2; p-, phosphorylated.
Figure 3.
LY294002 inhibits the activation of Akt during TGF-β2-induced EMT. Confocal cell immunofluorescence revealed that, compared with the (A–C) control, treatment with (D–F) TGF-β2 increased the expression of p-Akt. (G–I) LY294002 decreased the expression of p-Akt, compared with the control. Control group, cells incubated without TGF-β2 for 24 h; TGF-β2 group, cells incubated with 10 ng/ml TGF-β2 for 24 h; LY+TGF-β2 group, cells pretreated with 20 µM LY294002 for 1 h, prior to incubation with TGF-β2 for 24 h. The expression of p-Akt is shown as red fluorescence. Nuclei stained with diamidinophenylindole appear blue. Images C, F, and I show the merged images of A and B, D and E, and G and H, respectively. Representative blots of three independent experiments is shown (magnification, ×400). LY294002/LY, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Akt, protein kinase B; mTOR, mammalian target of rapamycin; TFG-β2, transforming growth factor β2; p-, phosphorylated.
Cytotoxicity of LY294002 in HLEB-3 cells
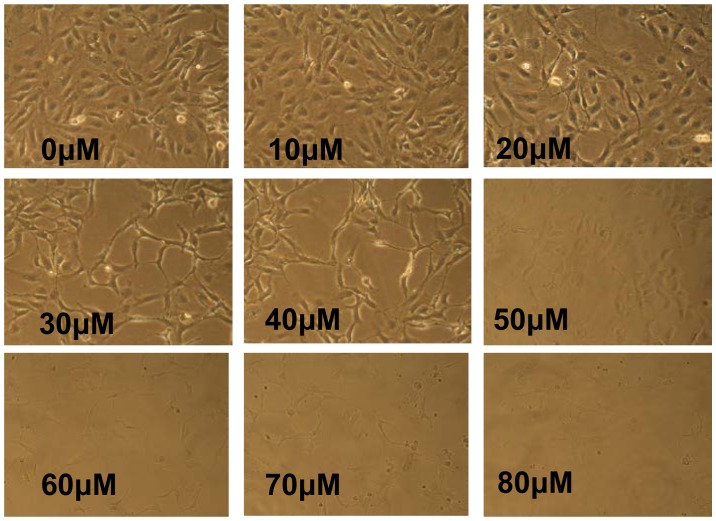
To determine an appropriate concentration of LY294002 for use in the present study, a CCK8 assay was used for the analysis of cytotoxicity. The results showed that LY294002 decreased cell viability in a concentration-dependent manner (Fig. 4). When the cells were treated with LY294002 at concentrations ≥30 µM, the cell viability was ≤ 80%. In addition, the HLEB-3 cells exhibited a change in morphology; with stretched, scattered and fragmented shapes, decreased cell density and increased cell gaps, when the concentration of LY294002 was ≥20 µM (Fig. 5). Therefore, 20 µM LY294002 was selected for use in the subsequent experiments.
Figure 4.
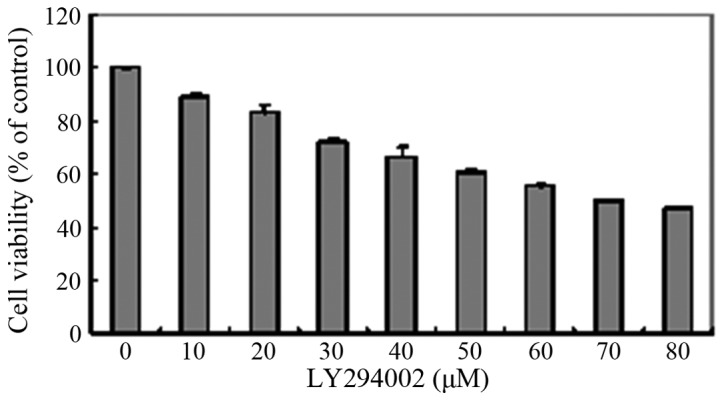
Cytotoxicity of LY294002 in HLEB-3 cells. The HLEB-3 cells were seeded at a density of 1×104 cells/well in 96-well plates and were then treated with LY294002 for 24 h at concentrations ranging between 0 and 80 µM. Cell viability was then determined using a Cell Counting Kit 8 assay. Data are expressed as the mean ± standard deviation, of measurements obtained from three independent experiments. LY29400, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one.
Figure 5.
Morphological observation of the HLECs following treatment with different concentrations of LY294002. When the cells were treated with LY294002 at concentrations ≥30 µM, the HLEB-3 cells exhibited a change in morphology, with stretched, scattered and fragmented shapes, reduced cell density and increased cell gaps.
LY294002 inhibits TGF-β2-induced EMT via the PI3K/Akt/mTOR signaling pathway
To examine the inhibitory effect of LY294002 on the PI3K/Akt/mTOR signaling pathway, the cells were pretreated with 20 µM LY294002 for 1 h prior to co-treatment with 10 ng/ml TGF-β2 for 24 h. Western blotting demonstrated that the levels of p-Akt and p-mTOR were significantly decreased, compared with the cells cultured with TGF-β2 only for 24 h (Fig. 2) and, as noted above, the confocal cell immunofluorescence confirmed that the expression of p-Akt was lower following co-treatment with LY294002 and TGF-β2 (Fig. 3G–I).
To further investigate whether LY294002 inhibited the EMT induced by TGF-β2, the expression levels of the associated protein markers, connexin 43 and fibronectin, were determined following treatment of the HLEB-3 cells with 20 µM LY294002 for 1 h and subsequent co-treatment with 10 ng/ml TGF-β2 for 24 h. The results showed that the protein level of connexin 43 increased, whereas the expression level of fibronectin decreased in the cells pretreated with LY294002, compared with the levels observed in the cells cultured with 10 ng/ml TGF-β2 only for 24 h (Fig. 1).
Discussion
Our previous study demonstrated that TGF-β2 can induce EMT in HLECs, and mTOR is activated during TGF-β2-induced EMT (10). In the present study, the role of the PI3K/Akt/mTOR signaling pathway during EMT was further investigated. The results revealed that the PI3K/Akt/mTOR signaling pathway was activated in the EMT induced by TGF-β2. Treatment with LY294002, an inhibitor of PI3K, effectively suppressed the activation of Akt and mTOR, and inhibited the development of EMT. The results suggested that TGF-β2 induced EMT through activation of the PI3K/Akt/mTOR signaling pathway in the cultured HLECs.
In the present study, the effect of TGF-β2 on EMT, and the activation of mTOR during TGF-β2-induced EMT in HLECs was described. The mTOR kinase integrates four major signals, including growth factors, energy status, oxygen and amino acids, to regulate several processes, and acts as a central regulator of cell metabolism, growth, proliferation and survival (11). In general, growth factors stimulate mTOR through the activation of Ras and PI3K/Akt (11). PI3K is an intracellular signaling molecul and, when activated, it generates 3′-phosphoinositides, which act as secondary messengers in the regulation of cell growth, proliferation and migration (13,14). As a downstream kinase of PI3K, Akt is phosphorylated at ser473 and is recruited to the membrane (15). A previous study demonstrated that platelet-derived growth factor can induce lenticular EMT through the Akt pathway (16). Another study reported that the inhibition of p-Akt reduced or prevented the formation of PCO in an ex vivo canine lens capsule model (17). In the present study, confocal cell immunofluorescence showed higher expression levels of p-Akt following treatment of the cells with TGF-β2. Consistent with this result, western blotting revealed a marked increase in the phosphorylation of the Akt protein. These results suggested that Akt and mTOR were activated during the TGF-β2-induced EMT process in the HLECs.
To further delineate whether the PI3K/Akt/mTOR signaling pathway was involved in TGF-β2 induced EMT in the HLECs, the present study used the PI3K inhibitor, LY294002. PI3K is a dimeric enzyme consisting of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit (18). LY294002 acts on the P110 subunit to specifically eliminate PI3K activity and subsequently inhibit the PI3K signaling pathway (19). Thus, LY294002 can potentially be used to provide a better understanding of the function and regulatory mechanisms of the enzyme and pathway mediated by PI3K. In the present study, the results showed that the phosphorylation levels of Akt and mTOR were significantly decreased following treatment of the cells with LY294002, which suggested that LY294002 effectively inhibited the activation of TGF-β2-induced PI3K/Akt/mTOR signaling. A previous study reported similar effects when growth factor-induced cells were treated with LY294002 in cultured rat hippocampal neurons (20). In addition, the present study found that the expression connexin 43 was upregulated, and that of fibronectin was downregulated in the cells pretreated with LY294002, indicating that the EMT induced by TGF-β2 was prevented when LY294002 inhibited the activation of the PI3K/Akt/mTOR pathway. Previous studies have demonstrated that PI3K/Akt signaling is required for TGF-β-induced EMT in mouse LECs and HLECs (21,22). Our previous study demonstrated that mTOR is involved in the regulation of TGF-β2-induced EMT. According to these results, it was hypothesized that TGF-β2 induces EMT by activating the PI3K/Akt/mTOR signaling pathway in HLECs.
In conclusion, the present study demonstrated that Akt and mTOR were activated during TGF-β2-induced EMT. In HLECs, treatment with 20 µM LY294002 inhibited the activation of Akt and mTOR, thus preventing the induction of EMT by TGF-β2. These results suggested that the PI3K/Akt/mTOR signaling pathway is involved in the regulation of TGF-β2-induced EMT in HLECs and may contribute to PCO. At present, pharmacological PCO prophylaxis has not been achieved (23). Although several approaches have been developed to prevent PCO using chemicals, none of these apporaches have been applied in a clinical setting (24–26). The results of the present study demonstrated that LY294002 and other PI3K/Akt/mTOR signaling pathway inhibitors, including rapamycin and Ku-0063794, may be potential agents for the prevention and treatment of PCO. However, further investigations are required to investigate whether other signaling pathways, including the Ras/mTOR pathway, also regulate EMT in HLECs.
Acknowledgments
This study was supported by funding provided by the Guangzhou Pearl River Nova of Science and Technology (grant. no. 2011J2200050), the Guangdong Natural Science Foundation (grant. no. S2011010000462) and the Guangzhou Science and Technology Commission (grant. no. Z032012245).
References
- 1.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: A problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–562. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 2.Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, Tsai JC, Castaneda VE, Hoggatt JP, Kostick AM. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell PJ, Krause W, Glaser BM. In vitro inhibition of lens epithelial cell proliferation and migration. Ophthalmic Surg. 1988;19:25–30. [PubMed] [Google Scholar]
- 4.de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- 5.Saika S, Yamanaka O, Flanders KC, Okada Y, Miyamoto T, Sumioka T, Shirai K, Kitano A, Miyazaki K, Tanaka S, Ikeda K. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr Metab Immune Disord Drug Targets. 2008;8:69–76. doi: 10.2174/187153008783928343. [DOI] [PubMed] [Google Scholar]
- 6.Barrallo-Gimeno A, Nieto MA. The snail genes as inducers of cell movement and survival: Implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawes LJ, Elliott RM, Reddan JR, Wormstone YM, Wormstone IM. Oligonucleotide microarray analysis of human lens epithelial cells: TGFbeta regulated gene expression. Mol Vis. 2007;13:1181–1197. [PubMed] [Google Scholar]
- 9.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 10.Meng Q, Guo H, Xiao L, Cui Y, Guo R, Xiao D, Huang Y. mTOR regulates TGF-β2 -induced epithelial-mesenchymal transition in cultured human lens epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2013;251:2363–2370. doi: 10.1007/s00417-013-2435-z. [DOI] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 13.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 14.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/S0955-0674(98)80149-X. [DOI] [PubMed] [Google Scholar]
- 15.Su CH, Wang CY, Lan KH, Li CP, Chao Y, Lin HC, Lee SD, Lee WP. Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase. Cell Signal. 2011;23:1824–1830. doi: 10.1016/j.cellsig.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Xiong W, Cheng BH, Jia SB, Tang LS. Involvement of the PI3K/Akt signaling pathway in platelet-derived growth factor-induced migration of human lens epithelial cells. Curr Eye Res. 2010;35:389–401. doi: 10.3109/02713680903584686. [DOI] [PubMed] [Google Scholar]
- 17.Chandler HL, Webb TR, Barden CA, Thangavelu M, Kulp SK, Chen CS, Colitz CM. The effect of phosphorylated Akt inhibition on posterior capsule opacification in an ex vivo canine model. Mol Vis. 2010;16:2202–2214. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl- 4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 20.Yang XP, Liu TY, Qin XY, Yu LC. Potential protection of 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside against staurosporine-induced toxicity on cultured rat hippocampus neurons. Neurosci Lett. 2014;576:79–83. doi: 10.1016/j.neulet.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Cho HJ, Baek KE, Saika S, Jeong MJ, Yoo J. Snail is required for transforming growth factor-beta-induced epithelial-mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem Biophys Res Commun. 2007;353:337–343. doi: 10.1016/j.bbrc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Yao K, Ye PP, Tan J, Tang XJ, Shen Tu XC. Involvement of PI3K/Akt pathway in TGF-beta2-mediated epithelial mesenchylmal transition in human lens epithelial cells. Ophthalmic Res. 2008;40:69–76. doi: 10.1159/000113884. [DOI] [PubMed] [Google Scholar]
- 23.Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;(2):CD003738. doi: 10.1002/14651858.CD003738.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas NR, Mongre PK, Das GK, Sen S, Angra SK, Vajpayee RB. Animal study on the effects of catalin on after cataract and posterior capsule opacification. Ophthalmic Res. 1999;31:140–142. doi: 10.1159/000055526. [DOI] [PubMed] [Google Scholar]
- 25.Chandler HL, Barden CA, Lu P, Kusewitt DF, Colitz CM. Prevention of posterior capsular opacification through cyclooxy-genase-2 inhibition. Mol Vis. 2007;13:677–691. [PMC free article] [PubMed] [Google Scholar]
- 26.Rabsilber TM, Auffarth GU. Pharmacological means to prevent secondary cataract. Klin Monbl Augenheilkd. 2006;223:559–567. doi: 10.1055/s-2006-926859. In German. [DOI] [PubMed] [Google Scholar]