Abstract
Previous studies have reported that long, non-coding RNAs (lncRNAs) are important in cardiovascular disease. However, the lncRNAs involved in myocardial infarction and their detailed mechanism have not been well characterized. In the present study, an affymetrix microarray associated with myocardial infarction was re-annotated, following which a myocardial infarction-related differential lncRNA-mRNA co-expression network (MILMN) was constructed. Subsequently, pathway enrichment analysis was used for all the mRNAs in the MILMN, and an lncRNA-pathway network was constructed. It was found that the mRNAs were predominantly involved in certain cardiovascular disease-associated pathway, for example the dilated cardiomyopathy and mitogen-activated protein kinase signaling pathway. Finally, a total of 39 key lncRNAs were identified, which regulate crucial pathways in myocardial infarction. Through pathway analysis of these 39 key lncRNAs, the novel function of an annotated lncRNAs-H19 was predicted, which may regulate apoptosis signal-regulating kinase, which is a protein that promotes pathological cardiac remodeling following myocardial infarction. The results of the present study not only provide potential non-coding RNA biomarkers, but also provide further insights into understanding the molecular mechanism of lncRNAs.
Keywords: myocardial infarction, lncRNA, network analysis, pathway, co-expression network
Introduction
Myocardial infarction, which is predominantly caused by coronary heart disease, and by coronary thrombosis in particularly, is a major contributor to mortality rates globally (1). With developments in research into myocardial infarction, the biological-psychological-social medicine nexus has provided a more detailed understanding of the risks of myocardial infarction. Previous studies have reported that long non-coding RNAs (lncRNAs) are involved in cardiac development (2,3). The lncRNAs comprise a group of non-coding RNAs, in addition to microRNA, PIWI-interacting RNAand endogenous small interfering RNA, and are defined as transcripts >200 nt in length with no known protein-coding function (4). They have a large range of functions, including in cell proliferation, apoptosis and cell invasion (5,6). It has been shown that lncRNAs are associated with cardiac hypertrophy (7), cardiovascular ageing (8) and cardiac tissues following myocardial infarction (9). Although there is no direct evidence between lncRNAs and myocardial infarction, certain lncRNAs are associated with the risks of myocardial infarction. For example, GAS5 functions as a starvation- or growth arrest-linked riborepressor (10), and this condition is similar to myocardial infarction. In addition, lncRNA-p21 and lncRNA PANDA are induced by DNA damage in a p53-dependent manner (11,12) which also occurs in the cardiomyocyte death that is associated with myocardial infarction.
MicroRNAs have been well demonstrated in the development of cardiovascular diseases (13), however, there are few reports on lncRNAs in myocardial infarction (14). RNA sequencing (RNA-seq) is a prevalent technique used to profile lncRNAs, however, the publicly available RNA-seq data are limited due to relatively high costs of the RNA-seq technique. In addition, RNA-seq data are lacking in sample numbers, compared with microarray expression profile data, which often contained dozens to hundreds of pair-matched samples (15). Therefore, the present study adopted a re-annotation method to identify lncRNAs associated with myocardial infarction. Furthermore, increasing evidence shows that lncRNAs may be important in regulating gene expression, and that the functions of lncRNAs are performed predominantly by their secondary structures, which is difficult to decipher (15). Due to the considerable challenges in investigating the functions of lncRNA, the present study used a co-expression-based method, in which lncRNA functions were predicted, based on the functions of their co-expressed protein-coding genes (15).
Therefore, the present study aimed to identify the lncRNAs involved in myocardial infarction. lncRNA functions can be predicted based on the functions of their co-expressed protein coding genes, and alterations in the associations between these genes between the different samples (normal or myocardial infarction) can be used to identify key lncRNAs in myocardial infarction. By re-annotating an affymetrix microarray associated with myocardial infarction, a myocardial infarction-related differential lncRNA-mRNA co-expression network (MILMN) was constructed in the current study, then pathway enrichment analysis was conducted. The present study aimed to identify potential non-coding RNA biomarkers, in addition to providing further insight into the understanding of the molecular mechanism of lncRNAs.
Materials and methods
Microarray data
The microarray data set, GSE48060, was downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48060). This dataset applied the methodology on the blood samples from 21 healthy control individuals and 31 patients with myocardial infarction using an Affymetrix HG-U133 Plus 2.0 Microarray (16). The whole-genome microarray profiling was performed on blood samples from control individuals with normal cardiac function and from patients with first-time acute myocardial infarction, within 48 h following myocardial infarction (16).
Functional re-annotation of lncRNAs
To re-annotate the microarray data obtained, a non-coding RNA function annotation server (ncFANs) was used to re-annotate the probes on a HG-U133 Plus 2.0 array, following the steps on its website (17). A total of 2,495 lncRNAs were re-annotated, and each lncRNA and mRNA probe was converted into gene Ensembl Gene IDs (http://www.ensembl.org/index.html). If one gene matched more than one probe, the expression value of this mRNA or lncRNA was computed by determining the average expression value of all its corresponding probes.
Construction of the MILMN
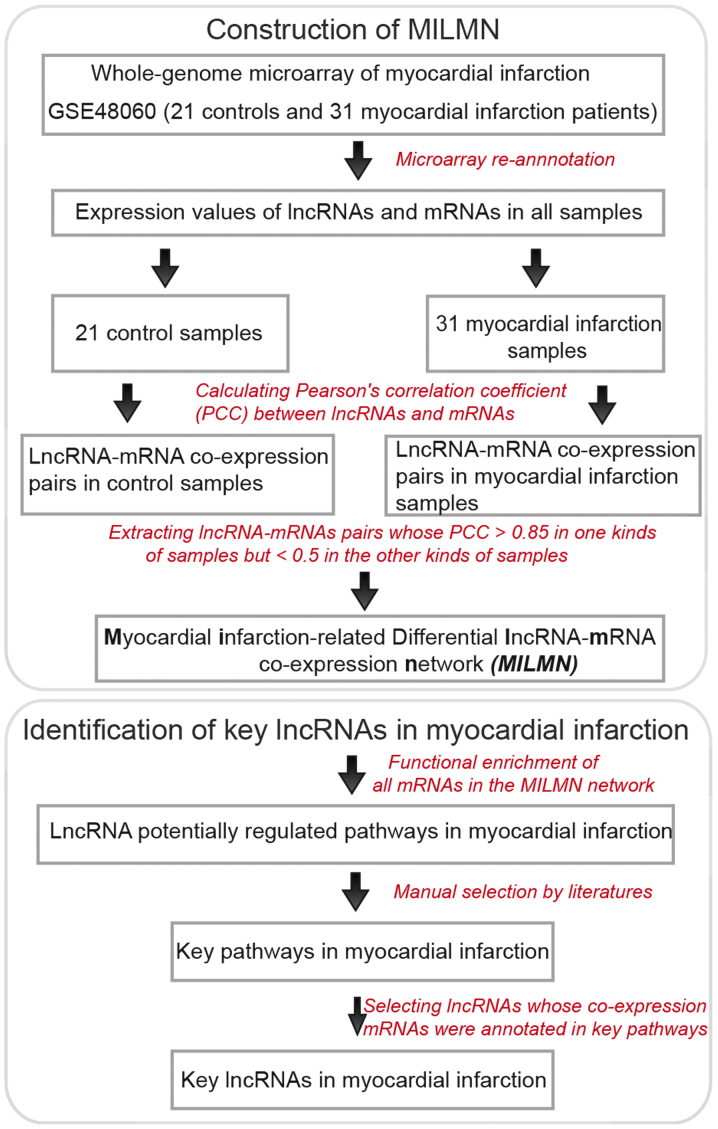
Following re-annotation of the microarray data, the expression values of the lncRNAs and mRNAs were obtained. Subsequently, Pearson's correlation coefficient (PCC) was calculated between the expression values of each of the lncRNA-mRNA pairs across the normal samples and the myocardial infarction samples, respectively (Fig. 1). The lncRNA-mRNA pairs with a PCC >0.85 in one sample group, but <0.5 in the other sample group were selected (Fig. 1), as these parameters indicated that the lncRNA-mRNA pairs were differentially co-expressed in the two sample groups. Finally, the MILMN was constructed, in which nodes were lncRNAs or mRNAs, and were connected if they were differentially co-expressed (Fig. 2). The top 20 mRNA nodes, which were those with the highest degree, were determined (Table I).
Figure 1.
Flow chart of the procedures used in the present study. lncRNA, long non-coding RNA.
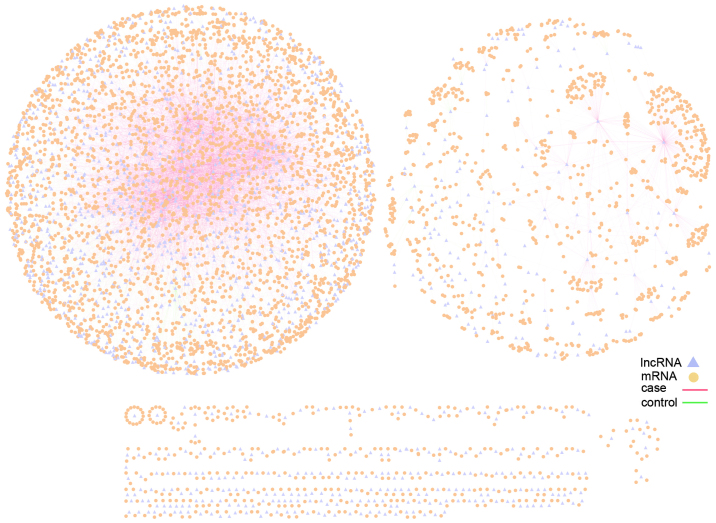
Figure 2.
Myocardial infarction-related differential lncRNA-mRNA co-expression network. The triangle nodes represent lncRNAs and the circular nodes represent mRNAs. Red edges represent an lncRNA-mRNA co-expression association in the case samples, whereas green edges represent an lncRNA-mRNA co-expression association in the control samples. lncRNA, long non-coding RNA.
Table I.
Top 20 mRNAs with the highest degree in the myocardial infarction-related differential long non-coding RNA-mRNA co-expression network.
| Symbol | Degree | Gene name |
|---|---|---|
| Cilp2 | 49 | Cartilage intermediate layer protein 2 |
| ANGPTL2 | 46 | Angiopoietin-like 2 |
| Ptgis | 45 | Prostaglandin I2 (prostacyclin) synthase |
| CORO6 | 40 | Coronin 6 |
| CLDN10 | 39 | Claudin 10 |
| piwil2 | 39 | Hydroxy-δ-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase 7; piwi-like 2 (Drosophila) |
| mfap4 | 37 | Microfibrillar-associated protein 4 |
| Hus1b | 35 | HUS1 checkpoint homolog b (S. pombe) |
| PCGF1 | 34 | Polycomb group ring finger 1 |
| Slc22a9 | 33 | Solute carrier family 22 (organic anion transporter), member 9 |
| TROAP | 32 | Trophinin associated protein (tastin) |
| zar1 | 30 | Zygote arrest 1 |
| CLDN19 | 30 | Claudin 19 |
| lmf2 | 30 | Lipase maturation factor 2 |
| ppyr1 | 28 | Pancreatic polypeptide receptor 1 |
| ephX1 | 28 | Epoxide hydrolase 1, microsomal (xenobiotic) |
| Fkbp1b | 28 | FK506 binding protein 1B, 12.6 kDa |
| SLC5A4 | 27 | Solute carrier family 5 (low affinity glucose cotransporter), member 4 |
| RNF207 | 25 | Ring finger protein 207 |
| HSPB8 | 25 | Heat shock 22 kDa protein 8 |
Identification of key lncRNAs in myocardial infarction
To identify the key lncRNAs in myocardial infarction, the present study initially implemented pathway enrichment of the mRNAs in the MILMN, using the Database for Annotation, Visualization and Integrated Discovery 6.7 (18). P<0.05 was considered to indicate a statistically significant difference. Subsequently an lncRNA-pathway network was constructed, in which nodes represented lncRNAs or pathways, and they were connected if the corresponding co-expressed mRNAs of an lncRNA were enriched in the corresponding pathway (Fig. 3), suggesting that these pathways were potentially regulated by the corresponding lncRNAs. From these pathways, crucial pathways in myocardial infarction were selected by a literature search using the following criteria: (i) cardiovascular disease pathway; (ii) important signaling pathway; (iii) cardiovascular muscle-related.
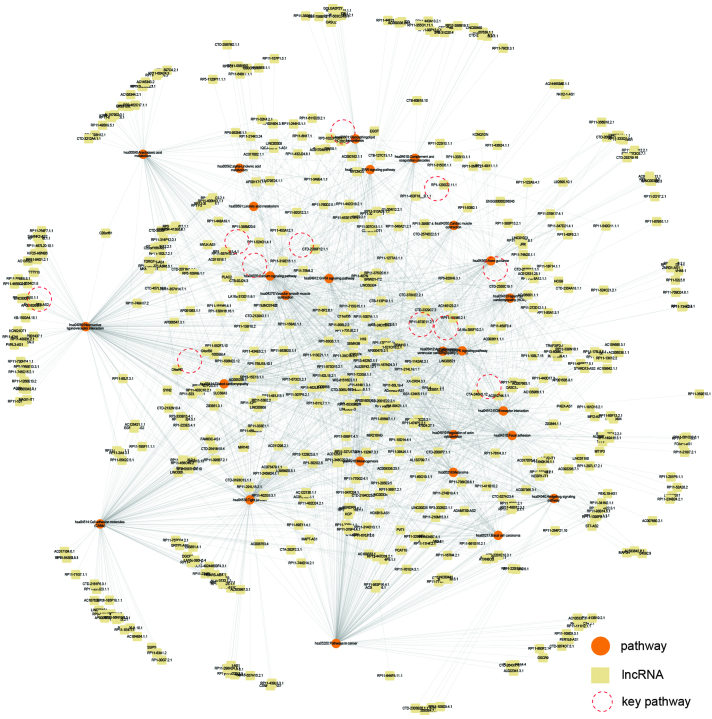
Figure 3.
lncRNA-pathway network. lncRNA, long non-coding RNA.
Finally, the lncRNAs which were linked with at least six of the 11 crucial pathways in the lncRNA-pathway network (Fig. 3) were considered to be key regulating lncRNAs in myocardial infarction. The key lncRNAs and corresponding crucial pathways are shown in Fig. 4A and in Tables II and III. To better illustrate the potentially regulated process, the co-expressing mRNAs were annotated into a dilated cardiomyopathy pathway and a mitogen-activated protein kinase (MAPK) signaling pathway (Figs 4B and 5). Pathway annotation was used performed using the search and color tools of the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.kegg.jp/kegg/tool/map_pathway2.html).
Figure 4.
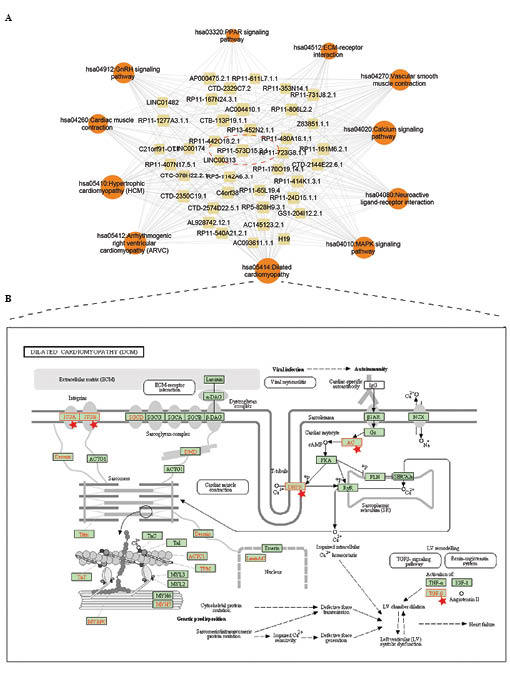
Regulatory mechanism of lncRNAs. (A) Sub-network of key lncRNAs and their regulatory pathways. (B) Dilated cardiomyopathy pathway, determined using the Kyoto Encyclopedia of Genes and Genomes database. Red stars indicate proteins, that are potentially regulated by lncRNAs. lncRNA, long non-coding RNA.
Table II.
Key pathways in myocardial infarction.
| Pathway | Regulatory lncRNAs (n) | PMID |
|---|---|---|
| hsa04010: MAPK signaling pathway | 38 | 25154304 |
| hsa04270: Vascular smooth muscle contraction | 35 | |
| hsa04260: Cardiac muscle contraction | 35 | |
| hsa03320: PPAR signaling pathway | 32 | 10073956 |
| hsa04512: ECM-receptor interaction | 30 | 19747544 |
| hsa05412: Arrhythmogenic right ventricular cardiomyopathy | 29 | |
| hsa04080: Neuroactive ligand-receptor interaction | 29 | 23916832 |
| hsa04020: Calcium signaling pathway | 28 | 24548334 |
| hsa05410: Hypertrophic cardiomyopathy | 25 | |
| hsa05414: Dilated cardiomyopathy | 19 | |
| hsa04912: GnRH signaling pathway | 12 | 19554077 |
lncRNA, long non-coding RNA; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferator-activated receptor; ECM, extracellular matrix; GnRH, gonadotropin-releasing hormone.
Table III.
Key lncRNAs in myocardial infarction.
| lncRNA | Regulatory pathways (n) | Function |
|---|---|---|
| AC004410.1 | 11 | Uncharacteristic |
| RP13-452N2.1.1 | 10 | Uncharacteristic |
| CTB-113P19.1.1 | 10 | Uncharacteristic |
| RP11-1277A3.1.1 | 9 | Uncharacteristic |
| RP11-731J8.2.1 | 9 | Uncharacteristic |
| RP5-828H9.3.1 | 9 | Uncharacteristic |
| CTD-2350C19.1 | 9 | Uncharacteristic |
| RP11-161M6.2.1 | 9 | Uncharacteristic |
| CTD-2144E22.6.1 | 9 | Uncharacteristic |
| RP11-806L2.2 | 9 | Uncharacteristic |
| RP11-573D15.2.1 | 9 | Uncharacteristic |
| RP11-414K1.3.1 | 9 | Uncharacteristic |
| AP000475.2.1 | 9 | Uncharacteristic |
| RP11-24D15.1.1 | 9 | Uncharacteristic |
| C4orf38 | 9 | Uncharacteristic |
| CTD-2329C7.2 | 9 | Uncharacteristic |
| AC145123.2.1 | 9 | Uncharacteristic |
| RP5-1142A6.3.1 | 8 | Uncharacteristic |
| RP11-540A21.2.1 | 8 | Uncharacteristic |
| H19 | 8 | Tumor suppressor |
| CTD-2574D22.5.1 | 8 | Uncharacteristic |
| RP11-407N17.5.1 | 8 | Uncharacteristic |
| CTC-378H22.2.1 | 8 | Uncharacteristic |
| RP11-723G8.1.1 | 8 | Uncharacteristic |
| RP11-167N24.3.1 | 8 | Uncharacteristic |
| AL928742.12.1 | 8 | Uncharacteristic |
| LINC00174 | 7 | Uncharacteristic |
| RP11-65L19.4 | 7 | Uncharacteristic |
| RP11-442O18.2.1 | 7 | Uncharacteristic |
| RP1-170O19.14.1 | 7 | Uncharacteristic |
| RP11-480A16.1.1 | 7 | Uncharacteristic |
| GS1-204I12.2.1 | 6 | Uncharacteristic |
| RP11-611L7.1.1 | 6 | Uncharacteristic |
| Z83851.1.1 | 6 | Uncharacteristic |
| RP11-353N14.1 | 6 | Uncharacteristic |
| LINC01482 | 6 | Uncharacteristic |
| AC093611.1.1 | 6 | Uncharacteristic |
| C21orf91-OT1 | 6 | Uncharacteristic |
| LINC00313 | 6 | Uncharacteristic |
lncRNA, long non-coding RNA.
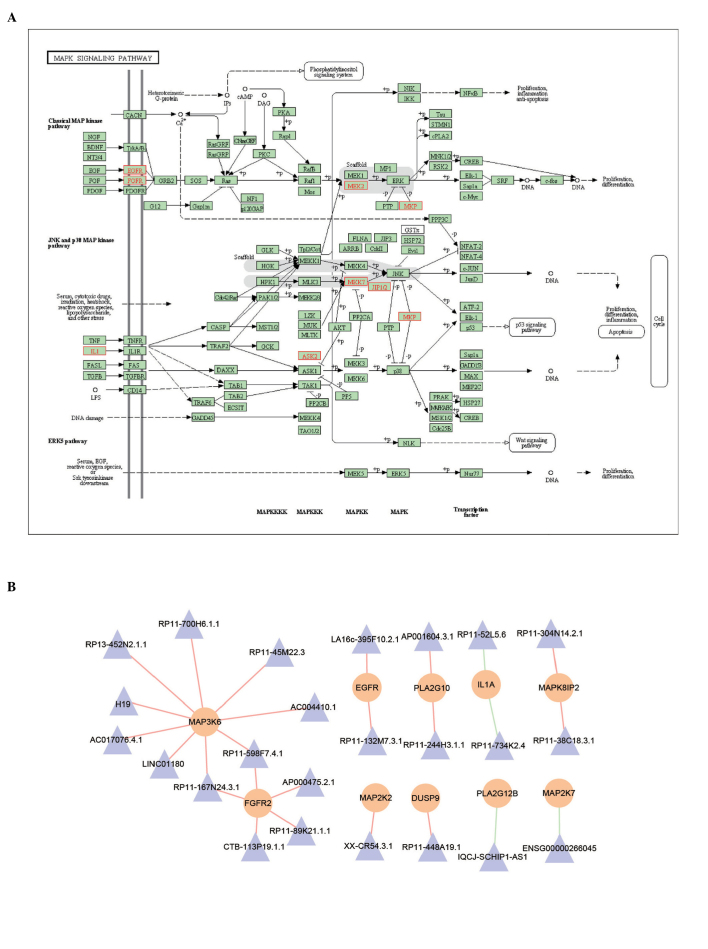
Figure 5.
(A) MAPK signaling pathway, determined using the Kyoto Encyclopedia of Genes and Genomes database. Red rectangles indicate proteins, which are potentially regulated by lncRNAs. (B) Sub-network to illustrate the potential lncRNA-mRNA co-expression associations in the MAPK signaling pathway. The triangle and circular nodes represent lncRNAs and mRNAs, respectively. lncRNA, long non-coding RNA; MAPK, mitogen-activated protein kinase.
Results
Construction of the MILMN
In the present study, the MILMN was constructed, based on the co-expression associations identified between the lncRNAs and mRNAs. This network contained a total of 1,476 lncRNAs, 4,444 mRNAs and 12,098 edges (Fig. 2). The top 20 mRNA nodes, which comprised those with the highest degree, are shown in Table I.
Detection of key lncRNAs regulating crucial pathways in myocardial infarction
To investigate the biological functions of lncRNAs during the development of myocardial infarction, the present study annotated the mRNAs in the MILMN into KEGG pathways. This resulted in a total of 26 pathways being detected (Fig. 3), which included certain cardiovascular disease pathways, including the Dilated cardiomyopathy and Hypertrophic cardiomyopathy pathways, and certain important signaling pathways, including the Calcium signaling pathway and MAPK signaling pathway. Subsequently an lncRNA-pathway network was constructed based on these pathways, in which edges indicated lncRNAs, which potentially regulated the corresponding pathways (Fig. 3). From these pathways, 11 crucial pathways were selected, following a literature review. These pathways were either cardiovascular disease pathways or were important signaling pathways in myocardial infarction (Table II). The key lncRNAs, which regulated at least six of the 11 crucial pathways were selected (Fig. 4A; Table III). A total of 39 key lncRNAs were identified (Table III), and three of the lncRNAs (AC004410.1, CTB-113P19.1.1 and RP13-452N2.1.1) were found to regulate the most crucial pathways (Table III).
Investigating the regulatory mechanism of lncRNAs in myocardial infarction
To examine the detailed regulatory mechanism of the key lncRNAs identified, the co-expressing mRNAs of the key lncRNAS were mapped into the Dilated cardiomyopathy pathway and MAPK signaling pathway (Figs. 4B and 5). In the Dilated cardiomyopathy pathway, certain key proteins were potentially regulated by lncRNAs, including dihydropteridine reductase (DHPR) and transforming growth factor (TGF)-β (Fig. 4B). In the MAPK signaling pathway, lncRNAs were also found, which potentially regulated certain key proteins, including MAPK kinase 2 (MEK2), MAPK kinase 7 (MKK7) and epidermal growth factor receptor (EGFR), as shown in Fig. 5A. Subsequently, the sub-network was extracted from the MILMN, within which were the mRNAs that were in the MAPK signaling pathway. mRNA-MAP3K6 was found to be differentially co-expressed with nine lncRNAs, including H19 (Fig. 5B).
Discussion
In previous years, the significant functional molecular mechanism of lncRNAs has been recognized, particularly in cardiovascular diseases. Furthermore, increasing evidence shows that lncRNAs may be important in regulating gene expression (15). Due to considerable challenges in examining the functions of lncRNA, a co-expression-based method was developed, in which lncRNA functions were predicted based on the functions of their co-expressed protein-coding genes (15), as genes that exhibit similar expression patterns under multiple conditions have a tendency to be involved in the same pathways (19). Therefore, these co-expressed protein-coding genes are potentially regulated by the corresponding lncRNAs (15,20).
Therefore, in the present study, an affymetrix microarray associated with myocardial infarction was re-annotated, following which an MILMN was constructed. This network contained a total of 1,476 lncRNAs and 4,444 mRNAs. In this network, a number of mRNAs were identified, which were linked with several lncRNAs, indicating that they are potentially regulated by these lncRNAs in myocardial infarction. The most connected mRNA was CLIP2, with a degree of 49 (Table I). Studies have shown that the minor T allele of the CLIP2 gene has a protective effect against elevated serum lipid and lipoprotein levels, thus being associated with the risk of cardiovascular diseases (21). Another highly-connected mRNA was PTGIS, which is a catalyst for the synthesis of PGI2 from prostaglandin H2, is widely distributed and is predominantly found in vascular endothelial cells and smooth muscle cells (22).
To examine the key lncRNAs and their potential functions in myocardial infarction, pathway enrichment of all the mRNAs in the MILMN was performed, from which an lncRNA-pathway network was constructed (Fig. 3). From a total of 26 pathways, 11 crucial pathways, which comprised cardiovascular disease pathways or important signaling pathways, were selected (Table II). For example, the Calcium signaling pathway (23), peroxisome proliferator-activated receptor signaling pathway (24) and MAPK signaling pathway (25) were found to be important in the development of myocardial infarction.
Subsequently, 39 key lncRNAs were identified that appeared to regulate the majority (6/11) of the crucial pathways (Table III). Of these lncRNAs, AC004410.1, CTB-113P19.1.1 and RP13-452N2.1.1 regulated almost all the crucial pathways (Table III). To examine the detailed regulatory mechanism of the key lncRNAs, the co-expressed mRNAs of the key lncRNAs were mapped into the Dilated cardiomyopathy pathway. A number of crucial membrane proteins were annotated (Fig. 4B). For example, DHPR, a dihydropyridine receptor, is essential in skeletal muscle excitation-contraction coupling, which leads to an increase in [Ca2+] via the activation of ryanodine receptors (26,27), and indicates that lncRNAs may be involved in inter/intra-cardiac cell communication. Of note, previous studies have suggested that several classes of RNA molecules are used for the horizontal transfer of information between different types of cells in the heart (28). Furthermore, the present study revealed that another important protein, TGF-β, which is considered a potential biomarker of myocardial infarction (29), was also regulated by the lncRNAs.
The present study subsequently focused on the MAPK signaling pathway, due to its vast implications in signaling and crosstalk with other signaling networks (Fig. 5A). For example, MAPK is involved in crosstalk with mitochondria, which are the powerhouses of the cell that provide >80% of the adenosine triphosphate required for normal cardiomyocyte function, and have a crucial role in cell death (30). The results of the present study showed that certain pivotal proteins in this pathway were regulated by lncRNAs. These proteins were found to be important in myocardial infarction, for example MKK cascades modulate the hypertrophic response of the heart to pressure overload (31,32) and, in the ras-Raf-MEK-ERK signaling cascade, overexpression of an activated form of MKK1 has been shown to lead to profound cardiac hypertrophy without fibrosis (33). In addition, these cascades also function as molecular switches in response to spatiotemporal-specific cell-cell communication in myocardial infarction (30,31). To better illustrate the potential mechanism of lncRNAs, the present study extracted the sub-network from the MILMN, which contained key lncRNAs and their regulating mRNAs in the MAPK signaling pathway. Of these lncRNAs, only H19 had functional annotation. Although it has been shown to be important in several types of cancer (34), its role in myocardial infarction remains to be fully elucidated. The present study found that H19 potentially regulated mRNA-MAP3K6, coding the ASK2 protein, which promotes pathological cardiac remodeling following myocardial infarction (35), indicating the potential roles of H19. Although the results of the present study require further experimental verification, the results provide further insight into understanding the roles of lncRNAs in myocardial infarction.
Acknowledgments
The present study was supported by grants from the National Science Foundation of China (grant no. 30873131) and the Jilin Province Development and Reform Commission (grant no. 3J113AX33426).
References
- 1.Wang Y, Zhang H, Chai F, Liu X, Berk M. The effects of escitalopram on myocardial apoptosis and the expression of Bax and Bcl-2 during myocardial ischemia/reperfusion in a model of rats with depression. BMC Psychiatry. 2014;14:349. doi: 10.1186/s12888-014-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129:1009–1021. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitra SA, Mitra AP, Triche TJ. A central role for long non-coding RNA in cancer. Front Genet. 2012;3:17. doi: 10.3389/fgene.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014;114:1377–1388. doi: 10.1161/CIRCRESAHA.114.302476. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SK, Piccoli MT, Thum T. Non-coding RNAs in cardiovascular ageing. Ageing Res Rev. 2014;17:79–85. doi: 10.1016/j.arr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M, Pezzuto I, Crippa S, Nemir M, Sarre A, Johnson R, et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J. 2015;36:353–368a. doi: 10.1093/eurheartj/ehu180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Choi E, Cha MJ, Park AJ, Yoon C, Hwang KC. Impact of miRNAs on cardiovascular aging. J Geriatr Cardiol. 2015;12:569–574. doi: 10.11909/j.issn.1671-5411.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calore M, De Windt LJ, Rampazzo A. Genetics meets epigenetics: Genetic variants that modulate noncoding RNA in cardiovascular diseases. J Mol Cell Cardiol. 2015 Nov 3; doi: 10.1016/j.yjmcc.2015.10.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Liao Q, Liu C, Yuan X, Kang S, Miao R, Xiao H, Zhao G, Luo H, Bu D, Zhao H, et al. Large-scale prediction of long non-coding RNA functions in a coding-non-coding gene co-expression network. Nucleic Acids Res. 2011;39:3864–3878. doi: 10.1093/nar/gkq1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suresh R, Li X, Chiriac A, Goel K, Terzic A, Perez-Terzic C, Nelson TJ. Transcriptome from circulating cells suggests dysregulated pathways associated with long-term recurrent events following first-time myocardial infarction. J Mol Cell Cardiol. 2014;74:13–21. doi: 10.1016/j.yjmcc.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Q, Xiao H, Bu D, Xie C, Miao R, Luo H, Zhao G, Yu K, Zhao H, Skogerbø G, et al. ncFANs: A web server for functional annotation of long non-coding RNAs. Nucleic Acids Res. 2011;39:W118–W124. doi: 10.1093/nar/gkr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Sacco L, Baldassarre A, Masotti A. Bioinformatics tools and novel challenges in long non-coding RNAs (lncRNAs) functional analysis. Int J Mol Sci. 2012;13:97–114. doi: 10.3390/ijms13010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luptáková L, Benčová D, Siváková D, Cvíčelová M. Association of CILP2 and ACE gene polymorphisms with cardiovascular risk factors in Slovak midlife women. Biomed Res Int. 2013;2013:634207. doi: 10.1155/2013/634207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama T. Genetic polymorphisms of prostacyclin synthase gene and cardiovascular disease. Int Angiol. 2010;29(Suppl 2):S33–S42. [PubMed] [Google Scholar]
- 23.Zhao Y, Hu HY, Sun DR, Feng R, Sun XF, Guo F, Hao LY. Dynamic alterations in the CaV1.2/CaM/CaMKII signaling pathway in the left ventricular myocardium of ischemic rat hearts. DNA Cell Biol. 2014;33:282–290. doi: 10.1089/dna.2013.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.ATV.19.3.546. [DOI] [PubMed] [Google Scholar]
- 25.Wei N, Zhang C, He H, Wang T, Liu Z, Liu G, Sun Z, Zhou Z, Bai C, Yuan D. Protective effect of saponins extract from Panax japonicus on myocardial infarction: Involvement of NF-kB, Sirt1 and mitogen-activated protein kinase signalling pathways and inhibition of inflammation. J Pharm Pharmacol. 2014;66:1641–1651. doi: 10.1111/jphp.12291. [DOI] [PubMed] [Google Scholar]
- 26.Eltit JM, Franzini-Armstrong C, Perez CF. Amino acid residues 489–503 of dihydropyridine receptor (DHPR) beta1a subunit are critical for structural communication between the skeletal muscle DHPR complex and Type-1 ryanodine receptor. J Biol Chem. 2014;289:36116–36124. doi: 10.1074/jbc.M114.615526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viola HM, Adams AM, Davies SM, Fletcher S, Filipovska A, Hool LC. Impaired functional communication between the L-type calcium channel and mitochondria contributes to metabolic inhibition in the mdx heart. Proc Natl Acad Sci USA. 2014;111:E2905–E2914. doi: 10.1073/pnas.1402544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intra-cardiac communication. Cardiovasc Res. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 29.Lax A, Sanchez-Mas J, Asensio-Lopez MC, Fernandez-Del Palacio MJ, Caballero L, Garrido IP, Pastor-Perez FJ, Januzzi JL, Pascual-Figal DA. Mineralocorticoid receptor antagonists modulate galectin-3 and interleukin-33/ST2 signaling in left ventricular systolic dysfunction after acute myocardial infarction. JACC Heart Fail. 2015;3:50–58. doi: 10.1016/j.jchf.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol Ther. 2014;144:202–225. doi: 10.1016/j.pharmthera.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muslin AJ. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foltz IN, Gerl RE, Wieler JS, Luckach M, Salmon RA, Schrader JW. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 33.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: A new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, et al. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]