Abstract
Background
Exteroception involves processes related to the perception of environmental stimuli important for an organism's ability to adapt to its environment. As such, exteroception plays a critical role in conditioned response. In addiction, behavioral and neuroimaging studies show that the conditioned response to drug-related cues is often associated with alterations in brain regions including the precuneus/posterior cingulate cortex, an important node within the default mode network dedicated to processes such as self-monitoring.
Objective
This review aimed to summarize the growing, but largely fragmented, literature that supports a central role of exteroceptive processes in addiction.
Methods
We performed a systematic review of empirical research via PubMed and Google Scholar with keywords including ‘addiction’, ‘exteroception’, ‘precuneus’, and ‘self-awareness’, to identify human behavioral and neuroimaging studies that report mechanisms of self-awareness in healthy populations, and altered selfawareness processes, specifically exteroception, in addicted populations.
Results
Results demonstrate that exteroceptive processes play a critical role in conditioned cue response in addiction and serve as targets for interventions such as mindfulness training. Further, a hub of the default mode network, namely, the precuneus, is (i) consistently implicated in exteroceptive processes, and (ii) widely demonstrated to have increased activation and connectivity in addicted populations.
Conclusion
Heightened exteroceptive processes may underlie cue-elicited craving, which in turn may lead to the maintenance and worsening of substance use disorders. An exteroception model of addiction provides a testable framework from which novel targets for interventions can be identified.
Keywords: Addiction, default mode network, exteroception, posterior cingulated cortex, precuneus, self-monitoring
Introduction
From a biochemical perspective, the mechanisms of drugseeking behavior are attributable to mesolimbic regions, specifically dopaminergic projections of the nucleus accumbens (1). Hence, models of addiction primarily revolve around functions related to the mesolimbic-dopaminergic pathway, such as the cognitive processing of reward and motivation. Animal research, however, has shown that lesioning these regions does not fully abate all drug-seeking behavior (2), implying involvement of other neurocircuitry and processes that contribute to the sequela of addiction. For example, repeated drug use has shown to attenuate frontal regulation of behavior that is associated with impulsivity and compulsive drug taking (3). Additionally, a seminal paper in patients with insula damage demonstrated important contributions of the insula, and consequently interoceptive processes in nicotine dependence (4). These findings propose that evaluative processes relating to internal physiological states or memory thereof are important cognitive processes related to addiction processes.
Two signaling sources for sentience: interoception and exteroception
Sources for sentience or sensory awareness can be parsed based on whether the neuronal signaling codes for internal bodily states (interoception) or the external environment (exteroception). In the addiction literature, of the two sources, interoception has been a topic of keen interest. Since Naqvi and Bechara's insular model of addiction (4), most of the literature related to sensory awareness and drug use has focused on interoceptive processes (e.g. using to avoid physiological withdrawal). Specifically, fMRI studies have demonstrated greater insula activity after presentation of cigarette (5) cocaine (6) alcohol (7) and heroin (8) cues in their respective using populations, indicating heightened internal awareness in the presence of these stimuli. In all, these studies suggest that the primary contribution of insula activity and interoceptive processes in drug addiction is in monitoring internal, bodily states such as craving or urge to consume a substance (9). In concordance with the aforementioned study in which insula-lesioned patients demonstrated significantly reduced craving for tobacco (4,10,11) these results indicate an important role of the insula in monitoring internal states that drive drug-seeking behavior.
As with interoception, drug craving can also be driven by exteroceptive processes (11,12). In contrast to sensory awareness of internal body states, exteroception refers to sensory awareness of stimuli outside of the body. In the context of drug-craving, heightened exteroception may occur under positive or negative valence. For example, heightened awareness of negative external stressors (e.g. an exam) may drive an individual to use a drug (negative valence). An example of positive valence are drug cues or paraphernalia that signal the impending pleasurable experience from drug use. This heightened awareness to conditioned drug cues, also referred to as cue-elicited craving, is one of the key mechanisms that leads to drug-seeking behavior. To date, the literature has focused largely on this positive incentivization, and, as such, is the focus of our proposed model of exteroception of addiction.
Exteroceptive processes have been postulated to lie within posterior cortical midline structures (pCMS), particularly the precuneus (13–17), which are considered fundamental regions in the perception of the physical body in space (18–21). In this light, pCMS regions are critical for bottom-up sensory processing that contributes to the initial recognition and assignment of salience to external stimuli. This subsequently affects top-down processes within frontal areas of the brain such as decision-making. Exteroception may also be modulated by other processes, including those that relate to the evaluation of self-relevant information. While exteroception refers to the perception of all external stimuli, the stimuli's relevance to the self is processed by the Relevance Detector Network (RDN) with the amygdala as its primary node (22). In the RDN, the amygdala serves as the gateway through which external information (via exteroception) is tagged as self-relevant via emotional arousal. In this light, the amygdala has been referred to as a “quick and dirty” evaluation of salient stimuli (23) and as such may be the first stage of self-relevant processing. This first level of self-relevant detection may then be integrated in pCMS regions. Dysfunction of this relevance detection system has been described as possibly displaying an aberrant “priority mapping” of salient stimuli in which inappropriate stimuli become tagged as self-relevant (24). In the context of addiction, this would involve heightened exteroception on drug-relevant stimuli.
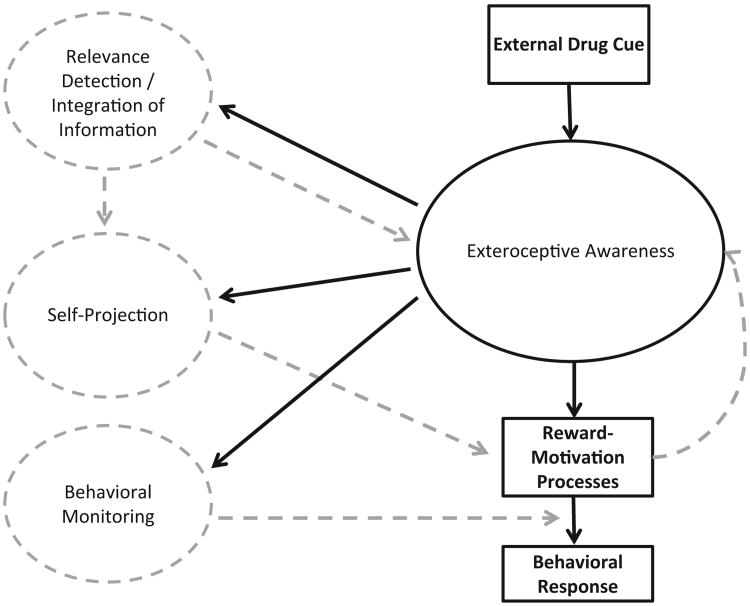
While the construct of exteroception has yet to be tested empirically in the context of addiction, it conceptually will involve exteroceptive awareness with afferent and efferent projections from RDN (self-relevant evaluation) and integration of information, self-projection (self-referential processing in future scenarios), as well as exteroceptive enhancement via reward-motivation signaling, all of which then influence downstream behavioral outcomes (see proposed model in Figure 1). In this model, we acknowledge that affective salience and episodic memory associated with the evaluation of self-relevant cues likely contribute to a heightened sensory awareness of drug-related cues, and in this way further influence downstream behavioral outcomes such as drug use. This is based on the notion that the individual's self-defined concept of the drug stimuli is tightly woven with the experiences evoked by the cues resulting in changes in sensory awareness. The corresponding neural regions in this model overlap with those previously outlined in a meta-analysis (25) suggesting that these heterogeneous processes lie primarily in cortical midline structures such as the dorsomedial prefrontal cortex (DMPFC), posterior cingulate cortex (PCC) and the precuneus. Notably, of these regions, the precuneus is more widely-reported across different paradigms such as cue-reactivity (20), self-centered mental imagery (26) and across different addicted populations (15–17,27).
Figure 1.
An exteroception model of addiction. In this proposed exteroception model of addiction, we suggest that processing of drug cues in the reward-motivation system (or final common pathway) (1) leading to a behavioral response is influenced by (i) Exteroceptive awareness of salient cues within the precuneus (path shown in black). Grayed lines indicate modulatory paths whereby evaluation of the exteroception signal may be further processed for (ii) Relevance – cues are tagged as self-relevant via rapid detection within the amygdala as well as integration of information via precunues/posterior cingulate area. The path between exteroceptive awareness (precuneus) and the reward-motivation processes (mesocorticolimbic) can also be modulated by (iii) Self-projection – self-referential processing in future scenarios that is a viable target for cognitive strategies, e.g. reappraisal, and, (iv) Behavioral monitoring – occurs after stimuli are tagged as self-relevant in anterior/frontal regions (anterior cingulate, mPFC) and guides the behavioral response. Finally, signaling during reward-motivation processes can enhance exteroceptive awareness for drug cues via feedback loop, thereby increasing exteroceptive awareness for drug cues.
Exteroception and the default mode network
Given that interoceptive and exteroceptive processes lie in cortical midline structures (CMS), it is therefore of interest that the CMS also play a role in the default mode network (DMN). The DMN consists of heterogeneous processes related to self-monitoring such as self-awareness and self-relevant processing; i.e. thoughts and knowledge about the self, and mentalization of experiences related to the self (25,28–30). Consequently, the DMN has been implicated in behaviors related to addiction such as impaired insight; either decreased awareness of one's self (9), or decreased awareness of stimuli relevant to one's self (31).
By and large, the DMN has been attributed to monitoring of internal states, such as in interoceptive processes. These include collecting and monitoring information about the self in order to plan behavior responses to internal stimuli (20,32). However, others suggest that sub-regions of the DMN have unique contributions of self-monitoring that are not specific to representations of the internal self. For example, Gusnard and Raichle (33) proposed functionally distinct sub regions of the DMN consisting of the medial prefrontal cortex (similar to anterior CMS) as well as the posterior medial cortex (similar to posterior CMS). This model posits that the DMN's role in monitoring of internal states is primarily via medial prefrontal cortex. Meanwhile, the posterior medial cortex, (specifically posterior cingulate, precuneus and retrosplenial cortex) was described to be primarily involved in monitoring the outside world; i.e. exteroception. The authors cited evidence of these regions' involvement in visuospatial processing to support this notion and further stated that the posterior cingulate gyrus has an evaluative role of what is being monitored. From this, they concluded that gathering (and evaluating) information about the external world is a defining feature of the DMN.
Monitoring and evaluating the external world via the DMN not only provides information about the external environment but also appears to heavily influence the sense of self. In a meta-analysis of fMRI studies, Schilbach and colleagues (34) showed remarkable overlap between regions of DMN and regions involved in social cognition, i.e. relating the self to others and the outside world. Additionally, a recent study using transcranial magnetic stimulation (TMS) sought to separate regions involved specifically in self-awareness and those which may be involved in both self-awareness and the DMN (30). TMS was applied to both medial prefrontal cortex as well as bilateral parietal cortex while participants performed a task involving “self-relevant” stimuli and “other” stimuli. The results showed that TMS interruption of cortical activation only disrupted task performance when applied to parietal regions, but not the medial prefrontal cortex. The authors suggested this to be evidence that while self-referential processing shares regions with the DMN, they do not completely overlap. This is in line with Gusnard and Raichle's suggestion that sub-regions of the DMN are specifically involved in such self-referential or exteroceptive processes. Notably, subsequent studies reveal activation in medial prefrontal cortex specific to self-referential processing (35,36). Thus, the degree to which regions of self-referential processing overlap with the DMN requires further investigation.
Taken together, the specific loci for exteroception may be in posterior, midline hubs of the default mode network, i.e. posterior cingulate cortex and precuneus, with likely contributions from medial prefrontal cortex and initial, amygdala relevance detection input. Their role in monitoring and evaluating the outside world, as well as in processing self-relevant information in the context of social cognition, suggest that these are the neural substrates for evaluating self-relevant information in the outside world.
Exteroception and addiction
The primary contribution of exteroception in addiction is in the hyper-sensitivity (sensory awareness) to external cues deemed self-relevant. In substance abusers, external cues associated with their drug of choice have heightened relevance, and, therefore, “turn up the volume” of exteroceptors (regions dedicated to processing external stimuli) (14). Moreover, memory and affective processes can also modulate relevance (37–39). Neuroimaging studies provide concordant findings that support the contributions of exteroception in symptoms of addiction. For example, increased neural response to drug cues in the precuneus have been observed in different substance abusing populations and have been associated with symptoms of addiction, such as risky decision-making and problems related to substance use (40– 43). Although increased activation observed in the precuneus was not directly addressed in these studies, it may reflect heightened sensory awareness in individuals with substance use disorders (SUDs) in response to cues. In a seed-based region of interest (ROI) study of functional connectivity comparing cannabis users and healthy controls, cannabis users demonstrated greater connectivity in brain regions associated with self-referential processing including ventral posterior cingulate cortex (PCC), anterior left insula, and bilateral supramarginal gyri (15). In cannabis users, ventral PCC-insula connectivity correlated with quantity of cannabis used, which suggests a role of exteroception in the onset and maintenance of substance use. Given that no directionality of this connection is implied (PCC-to-insula or insula-to-PCC), this association may speak to the integration of interoceptive and exteroceptive signaling that may, in turn, contribute to behaviors related to addiction, such as craving. Thus, in the context of addiction, aberrant connectivity/activation in exteroception regions may signal a bias for which external cues are being evaluated as self-relevant. This is illustrated in studies whereby response to drug cues is greater than response to salient non-drug stimuli (44,45). In all drug users, the meaning of drug-related cues is such that the drug and the drug user's sense of self become inextricably linked. In sum, we propose that processes of exteroception are hijacked such that drug cues become increased in self-relevance through a feedback loop involving heightened sensory awareness in the presence of relevant drug cues. While this model describes the process through which an individual transitions to a substance use disorder, we cannot discount the possibility that there may be enhanced exteroception (46,47) that is premorbid to substance use. Moreover, it is also likely that pre-morbid conditions of hyper-exteroception are exacerbated by exposure to substances. In which case, repeated conditioning of the rewarding experience with the drug cues may then titrate the individual's exteroceptors to the drug cues, thereby, making the cues more self-relevant.
It is possible that impaired attribution of self-relevance in drug-addicted individuals may result in a behavioral bias towards (i.e. error monitoring and appraisal) drug cues observed in the anterior cingulate gyrus and ventromedial prefrontal cortex (9). In the context of our present model, this behavioral bias would come into play in the downstream behavioral response after an initial exteroceptive bias to tag drug cues as relevant to one's sense of self. Importantly, identifying one's self with the drug of choice is a product of continued substance abuse. In this light, altered exteroceptive processes influence behavioral monitoring (see Figure 1) which in turn exacerbates the exteroceptive bias towards drug cues and thus contributes to the maintenance of drug use and relapse.
Roles of the precuneus in exteroception
The precuneus is considered a functional core of the DMN, although its specificity for exteroceptive processes is unique from other DMN-related processes. As a core of the DMN, it has been suggested that the precuneus may be highly adaptable, given the DMN's involvement in wide-ranging cognitive processes (33,48) (see Table 1). Thus, besides awareness of external stimuli, exteroceptive processes in the precuneus may also contribute to self-referential processes in future scenarios or “self-projection” (26). Theoretically, self-projection plays a critical role in higher-order decision-making (see [20] for review) as it evaluates the self in future scenarios. These simulations would be based largely on memory of past experiences through connections with medial temporal lobe regions for the consolidation and retrieval of memories. Such self-projections may influence reward-motivation processes during continued drug use by triggering a cue-elicited craving (i.e. imagining oneself using a drug of choice) or during abstinence via imagined behavioral changes (e.g. motivation to engage in intervention strategies). As such, these self-projections present a modifiable target for intervention.
Table 1.
Human studies reporting the putative role of the precuneus in exteroceptive processes.
| Authors | Modality | Participants | Putative mechanism of precuneus |
|---|---|---|---|
| Grant et al. (17) | PET
|
13 cocaine abusers vs. 5 healthy controls |
|
| Shulman et al. (48) | PET
|
9 PET studies including 132 healthy adults total |
|
| Greicius et al. (65) | fMRI
|
14 healthy participants |
|
| Tapert et al. (15) | fMRI
|
15 adolescents with alcohol use disorders |
|
| Vogeley et al. (49) | fMRI
|
11 healthy males |
|
| Fox et al. (66) | fMRI
|
10 healthy controls |
|
| Brody et al. (51) | fMRI
|
42 adult smokers |
|
| McClernon et al. (16) | fMRI
|
18 adult smokers |
|
| Lou, Luber, Stanford, Lisanby (30) | Transcranial Magnetic Stimulation (TMS) in bilateral parietal cortex (angular gyrus) During self-referential task | 18 healthy subjects |
|
| Claus et al. (42) | fMRI
|
326 heavy drinking individuals |
|
| Ersche et al. (67) | sMRI | 50 sibling pairs discordant for stimulant dependence vs. 50 healthy controls |
|
| Feldstein Ewing et al. (68) | fMRI
|
41 adolescent cannabis users |
|
| Filbey et al. (62) | fMRI
|
32 overweight (BMI > 25) adults |
|
| Claus et al. (64) | fMRI
|
116 adult smokers |
|
| Feldstein Ewing et al. (63) | fMRI
|
43 adolescent cannabis users |
|
| Pujol et al. (27) | fMRI
|
28 male cannabis users vs. 29 healthy controls |
|
| DeWitt et al. (69) | fMRI
|
18 risk-taking adolescents (RT),18 non-risk-taking adolescents (NR1) |
|
| Filbey and Dunlop (70) | fMRI
|
71 cannabis users |
|
| Utevsky et al. (71) | fMRI conjunction analysis between:
|
209 healthy participants |
|
DMN, default mode network; dPCC, dorsal posterior cingulate cortex.
Self-referential processes have also been examined using paradigms that require individuals to adopt either a first person or third person view of themselves. In these imaging studies, increased activation in the precuneus for third-person compared to first-person view was found (49). Activation of the precuneus in the context of such task demands is shown consistently across a number of paradigms. In a meta-analysis that investigated the neural substrates of theory of mind, Schurz and colleagues (50) observed increased activation in the precuneus in tasks related to false beliefs, rational actions, and, trait judgment. Each of these tasks, the researchers argued, required the individual to make judgments either about persons and the world around them, or about those persons' own perceptions of the world around them. These consistent findings have led researchers to suggest that the precuneus is a key region of mental imagery related to the perception of other individuals (20). Adopting the perception of other individuals helps formulate one's own perception, and contributes to the evaluation process during exteroception. In sum, as an integrated hub of a putative exteroception network, we propose that the precuneus may be a vulnerable target in addiction.
Modifying exteroception during addiction treatment
Activation of exteroceptive regions in the brain (i.e. precuneus) has also been observed in treatment studies for SUDs. With the goal of identifying mechanisms of change following cognitive reappraisal, Brody et al. (2007) examined response to cues in tobacco users trained to crave nicotine or resist the urge (crave vs. resist conditions) (51). Results showed greater activation in the precuneus during the crave condition for nicotine cues (vs. neutral cues), and that subjective craving levels also correlated with precuneus activation. Interestingly, precuneus activation was also greater during the resist condition vs. crave condition for nicotine cues. It is important to note that while instructions to resist craving do not explicitly tap into cognitive reappraisal strategies, individuals commonly report using such strategies in order to resist. Given the role of the precuneus in exteroceptive processes, these findings further support the idea that (i) self-relevant drug cues enhance exteroception response, and that (ii) cognitive reappraisal (i.e. resist condition) target exteroceptive processes, specifically self-referencing, as reflected by increased activation in precuneus when trying to resist. Thus, current interventions in addiction implicate reductions in self-relevant processing of drug cues.
In addition to cognitive reappraisal, mindfulness training (MT) is another treatment approach that targets behavior change via exteroception mechanisms (in addition to interoception). MT involves focusing attention in the present moment, without judgment of internal or external experiences (52). It has been shown to reduce craving for nicotine dependence (53), alcohol (54) and marijuana craving (55,56), and leads to a reduction in relapse rates for substance use (57). While cognitive reappraisal techniques involve self-awareness regions coming online to engage in self-referential reappraisal of a scenario/stimuli, MT appears to involve disengaging from subjective, evaluative experience. Interestingly, the integrity of these self-awareness regions is closely tied to success in mindfulness strategies.
fMRI studies show that mindfulness meditation is associated with decreases in BOLD signal in the precuneus and anterior insula that was attributed to successful “deidentification” or non-subjective judgments and perceptions associated with mindfulness (58). Concordant findings have also been reported in terms of increased connectivity and network efficiency of the precuneus within the DMN during mindfulness, as well as activation in precuneus associated with successful treatment using motivational interviewing; another popular cognitive intervention strategy (59–61). These latter findings suggest that optimal network connectivity in exteroception regions, specifically the precuneus, leads to greater success in disengaging from evaluative thinking during mindfulness meditation. Thus, the extent to which addictive behaviors stem from a dysregulation of exteroception would be crucial for identifying mechanisms of change as well as for predicting treatment response.
Conclusions
In this review, we introduce the construct of exteroception as evaluative processes of self-relevant external cues that integrate self-projections to guide behavior. Our review of the literature suggests that the addiction process increases sensitivity of exteroceptors to drug-related cues, and may be the underlying mechanism for cue-elicited craving. It is also possible that heightened exteroceptive processes may be a vulnerability factor for addiction. Evidence from treatment studies provides support for the importance of evaluating the self and external stimuli, such as in cognitive reappraisal and mindfulness training. Success likely depends upon the extent to which the exteroceptive regions of addicted individuals can be re-trained. Successful re-training would cause drug-related cues to be perceived as less relevant to the self, and therefore less integral to the recovered drug-user's sense of self. In sum, models of addiction should take into account these exteroceptive processes, given (i) behavioral and neuroimaging evidence for their role in addictive behavior, and (ii) that these processes underlie mechanisms of change following psychosocial treatment.
Footnotes
Shared first authorship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 6.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 7.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 8.Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 9.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cognit Sci. 2014;18:635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 11.Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front Psychiatry. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropatholog. 2014;127:91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel S. Drug tolerance, drug addiction, and drug anticipation. Curr Direct Psycholog Sci. 2005;14:296–300. [Google Scholar]
- 14.Filbey FM, DeWitt SJ. Cannabis cue-elicited craving and the reward neurocircuitry. Prog Neuro-psychopharmacol Biolog Psychiatry. 2012;38:30–35. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- 16.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosoph Transact Royal Soc London Series B, Biolog Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 20.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 21.Heilman KM, Barrett AM, Adair JC. Possible mechanisms of anosognosia: a defect in self-awareness. Philosoph Transact Royal Soc London Series B, Biolog Sci. 1998;353:1903–1909. doi: 10.1098/rstb.1998.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 23.LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 24.Zalla T, Sperduti M. The amygdala and the relevance detection theory of autism: an evolutionary perspective. Front Human Neurosci. 2013;7:894. doi: 10.3389/fnhum.2013.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cognit Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, Crippa JA, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatric Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 28.D'Argembeau A, Salmon E. The neural basis of semantic and episodic forms of self-knowledge: insights from functional neuroimaging. Adv Experim Med Biol. 2012;739:276–290. doi: 10.1007/978-1-4614-1704-0_18. [DOI] [PubMed] [Google Scholar]
- 29.Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity – implications for the default mode network, self-reference and attention. Clinical Neurophysiol. 2012;123:700–710. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Lou HC, Luber B, Stanford A, Lisanby SH. Self-specific processing in the default network: a single-pulse TMS study. Experim Brain Res. 2010;207:27–38. doi: 10.1007/s00221-010-2425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, et al. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 34.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness Cognit. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Kim K, Johnson MK. Extended self: spontaneous activation of medial prefrontal cortex by objects that are ‘mine’. Soc Cognit Affective Neurosci. 2014;9:1006–1012. doi: 10.1093/scan/nst082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray RJ, Schaer M, Debbane M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- 38.Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. J Consult Clin Psychol. 1991;59:558–565. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- 39.Hull JG, Young RD, Jouriles E. Applications of the self-awareness model of alcohol consumption: predicting patterns of use and abuse. J Personality Soc Psychol. 1986;51:790–796. doi: 10.1037//0022-3514.51.4.790. [DOI] [PubMed] [Google Scholar]
- 40.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcoholism, Clin Experim Res. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biolog Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Henry EA, Kaye JT, Bryan AD, Hutchison KE, Ito TA. Cannabis cue reactivity and craving among never, infrequent and heavy cannabis users. Neuropsychopharmacology. 2014;39:1214–1221. doi: 10.1038/npp.2013.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filbey FM, Dunlop J, Baine J, Kuhn B, Alvi T, DeWitt S, Ketcherside A. Potentiating effects of cannabis in the brain's hedonic response: fMRI of cue-reactivity in daily, long-term cannabis users. under review. [Google Scholar]
- 46.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 48.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 49.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cognit Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 50.Schurz M, Aichhorn M, Martin A, Perner J. Common brain areas engaged in false belief reasoning and visual perspective taking: a meta-analysis of functional brain imaging studies. Front Human Neurosci. 2013;7:712. doi: 10.3389/fnhum.2013.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, et al. Neural substrates of resisting craving during cigarette cue exposure. Biolog Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabat-Zinn J. Wherever you go, there you are. New York: Hyperion; 1994. [Google Scholar]
- 53.Vidrine JI, Businelle MS, Cinciripini P, Li Y, Marcus MT, Waters AJ, Reitzel LR, et al. Associations of mindfulness with nicotine dependence, withdrawal, and agency. Substance Abuse. 2009;30:318–327. doi: 10.1080/08897070903252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy CM, MacKillop J. Mindfulness as a strategy for coping with cue-elicited cravings for alcohol: an experimental examination. Alcoholism, Clin Experim Res. 2014;38:1134–1142. doi: 10.1111/acer.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychol Addict Behav. 2013;27:329–335. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Dios MA, Herman DS, Britton WB, Hagerty CE, Anderson BJ, Stein MD. Motivational and mindfulness intervention for young adult female marijuana users. J Substance Abuse Treat. 2012;42:56–64. doi: 10.1016/j.jsat.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slomski A. Mindfulness Based intervention and substance abuse relapse. JAMA. 2014;311:2472. [Google Scholar]
- 58.Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Social Neurosci. 2011;6:231–242. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- 59.Prakash RS, De Leon AA, Klatt M, Malarkey W, Patterson B. Mindfulness disposition and default-mode network connectivity in older adults. Soc Cognit Affective Neurosci. 2013;8:112–117. doi: 10.1093/scan/nss115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paolini B, Burdette JH, Laurienti PJ, Morgan AR, Williamson DA, Rejeski WJ. Coping with brief periods of food restriction: mindfulness matters. Front Aging Neurosci. 2012;4:13. doi: 10.3389/fnagi.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldstein Ewing SW, Filbey FM, Hendershot CS, McEachern AD, Hutchison KE. Proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: the example of motivational interviewing. J Stud Alcohol Drugs. 2011;72:903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filbey FM, Myers US, Dewitt S. Reward circuit function in high BMI individuals with compulsive overeating: similarities with addiction. NeuroImage. 2012;63:1800–1806. doi: 10.1016/j.neuroimage.2012.08.073. [DOI] [PubMed] [Google Scholar]
- 63.Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychol Addict Behav. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 68.Feldstein Ewing SW, Mead HK, Yezhuvath U, Dewitt S, Hutchison KE, Filbey FM. A preliminary examination of how serotonergic polymorphisms influence brain response following an adolescent cannabis intervention. Psychiatry Res. 2012;204:112–116. doi: 10.1016/j.pscychresns.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeWitt SJ, Aslan S, Filbey FM. Adolescent risk-taking and resting state functional connectivity. Psychiatry Res. 2014;222:157–164. doi: 10.1016/j.pscychresns.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Filbey FM, Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 2014;140:101–111. doi: 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]