Abstract
Prenatal nicotinic exposure (PNE) prolongs bronchopulmonary C-fiber (PCF)-mediated apneic response to intra-atrial bolus injection of capsaicin in rat pups. The relevant mechanisms remain unclear. Pulmonary substance P and adenosine and their receptors (neurokinin-A receptor, NK1R and ADA1 receptor, ADA1R) and transient receptor potential cation channel subfamily V member 1 (TRPV1) expressed on PCFs are critical for PCF sensitization and/or activation. Here, we compared substance P and adenosine in BALF and NK1R, ADA1R, and TRPV1 expression in the nodose/jugular (N/J) ganglia (vagal pulmonary C-neurons retrogradely labeled) between Ctrl and PNE pups. We found that PNE failed to change BALF substance P and adenosine content, but significantly upregulated both mRNA and protein TRPV1 and NK1R in the N/J ganglia and only NK1R mRNA in pulmonary C-neurons. To define the role of NK1R in the PNE-induced PCF sensitization, the apneic response to capsaicin (i.v.) without or with pretreatment of SR140333 (a peripheral and selective NK1R antagonist) was compared and the prolonged apnea by PNE significantly shortened by SR140333. To clarify if the PNE-evoked responses depended on action of nicotinic acetylcholine receptors (nAChRs), particularly α7nAChR, mecamylamine or methyllycaconitine (a general nAChRs or a selective α7nAChR antagonist) was administrated via another mini-pump over the PNE period. Mecamylamine or methyllycaconitine eliminated the PNE-evoked mRNA and protein responses. Our data suggest that PNE is able to elevate PCF NK1R expression via activation of nAChRs, especially α7nAChR, which likely contributes to sensitize PCFs and prolong the PCF-mediated apneic response to capsaicin.
Keywords: SIDS, NK1R, TRPV1, Adenosine, nAChRs, Nodose/Jugular Ganglion
Introduction
Maternal smoking during pregnancy is closely associated with the risk of sudden infant death syndrome (SIDS) (Shea and Steiner, 2008) characterized by cardiorespiratory failure including lethal apnea (Harper et al., 2000; Hafstrom et al., 2002). Cigarette smoke contains numerous compounds emitted as gases and condensed tar particles, in which nicotine is the major neurotoxic chemical (Kalra et al., 2002). More importantly, prenatal nicotinic exposure (PNE) has been extensively used to reveal the adverse impacts of cigarette smoke on cardiorespiratory functions and its potential linkage with SIDS pathogenesis (Fregosi and Pilarski, 2008; Campos et al., 2009; Stephan-Blanchard et al., 2013). Our recent study showed a plasticity of bronchopulmonary C-fibers (PCFs) in PNE rat pups (Zhuang et al., 2015). This plasticity is characterized by a remarkable prolongation of the apneic response and augmentation of pulmonary C-neural response to right atrial injection of capsaicin, a selective stimulant to PCFs. However, to date, it remains unclear how PNE prolongs the PCF-mediated apnea.
Cigarette smoke (maternal) could increase synthesis and release of pulmonary substance P and adenosine in mice and mouse pups (Wu et al., 2009; Lu et al., 2013). Both substance P and adenosine are potent in sensitizing/activating PCFs via binding with corresponding PCF receptors, neurokinin-1 receptor (NK1R) and ADA1 receptor (ADA1R), respectively (Hong et al., 1998; Lee and Pisarri, 2001; Bergren, 2006). In addition, PCFs abundantly express capsaicin receptor, transient receptor potential vanilloid type 1 (TRPV1) that is responsible for the PCF-mediated cardiorespiratory responses (Gu et al., 2005). These, along with pulmonary inflammation induced by maternal cigarette smoke (Czekaj et al., 2002), induce our hypothesis that PNE would promote pulmonary substance P and adenosine release and upregulate TRPV1, NK1R, and ADA1R expression in PCFs associated with elevation of pulmonary inflammation in rat pups. An upregulation of NK1R, but not TRPV1 and ADA1R, gene expression in vagal pulmonary C-neurons was observed in our preliminary study. Owing to the potentiating effect of substance P via activation of NK1R on the capsaicin-induced currents in these neurons (Zhang et al., 2007), we tested the role of PCF NK1R in prolonging the capsaicin-induced apnea in PNE pups by using a peripheral NK1R blocker.
Recent reports have shown that nicotinic effect is either dependent on or independent of activating neuronal nicotinic acetylcholine receptors (nAChRs). For example, nicotine induced a several-fold increase in the capsaicin-activated currents in rat trigeminal ganglion neurons via facilitating TRPV1 currents, which was independent of the activation of neuronal nAChRs (Liu et al., 2004). On the other hand, nicotine increased calcitonin gene related peptide release in response to capsaicin in rat buccal mucosa, which was blocked by the nAChR antagonist (Dussor et al., 2003). Because α7nAChR among nAChRs is the most abundantly expressed in the lungs and plays a key role in the PNE-induced impairment of pulmonary development (Sekhon et al., 1999; Wongtrakool et al., 2012), we hypothesized that nAChRs, especially α7nAChRs, are critical for the PNE-induced mRNA and protein changes as proposed above.
Materials and Methods
Pathogen-free Sprague-Dawley rats (250–350 g) were purchased from Charles River Laboratories, Inc. (Wilmington, MA); housed in the animal facility at Lovelace Respiratory Research Institute in filter top cages; and provided with water and food ad libitum. The room was constantly ventilated and the temperature was kept at 23°C. The animals were quarantined for 2 weeks before experiments. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, USA.
Animal Preparation
PNE
The PNE pretreatment was the same as previously reported (Zhuang et al., 2015). Briefly, the adult females were randomly designated to receive vehicle (n = 25) and nicotine (n = 44) respectively via a mini-osmotic pump (0.25 μL/hr for 28 days, Alza Corp., Palo Alto, CA). The latter was subcutaneously implanted in the females to deliver vehicle or nicotine tartrate (6 mg/kg/day) that produces nicotine blood levels approximately equivalent to those that occur in moderate to heavy smokers (Slotkin et al., 1997). Ten days after the implantation, each female rat was placed in a breeding cage with a male rat for up to 4 days. Those with vaginal plugs were considered pregnant and separated from the male. On the 7th day of gestation, the pump was replaced with a new one filled accordingly with vehicle or nicotine.
Pretreatment with nAChRs or α7nAChR blockade
Of 44 females with nicotinic exposure, 18 were treated with mecamylamine (MM, n = 9) or methyllycaconitine (MLA, n = 9). MM is a noncompetitive antagonist for all known nAChR subunits (Singh et al., 2013). Although MLA also interacts with α4β2 and α6β2 receptors at high concentration, the dose (3 mg/kg/d) we used is reported to selectively antagonize a7nAChR subunit (Northrop et al., 2011; Rezvani et al., 2012). In these females, besides the nicotinic pump, another mini-osmotic pump containing either MM (0.03 mg/kg/d) or MLA (3 mg/kg/d) was subcutaneously implanted.
Usage of rat pups
Rat pups born by spontaneous vaginal delivery were housed with their mother and siblings (24–25°C, and 12:12 h light/dark cycle). In all experiments, no more than three male pups from each litter with a similar overall litter size were used in each study to minimize the possible effect of genetic difference between litters on the results. Males at postnatal day 11 to day 14 (P11–14) were chosen in this study because males are much more vulnerable than females in human SIDS (Adams et al., 1998) and pups’ brain development at this period is equivalent to newborn infants at 2–4 month (Klaus, 2004), the highest risk time window for SIDS. All studies were performed during 9:00 and 17:00 hours to avoid any influence from the circadian rhythm (Stephenson et al., 2001). Pups from vehicle-and nicotine-treated dams, and the latter coupled with MM or MLA pretreatment were grouped as Ctrl, PNE, MM+PNE, and MLA+PNE that were randomly assigned to the following six Study Series.
Experimental protocols
Study Series I was performed to test whether PNE altered substance P and adenosine in BALF (n = 10 for Ctrl and PNE, respectively), mRNA and protein levels of NK1R, ADA1R, and TRPV1 in N/J ganglionic neurons (n = 20 per group). To this end, substance P was detected by duplicate use of ELISA kit (Phoenix, EK-061–05), while adenosine was measured by amperometric measurements with needle-shaped biosensors (Bekar et al., 2008). The mRNA and protein levels of NK1R, ADA1R, and TRPV1 in N/J ganglionic neurons were analyzed by TaqMan real time PCR and Western blot, respectively.
Study Series II was designed to define PNE effect on NK1R, ADA1R, and TRPV1 expression in vagal pulmonary C-neurons. Ctrl and PNE pups (n = 12/group) at P3 were anesthetized by isoflurane (3–5%)to sufficiently suppress corneal and withdrawal reflexes. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, 0.25 mg/ml; 1% ethanol concentration) was applied via intratracheal instillation (0.05 ml X 2) to retrogradely trace vagal pulmonary C-neurons within the nodose/jugular (N/J) ganglia. As previously reported (Hu et al., 2010), ten to twelve days later, the animals were euthanized to collect the N/J ganglia and then pulmonary C-neurons. mRNA levels of NK1R, ADA1R, and TRPV1 in vagal pulmonary C-neurons were detected by TaqMan real time PCR, while Beta-actin (NM_031144.2) was used as the endogenous control.
Study Series III was conducted to examine the effects of MM (a general antagonist of nAChR) or MLA (a selective antagonist of α7nAChR) pretreatment on the PNE-induced changes in gene and protein levels of NK1R, ADA1R, and TRPV1 in N/J ganglionic neurons, and pulmonary inflammatory cells. Four groups of pups (Ctrl, PNE, MM+PNE, MLA+PNE; N = 20, 20, 20, and 25) were used. After euthanasia, BLAF was collected from 10 pups in each group for counting the total cells and cells differentiation (macrophages, neutrophils, lymphocytes, or eosinophils). Subsequently, N/J ganglia were harvested in all pups used in this series. The procedures and methods to measure the mRNA and protein levels were the same as described in Series I – II. Owing to the lack of change in substance P and adenosine in BALF in our preliminary study, we did not measure substance P and adenosine here.
Study Series IV was performed to clarify if MLA was able to block the PNE-induced elevation of NK1R mRNA in pulmonary C-neuron as our preliminary results showed a unique PNE effect on elevating NK1R mRNA in pulmonary C-neurons and the similarity of blocking effects of MM or MLA in Study Series III. The procedures were the same as described in Study Series II with the exception that only NK1R mRNA in pulmonary C-neurons was compared among Ctrl, PNE, and MLA+PNE pups (N = 5/group).
Study Series V was aimed at confirming the role of peripheral blockade of NK1R in the PNE-induced prolongation of apneic response to capsaicin. We targeted peripheral NK1R because PNE only increased NK1R expression in pulmonary C-neurons in our preliminary study. Ctrl (n = 6) and PNE pups (n = 10) were anesthetized and the right jugular vein was cannulated as reported before (Zhuang J, 2014). The ventilatory responses to bolus injection of capsaicin (10 μg/kg) into the right atrium were compared without and with right atrial injection of SR140333 (100 μg/kg), a peripheral and selective NK1R antagonist that completely blocked neurogenic inflammation (Emonds-Alt et al., 1993; Amann et al., 1995; Rupniak et al., 2003). SR140333 pretreatment was made 10 min before capsaicin injection. After completion of the ventilatory test, the animals were euthanized and the caudal nucleus tractus solitaries of the medulla harvested to detect local substance P and NK1R expression. A previous report has demonstrated that microinjection of substance P into the caudal nucleus tractus solitaries prolongs the capsaicin-induced apnea in guinea pigs (Mutoh et al., 2000). Thus, we investigated if PNE could elevate substance P and NK1R expression in the caudal nucleus tractus solitaries to centrally contribute to the prolongation of the apneic response to capsaicin.
Tissues collection and analysis/measurement
Collection of bronchoalveolar lavage fluid (BALF)
Briefly (Xu et al., 2008), pups were sacrificed by an overdose of Euthasol, the trachea cannulated and BALF collected following 2 times lavage washes with 0.5 ml ice-cold PBS. Then, the total cells in BALF were counted. BALF was then centrifuged (200 g × 5min) and supernatants stored at −70°C for substance P and adenosine measurement and the cell pellet used for different cell analysis by cytospin. Slides were fixed and stained with Giemsa for leucocyte analysis and the count of monocytes, lymphocytes, neutrophils, and eosinophils in total of 200 cells determined per slide.
Collection of the caudal nucleus tractus solitaries
Briefly (Gozal et al., 1998), the caudal nucleus tractus solitaries will be identified in the extracted brainstem under a surgical microscope according to the stereotaxic coordinate of the rat brain (Paxinos and Watson, 1998). the caudal nucleus tractus solitaries tissues were carefully collected with a 17-gauge, thin-walled hypodermic needle by punch sampling and stored at −80°C for following substance P and NK1R protein tests.
Pulmonary C neurons’ collection and Single cell real-time PCR
Harvest of the N/J ganglia was the same as previously reported (Hu et al., 2010). Immediately after euthanasia, the N/J ganglia were extracted and placed in ice-cold DMEM solution containing 0.1% type IV collagenase and 0.1% Trypsin. Then the N/J ganglia were cut into ~10 pieces and incubated in a CO2 incubator containing 5% CO2 at 37°C for 1 h. The tissue digestion was stopped by adding DMEM solution containing trypsin inhibitor (2 mg/ml). Subsequently, the cell suspension was centrifuged at 150 g for 5 min, the supernatant was aspirated, and the cells were resuspended in modified Neurobasal medium containing 10% FBS, 1% Glucose, 1 mM L-Glutamine, 2% B-27, and 100 U/ml penicillin-streptomycin. The suspended cells were plated on poly-L-lysine coated 13 mm cover slips and in 5% CO2 incubated until cells were attached (overnight).
Vagal pulmonary C-neurons were identified by retrograde-labeling and by the neural size (< 25 μm) (Lee et al., 2003) with fluorescence microscopy (Potenzieri et al., 2012). With a negative pressure, single cells were harvested into a glass-pipette (tip diameter: 25 μm) that was pulled by a DMZ-universal (Dagan Corporation, MN, USA). The pipette tip was then broken in a PCR tube containing 9 ul single cell lysis solution and 1 μl DNAase I (Single cell-to-CT™ kit, 4458237, Ambion), and the PCR tube was immediately snap frozen. A sample of the bath solution from the vicinity of a labelled neuron was collected from each coverslip for no-template experiments (bath negative control). As kit’s manual instructions, after reverse transcription, TRPV1 (NM_031982.1), NK1R (NM_012667.1), ADA1R (NM_017155.2) and beta-actin (NM_031144.3) primers were mixed for preamplification based on the instructions. The products from the preamplification stage were firstly used to detect the TRPV1 and then analyze NK1R and ADA1R expression by TaqMan gene assay in those neurons with positive signal of TRPV1.
Total mRNA isolation from N/J ganglia
Total mRNA in the N/J ganglia was isolated using RNeasy Mini Kit (Qiagen 74104, German). cDNA was synthesized by reverse transcription using Sensiscript RT Kit (Qiagen 205211, German).
Real-time analysis
TaqMan real time PCR was conducted on ABI PRISM 7900 HT system (Applied Biosystems, USA) to measure TRPV1, NK1R, and ADA1R in cDNA using the ΔΔCT method. GAPDH (NM_017008.3) and beta-actin were used as the endogenous control for N/J ganglia and pulmonary C-neurons, respectively. Reaction conditions were carried out as follows: 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 72°C for 30 s.
Western blot
For N/J ganglia, seven pups’ tissues were pooled as one trial, and for the caudal nucleus tractus solitaries tissue, one pup’s tissue was as one trial. The tissue were homogenized with M2 buffer (20 mM Tris-HCl, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM-glycerophosphate, 1 mM sodium vanadate, and 1 μg/ml leupeptin). 100 μg of each protein homogenates was run in 12% SDS-polyacrylamide gel electrophoresis, transferred to a PVDF membrane and probed with goat anti-NK1R polyclonal antibody (1:100, Santa Cruz), goat anti-TRPV1 polyclonal antibody (1:100, Santa Cruz), and rabbit anti-ADA1R polyclonal antibody (1:200, Santa Cruz), respectively, overnight at 4°C. After incubation with respective horseradish peroxidase–conjugated second antibody (1:2,000), the signals were detected by enhanced chemiluminescence according to the manuals (Millipore, Billerica, MA). The band density was quantified with ImageJ software (NIH, Bethesda, MD, USA) and normalized to corresponding loading control. Then all values were normalized to the mean of Ctrl group.
Measurement of ventilation
The animals were anesthetized (urethane, 1400 mg/kg, i.p.) to record the PCF-mediated apnea. Supplemental doses of anesthetics (120–240 mg/kg, urethane, i.v.) were provided as needed to suppress corneal and withdrawal reflexes. As previously reported (Zhang et al., 2013), the trachea was cannulated and connected to a pneumotachograph (Frank’s Mfg. Co., Albuquerque, NM) to record airflow. The airflow signals were integrated to generate tidal volume (VT), respiratory frequency (fR), and minute ventilatory volume (VE). The right femoral artery and jugular vein were cannulated for monitoring and recording arterial blood pressure/heart rate and delivering drug into the pulmonary circulation, respectively. The animal was exposed to 30% O2 throughout the experiment to prevent hypoxia. The core temperature of the animal was monitored with a rectal probe and maintained at ~37.5°C with a heating pad and radiant heat.
Data Acquisition and Statistical Analysis
All variables were expressed as absolute values with the exception that the apneic duration evoked by capsaicin was represented as Δ% of baseline expiratory duration (TE). Group data were reported as means ± SE. Student’s t-test was used to analyze the significant differences between the two groups (Ctrl vs. PNE), while one-way ANOVA was used to analyze the significant difference of PNE-evoked mRNA and protein responses before and after MM or MLA pretreatment. Two-way ANOVA was used to analyze the significant difference of apneic responses to capsaicin without and with SR140333 in Ctrl and PNE pups. If an overall test was significant, Tukey’s test was utilized for specific comparisons between individual groups. P-values < 0.05 were considered significant.
Results
PNE has little effect on pups’ behaviors
The pregnant rats undergoing nicotinic alone or coupled with MM or MLA exposure showed no discernible behavior abnormalities, such as agitation, loss of appetite, or shortness of breath. There was no significant difference in birth numbers among Ctrl, PNE, MM+PNE, and MLA+PNE groups (10.3 ± 1.9; 12.7 ± 2.2; 10.9 ± 1.7; and 11.2 ± 2.1; P > 0.05). All of the pups in the four groups were delivered vaginally at full term of gestational day 21 with no dead fetuses found and no differences in body weight (29.3 ± 3.0 g; 31.2 ± 4.2 g; 29.7 ± 2.8 g; and 30.4 ± 4.4 g; P > 0.05).
PNE fails to significantly change substance P and adenosine in BALF
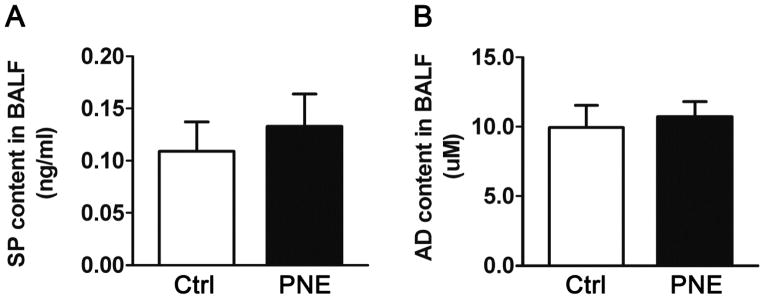
We compared substance P and adenosine protein in BALF between Ctrl and PNE pups. PNE did not markedly alter substance P and adenosine content in BALF (Fig. 1), supporting a lack of PNE effect on pulmonary release of SP and adenosine.
Figure 1.
PNE impacts on SP (A) and AD (B) concentration in BALF. PNE fails to alter SP and AD content (n = 10/group). Data present as Mean ± SE.
PNE elevates TRPV1 and NK1R in the N/J ganglia, and NK1R mRNA in vagal pulmonary C-neuron
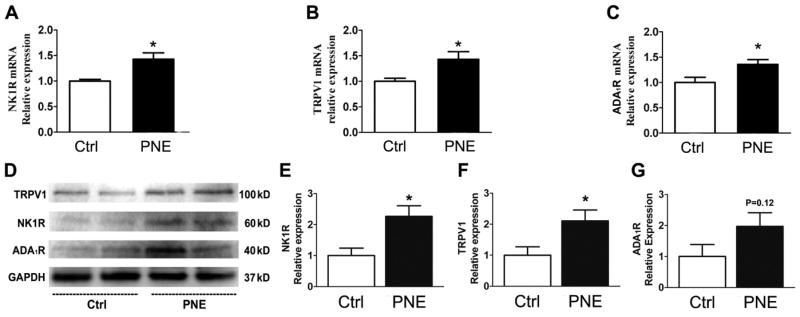
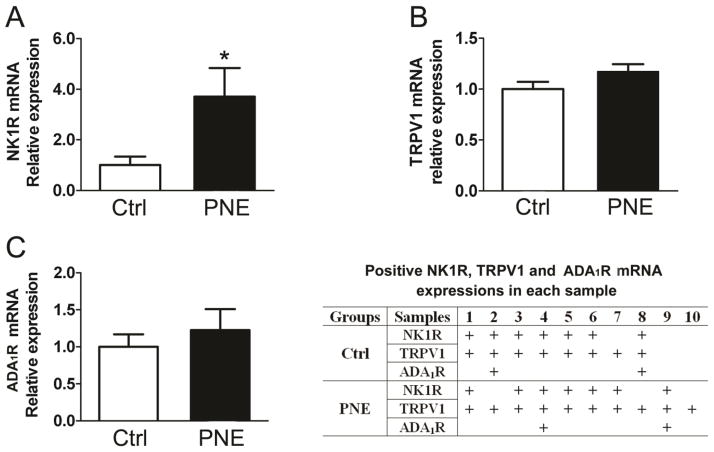
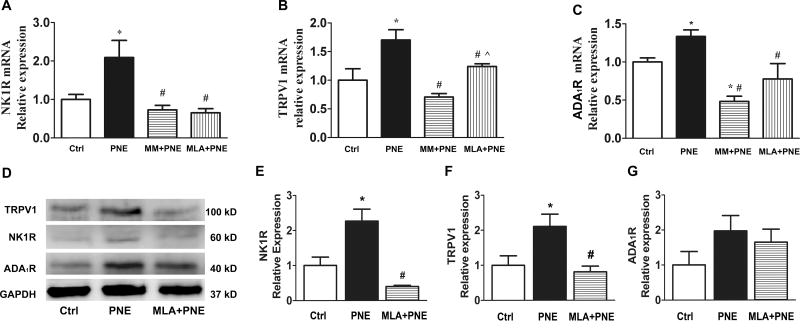
To reveal PNE impact on vagal expression of TRPV1, NK1R, and ADA1R, we compared their mRNA and protein expressions in the N/J ganglia between Ctrl and PNE pups. As exhibited in Fig. 2, PNE significantly increased mRNA level of TRPV1, NK1R, and ADA1R and protein levels of NK1R and TRPV1 in N/J ganglia. The N/J ganglia contain different types of neurons, including, but not being limited to, vagal pulmonary C-neurons (the cell bodies of PCFs). To further define whether these changes were reflected in vagal pulmonary C-neurons, we collected vagal pulmonary C-neurons retrogradely labeled by DiI and compared gene expression of TRPV1, NK1R, and ADA1R using signal cell RT-PCR. As presented in Fig. 3, of 24 vagal pulmonary C-neurons, TRPV1 positive neurons were found in 8/12 Ctrl and 10/12 PNE pups. The majority (≥ 80%) of TRPV1 positive neurons co-expressed NK1R in both groups and NK1R mRNA was significantly higher in PNE than Ctrl pups. In sharp contrast, only 2 pulmonary C-neurons showing TRPV1 co-presented ADA1R and NK1R mRNA (triple expression) and the levels of ADA1R mRNA appeared similar between Ctrl and PNE pups.
Figure 2.
Comparison of gene (A–C) and protein expression (D, examples and E–G quantified data) of NK1R, ADA1R, and TRPV1 in the N/J ganglia between Ctrl and PNE pups. PNE significantly upregulates gene of NK1R, ADA1R, and TRPV1 in the N/J ganglia (n = 6 in each group for gene detection). In D, the samples from both groups showed an equal amount of protein homogenates. GAPDH (37kD) functioned as a loading control. After quantification (E–G), all values were normalized to the mean of control groups. PNE significantly upregulated NK1R and TRPV1 protein expression in N/J ganglia (4 trial samples for each group). Data present as Mean ± SE; * P < 0.05 compared with Ctrl group.
Figure 3.
The effects of PNE on gene NK1R (A), TRPV1 (B), and ADA1R (C) in vagal pulmonary C-neurons in Ctrl and PNE groups by single cell PCR. Data present as Mean ± SE; PNE upregulates NK1R mRNA expression in vagal pulmonary C-neurons. The table lists 8/12 neurons in Ctrl group and 10/12 neurons in PNE group showing TRPV1 mRNA and their co-expression of NK1R and/or ADA1R mRNA. “+” represents the positive result. Majority of pulmonary C neurons expressing TRPV1 are NK1R positive (n = 7 and 8 for Ctrl and PNE group respectively), while ADA1R mRNA only expresses in 2 TRPV1+NK1R co-expression cells in both group (n = 2 in each group). Statistical analysis was not performed for ADA1R mRNA.
PNE increases inflammatory cells in BALF
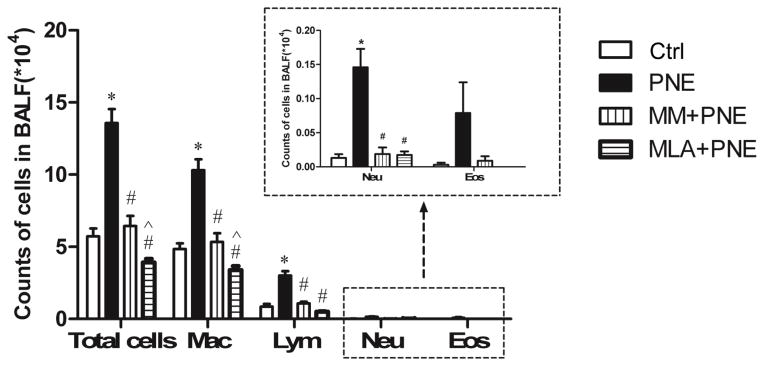
We evaluated the impact of PNE on pulmonary inflammatory cells. As illustrated in Fig. 4, total cells were significantly increased by PNE with remarkable elevation in macrophages, lymphocytes, and neutrophils without significant change in eosinophils.
Figure 4.
Inflammatory cells in BALF of Ctrl and PNE pups. PNE significantly increases the total inflammatory cells, especially macrophages, lymphocytes, and neutrophils, while MM and MLA eliminates these responses. N = 10/group. Data present as Mean ± SE; *, #, and ^ P < 0.05 compared with Ctrl, PNE, and MM+PNE group, respectively. Mac = macrophages; Lym = lymphocytes; Neu = neutrophils; and Eos = eosinophils.
MM or MLA blocks the PNE-induced changes in mRNA and protein and inflammatory cells
Our above studies showed that PNE upregulated TRPV1, NK1R, and ADA1R in the N/J ganglia and inflammatory cells in the airways/lungs. The goal of this experiment was to verify if these PNE-induced changes were dependent on nAChRs, particularly α7nAChR. MM and MLA were used to block nAChRs and α7 nAChR respectively. As shown in Fig. 5, the upregulation of TRPV1, NK1R, and ADA1R mRNA in the N/J ganglia by PNE was abolished by MM or MLA. Interestingly, a significantly lower level of ADA1R mRNA in the N/J ganglia was found after MM pretreatment in PNE pups compared to Ctrl pups, implying that MM not only blocks the ADA1R mRNA response to PNE but also reduces its expression in Ctrl pups. Because of the similarity of MM and MLA effects on the mRNA expressions (Fig. 5A–C), we focused on MLA impact on the protein changes. MLA blocked the upregulation of NK1R and TRPV1 protein expression by PNE with the typical examples presented in Fig. 5D and the quantified data in Fig. 5E–G. Moreover, the responses of inflammatory cells to PNE were blocked after blockade of nAChRs or α7 nAChR (Fig. 4). The values of total cells and macrophages in MLA+PNE were significantly lower than MM+PNE group, pointing to α7nAChR involvement in maintaining the normal level of macrophages in the airways/lungs.
Figure 5.
Effects of MM or MLA on the PNE-induced changes in gene (A–C) and protein expression (D for the examples and E–G for quantified data) of NK1R, ADA1R, and TRPV1 in the N/J ganglia. MM or MLA blocks the PNE-induced gene upregulation of NK1R, ADA1R, and TRPV1 in the N/J ganglia (n = 6 in each group for gene detection). PNE also increased the protein levels of TRPV1 (100 kD) and NK1R (60 kD) by Western blot with equal amount of protein homogenates of samples from the pups’ N/J ganglia. GAPDH (37 kD) functioned as a loading control. After quantification (E–G), all values were normalized to the mean of control groups (n = 4, 4, and 2 trial samples for Ctrl, PNE, and MLA+PNE group). MLA blocks the protein upregulation of NK1R and TRPV1 by PNE. Data present as Mean ± SE; *, #, and ^ P < 0.05 compared with Ctrl, PNE, and MM+PNE group respectively.
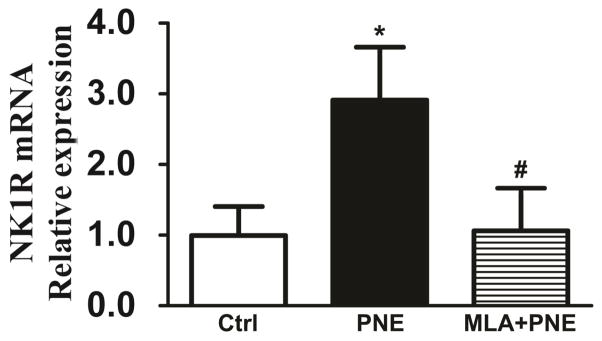
MLA eliminates the PNE-induced elevation of NK1R mRNA in pulmonary C-neurons
Our above results showed that PNE only elevated NK1R mRNA in pulmonary C-neurons and MLA (α7 nAChR antagonist) blocked the PNE-induced responses similarly to MM (nAChRs antagonist). Thus, we tested if the PNE-induced elevation of NK1R mRNA in pulmonary C-neurons was dependent on activation of α7nAChR and found that the pulmonary C-neural NK1R mRNA response to PNE was abolished after blockade of α7nAChR (Fig. 6).
Figure 6.
The effects of MLA treatment on NK1R mRNA expression in pulmonary C-neurons in Ctrl and PNE pups. MLA blocks the PNE-induced NK1R upregulation in vagal pulmonary C-neurons. Data present as Mean ± SE; * and # P < 0.05 compared to Ctrl and PNE, respectively. N = 5 in Ctrl, PNE, and MLA+PNE group.
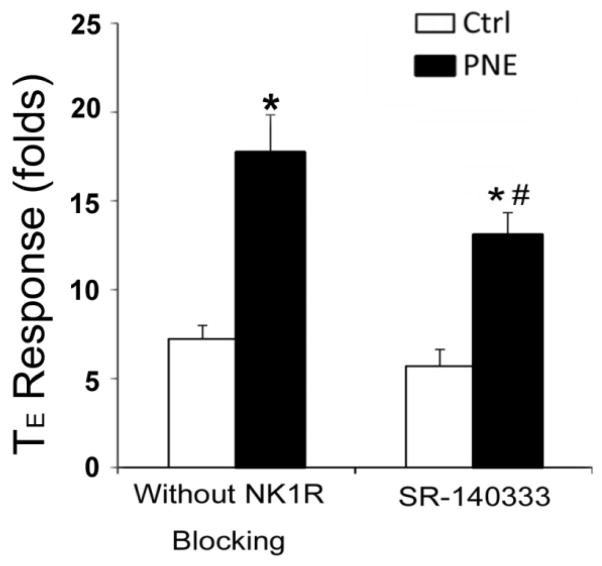
SR140333 shortens the PNE-induced prolongation of the apneic response to capsaicin
The fact that PNE induces a remarkable elevation of only NK1R expression in vagal pulmonary C-neurons raised a fundamental question as to whether PCF NK1R plays an important role in the PNE-induced prolongation of the apneic response to capsaicin. To address this issue, we compared the apneic response to right atrial bolus injection of capsaicin (10 μg/kg) before and 10 min after right atrial injection of SR140333, a peripheral NK1R antagonist, in Ctrl and PNE pups. As illustrated in Fig. 7, capsaicin-induced apnea (baseline TE = 0.34 sec ± 0.04 sec vs. apneic TE = 2.50 ± 0.32 sec) was prolonged by 1.5-fold by PNE (baseline TE values = 0.35 ± 0.05 sec vs. apneic TE = 6.33 ± 0.84 sec). Pretreatment with SR140333 did not strikingly change the capsaicin-induced apnea in Ctrl pups, but significantly shortened the PNE-induced prolongation of the apneic response to capsaicin by ~30%. It should be noted that similar to conscious pups (Zhuang et al., 2014), PNE did not change baseline ventilation in anesthetized pups and SR140333 did not alter baseline respiratory variables in both Ctrl and PNE pups (Table 1). Considering that substance P in the caudal nucleus tractus solitaries is capable of amplifying the PCF-mediated apneic response (Mutoh et al., 2000), we asked whether PNE affected substance P and NK1R expression in the caudal nucleus tractus solitaries. Surprisingly, both substance P and NK1R protein expression in the caudal nucleus tractus solitaries were significantly lower in PNE than Ctrl pups (Fig. 8).
Figure 7.
The effects of treatment with SR140333, a peripheral NK1R antagonist (100 μg/kg) on the apneic response to capsaicin (CAP, 10 μg/kg) in Ctrl and PNE pups. SR140333 significantly shortens the PNE-induced prolongation of the apneic response to CAP with no effect on the CAP-induced apnea in Ctrl pups. Data present as Mean ± SE; N = 6 and 10 for Ctrl and PNE group. * and # P < 0.05 compared to Ctrl and PNE before the blockade respectively.
Table 1.
Effects of PNE on baseline VE, fR, and VT without and with peripheral NK1R blocker (SR140333) in anesthetized rats’ pups
| Treatment | Animal Group | VE (ml/min) | fR (breaths/min) | VT (ml) |
|---|---|---|---|---|
| Without blocker | Ctrl | 25.2 ± 1.7 | 108.1 ± 6.7 | 0.23 ± 0.03 |
| PNE | 24.8 ± 1.2 | 111.6 ± 5.0 | 0.22 ± 0.02 | |
| SR140333 | Ctrl | 24.8 ± 1.3 | 102.0 ± 5.2 | 0.22 ± 0.02 |
| PNE | 25.0 ± 0.7 | 116.0 ± 4.7 | 0.21 ± 0.01 |
Figure 8.
Comparison of NTS SP and NK1R protein expression between Ctrl and PNE pups. A: Gel images of western blotting between Ctrl and PNE groups. GAPDH (37kD) functioned as a loading control. B and C: group data showing that PNE downregulates protein expressions of SP (15 kD) and NK1R (60 kD) in the NTS. Data present as Mean ± SE; with N = 8 and 12 for Ctrl and PNE. * P < 0.05 and ** P < 0.01 compared with Ctrl group.
Discussion
Our recent studies have shown that PNE sensitizes PCFs and prolongs the apneic response to right atrial bolus injection of capsaicin (Zhuang J, 2014). However, the mechanisms underlying this PNE modulation is unknown. Considering the potent sensitization and activation of PCFs by substance P/NK1R, adenosine/ADA1R, and TRPV1, we tested PNE effect on expression of substance P and adenosine in BALF and NK1R, ADA1R, and TRPV1 in the N/J ganglia, particularly vagal pulmonary C-neurons. One of our major findings in this study is that PNE upregulates mRNA of the three receptors and protein expression of TRPV1 and NK1R in N/J ganglionic neurons, and NK1R mRNA in vagal pulmonary C-neurons. Although lack of direct protein evidence, our data showing that the prolonged apneic response to capsaicin by PNE is shortened by blockade of PCFs’ NK1R support our assumption that PNE upregulates NK1R expression in vagal pulmonary C-neurons. It is surprising to us that TRPV1 mRNA is elevated by PNE in N/J ganglionic neurons but not in vagal pulmonary C-neurons. This discrepancy may be due to the fact that the single-cell RT-PCR used in this study cannot fully reflect TRPV1 response of pulmonary C-neurons to PNE. Alternatively, the enhanced TRPV1 and ADA1R in N/J ganglionic neurons could result from other sources of C-type neurons, such as those innervate gastrointestinal organs. Synthesis and release of pulmonary substance P and adenosine are increased by cigarette smoke (maternal) in mice and mouse pups (Wu et al., 2009; Lu et al., 2013). However, both substance P and adenosine content in BALF were not significantly increased in our study. The different exposure (PNE vs. maternal cigarette smoke) and species (rat pups vs. mouse pups) used in our lab and other investigators may have contributed to this difference. On the other hand, this negative result may be due to that the evoked release of substance P and adenosine, if it occurs, is trapped in the tissue with limited release into the BALF. Additionally, we found in this study that PNE increased inflammation cells in BALF. This result indicates a deleterious effect of PNE on the lungs, consistent with other reports (Singh et al., 2013; Huang et al., 2014).
NK1R is able to sensitize and activate C-fibers (Xu et al., 1992; Ustinova et al., 2006), especially PCFs (Bergren, 2006), consistent with our mRNA finding that the majority of TRPV1 positive neurons (≥80%) co-expressed NK1R and PNE only elevated NK1R mRNA in pulmonary C-neurons. Thus, we tested the possible NK1R involvement in the prolongation of the PCF-mediated apnea and found that this prolongation was significantly shortened by peripheral blockade of NK1R with SR140333. We are aware that SR140333 is a peripheral NK1R antagonist that does not only block PCFs’ NK1R. However, it is generally accepted that right atrial bolus injection of capsaicin could stimulate PCFs via selective activation on TRPV1 (Lee and Pisarri, 2001) and NK1R expressed on PCFs is capable of sensitizing TRPV1 (Zhang et al., 2007; Hazari et al., 2008). Thus, our results of peripheral NK1R blockade suggest that PCF NK1R likely upregulated by PNE contributes to the prolongation of the PCF-mediated apneic response. Previous study has shown that microinjection of substance P into the caudal nucleus tractus solitaries where PCFs project to could prolong the PCF-mediated apnea (Mutoh et al., 2000). Moreover, a recent study indicates an upregulation of substance P in the brainstem after PNE (Berner et al., 2008). These results encourage us to explore PNE effect on substance P and NK1R expression in the caudal nucleus tractus solitaries. Unexpectedly, we found a higher substance P and NK1R expression of the caudal nucleus tractus solitaries in Ctrl than PNE pups. We reason that PNE enhances PCF input partially via increasing PCF NK1R that overcomes the downregulation of substance P and NK1R expression in the caudal nucleus tractus solitaries that could inhibit local neurons receiving PCF afferent inputs. The observation that SR140333 diminished but did not block the prolongation of the apneic response to capsaicin by PNE suggests involvements of other peripheral and central mechanisms, which is pending further investigation. One may ask whether PNE effect on PCFs’ plasticity and the capsaicin-induced apnea are age-dependent. A previous study has reported that PNE-induced blunted heart rate response to hypoxia only occurs in P13 but not in P26 rat pups (Boychuk and Hayward, 2011), suggesting an age-dependent impact of PNE on cardiac response to hypoxia. Clearly, further studies are needed to determine if PNE effect on PCFs in the pups persists through their adult life.
The mechanism by which PNE upregulates PCF NK1R to potentiate the capsaicin-induced apnea is unclear. Two lines of recent studies suggest dual impacts of NK1R on C-fibers including PCFs. First, substance P has been reported to depolarize dorsal root ganglionic neurons in rats and cats (Dray and Pinnock, 1982; Inoue et al., 1995) and trigeminal ganglion neurons of guinea pigs by intracellular or whole-cell patch recordings (Li and Zhao, 1998) via action on NK1R. Second, NK1R could interact with TRPV1 to amplify PCF response to capsaicin. In vitro, NK1Rs were found to be co-expressed with TRPV1 in dorsal root ganglionic neurons and substance P significantly potentiates the capsaicin-induced currents in these neurons via action of NK1R (Zhang et al., 2007). In vivo, the apneic response to capsaicin was significantly prolonged after whole-body exposure to acrolein in rats, and this prolongation was abolished by pretreatment with TRPV1 antagonist or NK1R antagonist (Hazari et al., 2008). Intrarectal trinitrobenzenesulfonic acid substantially increased single-unit pelvic C-fiber firing response to intravesical infusion of capsaicin and this response was greatly blunted after depletion of substance P (Ustinova et al., 2007). Collectively, it is possible that PNE upregulation of PCF NK1R expression potentiates TRPV1 and thereby contributes to the prolongation of the PCF-mediated apneic response to capsaicin.
Logically, the PNE-induced changes should be achieved by action on nAChRs. However, this conception has been challenged by a recent report showing an independence of nicotine-induced increase in capsaicin-activated currents in rat trigeminal ganglion neurons of nAChRs (Liu et al., 2004). Thus, we asked if nAChRs, especially α7nAChRs, were essential for the PNE-induced changes in NK1R, ADA1R, and TRPV1 expressions in N/J ganglionic neurons and pulmonary C-neurons and pulmonary inflammation. Our data showed that the PNE-induced elevation of these receptors in N/J ganglionic neurons, and only NK1R mRNA in pulmonary C-neurons, and pulmonary inflammatory cells were eliminated after blocking nAChRs or α7nAChRs. In agreement with our observation, it was reported that nicotine-induced increase of calcitonin gene related peptide release from rat buccal mucosa in response to capsaicin relied on nAChRs (Dussor et al., 2003). Our results lead to the conclusion that α7nAChRs are necessary for PNE impacts on NK1R, ADA1R, and TRPV1 expression in N/J ganglionic neurons, NK1R mRNA in pulmonary C-neurons, and pulmonary inflammatory cells. In fact, the role of α7nAChR in impairing the airways/lung in pups with maternal exposure to cigarette smoke or nicotine has been reported. For example, the maternal exposure could increase the susceptibilities for the development of pediatric lung disorders (Schuller et al., 2000) and decrease forced expiratory flows in offspring largely through the action of α7nAChRs (Wongtrakool et al., 2012). Additionally, the effects of MM and MLA on the PNE-induced responses were not the same. MM not only eliminated ADA1R response to PNE, but also led to a significantly lower ADA1R compared to Ctrl (see Fig. 5). Similarly, MLA not only blocked macrophage response to PNE, but also reduced it to a level lower than Ctrl (see Fig. 4). These data point to a contribution of nAChRs to maintain the normal level of ADA1R expression in N/J ganglionic neurons and α7nAChRs to keep normal pulmonary macrophage level.
Perspective
The risk of exposure to cigarette smoke is highest during fetal and early postnatal life (DiFranza et al., 2004), but nearly one third of mothers still keep smoking during pregnancy (Hylkema and Blacquiere, 2009). The typical adverse consequence in these maternal cigarette smokers is that their offspring has the highest vulnerability of suffering from SIDS featured by cardiorespiratory failure (Trachtenberg et al., 2012). Nicotine is the major neurotoxic compound of cigarette smoke and responsible for pathogenesis of SIDS (Harper et al., 2000; Hafstrom et al., 2002). We previously showed that PNE significantly prolonged the apneic response and augmented pulmonary C-neural response to right atrial injection of capsaicin, proving the PNE-induced PCF plasticity (Zhuang J, 2014). Because PCF activation depresses hypoxic and hypercapnic ventilation that could be responsible for respiratory failure (see discussion in ref. (Zhuang J, 2014)), it is important to investigate how PNE sensitizes and activates PCFs. Our results provide the first evidence to show that PCF NK1R upregulation by PNE is likely responsible for prolongation of the PCF-mediated apneic response. These results not only allow us to better understand how PNE augments the PCF-mediated apneic response, but also gain insight into PCF cardiorespiratory pathophysiology. Most importantly, these results are relevant to pathogenesis of SIDS as an overexpression of C-fibers has been observed in SIDS victims (Becker et al., 1993). Our previous study has shown that PNE-induced lethal apnea during hypoxia is causal to animal death (Zhuang et al., 2014). However, questions remain as to whether upregulation of PCF NK1R by PNE is uniquely involved in the respiratory failure in our study, and if so, how.
Research Highlights.
PNE upregulated NK1R and TRPV1 gene and protein expression in the N/J ganglia.
PNE only elevated NK1R mRNA in vagal pulmonary C-neurons.
Blockage of peripheral NK1R reduced the PNE-induced PCF sensitization.
PNE induced gene and protein changes in NK1R and TRPV1 dependent on action of α7nAChR.
Acknowledgments
The authors are grateful to Ellen Blake for editing and Dr. Shuguang Leng for his technical assistance.
Grants
This study was supported in part by NIH grants HL-107462 and HL119683 (to F.X.) and HL-96914 (to L.Y.L.).
Abbreviations
- AD
Adenosine
- ADA1R
Adenosine A1 receptor
- BALF
Bronchoalveolar lavage fluid
- fR
Respiratory frequency
- MLA
Methyllycaconitine (a selective antagonist of α7nAChR)
- MM
Mecamylamine (a general antagonist of nAChR)
- nAChRs
Nicotine acetylcholine receptors
- NK1R
Neurokinin-A receptor
- N/J
Nodose/jugular
- PCF
Bronchopulmonary C-fiber
- PNE
Prenatal nicotinic exposure
- SIDS
Sudden infant death syndrome
- SP
Substance P
- TRPV1
Transient receptor potential cation channel subfamily V member 1
- VE
Minute ventilatory volume
- VT
Tidal volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams EJ, Chavez GF, Steen D, Shah R, Iyasu S, Krous HF. Changes in the epidemiologic profile of sudden infant death syndrome as rates decline among California infants: 1990–1995. Pediatrics. 1998;102:1445–1451. doi: 10.1542/peds.102.6.1445. [DOI] [PubMed] [Google Scholar]
- Amann R, Schuligoi R, Holzer P, Donnerer J. The non-peptide NK1 receptor antagonist SR140333 produces long-lasting inhibition of neurogenic inflammation, but does not influence acute chemo-or thermonociception in rats. Naunyn-Schmiedeberg’s archives of pharmacology. 1995;352:201–205. doi: 10.1007/BF00176775. [DOI] [PubMed] [Google Scholar]
- Becker LE, Zhang W, Pereyra PM. Delayed maturation of the vagus nerve in sudden infant death syndrome. Acta neuropathologica. 1993;86:617–622. doi: 10.1007/BF00294301. [DOI] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, Bakos R, Nedergaard M. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nature medicine. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- Bergren DR. Prostaglandin involvement in lung C-fiber activation by substance P in guinea pigs. Journal of applied physiology. 2006;100:1918–1927. doi: 10.1152/japplphysiol.01276.2005. [DOI] [PubMed] [Google Scholar]
- Berner J, Ringstedt T, Brodin E, Hokfelt T, Lagercrantz H, Wickstrom R. Prenatal exposure to nicotine affects substance p and preprotachykinin-A mRNA levels in newborn rat. Pediatric research. 2008;64:621–624. doi: 10.1203/PDR.0b013e318186e5f5. [DOI] [PubMed] [Google Scholar]
- Boychuk CR, Hayward LF. Prenatal nicotine exposure alters postnatal cardiorespiratory integration in young male but not female rats. Experimental neurology. 2011;232:212–221. doi: 10.1016/j.expneurol.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M, Bravo E, Eugenin J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clinical and experimental pharmacology & physiology. 2009;36:1205–1217. doi: 10.1111/j.1440-1681.2009.05214.x. [DOI] [PubMed] [Google Scholar]
- Czekaj P, Palasz A, Lebda-Wyborny T, Nowaczyk-Dura G, Karczewska W, Florek E, Kaminski M. Morphological changes in lungs, placenta, liver and kidneys of pregnant rats exposed to cigarette smoke. International archives of occupational and environmental health. 2002;75(Suppl):S27–35. doi: 10.1007/s00420-002-0343-3. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Dray A, Pinnock RD. Effects of substance P on adult rat sensory ganglion neurones in vitro. Neuroscience letters. 1982;33:61–66. doi: 10.1016/0304-3940(82)90130-6. [DOI] [PubMed] [Google Scholar]
- Dussor GO, Leong AS, Gracia NB, Kilo S, Price TJ, Hargreaves KM, Flores CM. Potentiation of evoked calcitonin gene-related peptide release from oral mucosa: a potential basis for the pro-inflammatory effects of nicotine. The European journal of neuroscience. 2003;18:2515–2526. doi: 10.1046/j.1460-9568.2003.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonds-Alt X, Doutremepuich JD, Heaulme M, Neliat G, Santucci V, Steinberg R, Vilain P, Bichon D, Ducoux JP, Proietto V, et al. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. European journal of pharmacology. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respiratory physiology & neurobiology. 2008;164:80–86. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal E, Roussel AL, Holt GA, Gozal L, Gozal YM, Torres JE, Gozal D. Protein kinase C modulation of ventilatory response to hypoxia in nucleus tractus solitarii of conscious rats. J Appl Physiol. 1998;84:1982–1990. doi: 10.1152/jappl.1998.84.6.1982. [DOI] [PubMed] [Google Scholar]
- Gu Q, Lin RL, Hu HZ, Zhu MX, Lee LY. 2-aminoethoxydiphenyl borate stimulates pulmonary C neurons via the activation of TRPV channels. American journal of physiology. Lung cellular and molecular physiology. 2005;288:L932–941. doi: 10.1152/ajplung.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. American journal of respiratory and critical care medicine. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respiration physiology. 2000;119:123–132. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Rowan WH, Winsett DW, Ledbetter AD, Haykal-Coates N, Watkinson WP, Costa DL. Potentiation of pulmonary reflex response to capsaicin 24h following whole-body acrolein exposure is mediated by TRPV1. Respiratory physiology & neurobiology. 2008;160:160–171. doi: 10.1016/j.resp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. The Journal of physiology. 1998;508( Pt 1):109–118. doi: 10.1111/j.1469-7793.1998.109br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-{alpha} in rat pulmonary sensory neurons. American journal of physiology. Lung cellular and molecular physiology. 2010;299:L483–492. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LT, Chou HC, Lin CM, Yeh TF, Chen CM. Maternal nicotine exposure exacerbates neonatal hyperoxia-induced lung fibrosis in rats. Neonatology. 2014;106:94–101. doi: 10.1159/000362153. [DOI] [PubMed] [Google Scholar]
- Hylkema MN, Blacquiere MJ. Intrauterine effects of maternal smoking on sensitization, asthma, and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2009;6:660–662. doi: 10.1513/pats.200907-065DP. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakazawa K, Inoue K, Fujimori K. Nonselective cation channels coupled with tachykinin receptors in rat sensory neurons. Journal of neurophysiology. 1995;73:736–742. doi: 10.1152/jn.1995.73.2.736. [DOI] [PubMed] [Google Scholar]
- Kalra R, Singh SP, Kracko D, Matta SG, Sharp BM, Sopori ML. Chronic self-administration of nicotine in rats impairs T cell responsiveness. The Journal of pharmacology and experimental therapeutics. 2002;302:935–939. doi: 10.1124/jpet.302.3.935. [DOI] [PubMed] [Google Scholar]
- Klaus B. Neuromodulation of the Perinatal Respiratory Network. Current Neuropharmacology. 2004 Apr;2(2):221–243. doi: 10.2174/1570159043476828. 23. [DOI] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respiration physiology. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- Li HS, Zhao ZQ. Small sensory neurons in the rat dorsal root ganglia express functional NK-1 tachykinin receptor. The European journal of neuroscience. 1998;10:1292–1299. doi: 10.1046/j.1460-9568.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu W, Zhang ZS, Yang T, Grant A, Oxford G, Simon SA. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. Journal of neurophysiology. 2004;91:1482–1491. doi: 10.1152/jn.00922.2003. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sakhatskyy P, Newton J, Shamirian P, Hsiao V, Curren S, Gabino Miranda GA, Pedroza M, Blackburn MR, Rounds S. Sustained adenosine exposure causes lung endothelial apoptosis: a possible contributor to cigarette smoke-induced endothelial apoptosis and lung injury. American journal of physiology. Lung cellular and molecular physiology. 2013;304:L361–370. doi: 10.1152/ajplung.00161.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Bonham AC, Joad JP. Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. American journal of physiology. Regulatory, integrative and comparative physiology. 2000;279:R1215–1223. doi: 10.1152/ajpregu.2000.279.4.R1215. [DOI] [PubMed] [Google Scholar]
- Northrop NA, Smith LP, Yamamoto BK, Eyerman DJ. Regulation of glutamate release by alpha7 nicotinic receptors: differential role in methamphetamine-induced damage to dopaminergic and serotonergic terminals. The Journal of pharmacology and experimental therapeutics. 2011;336:900–907. doi: 10.1124/jpet.110.177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Potenzieri C, Meeker S, Undem BJ. Activation of mouse bronchopulmonary C-fibres by serotonin and allergen-ovalbumin challenge. The Journal of physiology. 2012;590:5449–5459. doi: 10.1113/jphysiol.2012.237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Timofeeva O, Sexton HG, DeCuir D, Xiao Y, Gordon CJ, Kellar KJ, Levin ED. Effects of sazetidine-A, a selective alpha4beta2* nicotinic receptor desensitizing agent, on body temperature regulation in mice and rats. European journal of pharmacology. 2012;682:110–117. doi: 10.1016/j.ejphar.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NM, Fisher A, Boyce S, Clarke D, Pike A, O’Connor D, Watt A. P-Glycoprotein efflux reduces the brain concentration of the substance P (NK1 receptor) antagonists SR140333 and GR205171: a comparative study using mdr1a−/− and mdr1a+/+ mice. Behavioural pharmacology. 2003;14:457–463. doi: 10.1097/01.fbp.0000087734.21047.ae. [DOI] [PubMed] [Google Scholar]
- Schuller HM, Jull BA, Sheppard BJ, Plummer HK. Interaction of tobacco-specific toxicants with the neuronal alpha(7) nicotinic acetylcholine receptor and its associated mitogenic signal transduction pathway: potential role in lung carcinogenesis and pediatric lung disorders. European journal of pharmacology. 2000;393:265–277. doi: 10.1016/s0014-2999(00)00094-7. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. The Journal of clinical investigation. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- Singh SP, Gundavarapu S, Smith KR, Chand HS, Saeed AI, Mishra NC, Hutt J, Barrett EG, Husain M, Harrod KS, Langley RJ, Sopori ML. Gestational exposure of mice to secondhand cigarette smoke causes bronchopulmonary dysplasia blocked by the nicotinic receptor antagonist mecamylamine. Environmental health perspectives. 2013;121:957–964. doi: 10.1289/ehp.1306611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Stephan-Blanchard E, Bach V, Telliez F, Chardon K. Perinatal nicotine/smoking exposure and carotid chemoreceptors during development. Respiratory physiology & neurobiology. 2013;185:110–119. doi: 10.1016/j.resp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. The Journal of physiology. 2001;536:225–235. doi: 10.1111/j.1469-7793.2001.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics. 2012;129:630–638. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. American journal of physiology. Renal physiology. 2006;290:F1478–1487. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. American journal of physiology. Renal physiology. 2007;292:F123–130. doi: 10.1152/ajprenal.00162.2006. [DOI] [PubMed] [Google Scholar]
- Wongtrakool C, Wang N, Hyde DM, Roman J, Spindel ER. Prenatal nicotine exposure alters lung function and airway geometry through alpha7 nicotinic receptors. American journal of respiratory cell and molecular biology. 2012;46:695–702. doi: 10.1165/rcmb.2011-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environmental health perspectives. 2009;117:1434–1440. doi: 10.1289/ehp.0800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu F, Barrett E. Metalloelastase in lungs and alveolar macrophages is modulated by extracellular substance P in mice. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L162–170. doi: 10.1152/ajplung.00282.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Intrathecal CP-96,345 blocks reflex facilitation induced in rats by substance P and C-fiber-conditioning stimulation. European journal of pharmacology. 1992;216:337–344. doi: 10.1016/0014-2999(92)90428-7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhuang J, Zhang C, Xu F. Isoflurane depolarizes bronchopulmonary C neurons by inhibiting transient A-type and delayed rectifier potassium channels. Respiratory physiology & neurobiology. 2013;186:164–172. doi: 10.1016/j.resp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Zhao L, Xu F. Maternal nicotinic exposure produces a depressed hypoxic ventilatory response and subsequent death in postnatal rats. Physiological reports. 2014;2(5):1–12. doi: 10.14814/phy2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Zhao L, Zang N, Xu F. Prenatal Nicotinic Exposure Augments Cardiorespiratory Responses to Activation of Bronchopulmonary C-fibers. American journal of physiology. Lung cellular and molecular physiology, ajplung 00241 02014. 2015 doi: 10.1152/ajplung.00241.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Zhao L, Zang N, Xu F. Prenatal nicotinic exposure increases pulmonary C neural response to capsaicin associated with upregulation of TRPV1 in nodose ganglia neurons. FASEB J. 2014;28:713.4. [Google Scholar]