Abstract
Extreme prematurity is a major risk factor for perinatal and neonatal brain injury, and can lead to white matter injury that is a precursor for a number of neurological diseases, including cerebral palsy (CP) and autism. Neuroinflammation, mediated by activated microglia and astrocytes, is implicated in the pathogenesis of neonatal brain injury. Therefore, targeted drug delivery to attenuate neuroinflammation may greatly improve therapeutic outcomes in models of perinatal white matter injury. In this work, we use a mouse model of ischemia-induced neonatal white matter injury to study the biodistribution of generation 4, hydroxyl-functionalized polyamidoamine dendrimers. Following systemic administration of the Cy5-labeled dendrimer (D-Cy5), we demonstrate dendrimer uptake in cells involved in ischemic injury, and in ongoing inflammation, leading to secondary injury. The sub-acute response to injury is driven by astrocytes. Within five days of injury, microglial proliferation and migration occurs, along with limited differentiation of oligodendrocytes and oligodendrocyte death. From one day to five days after injury, a shift in dendrimer co-localization occurred. Initially, dendrimer predominantly co-localized with astrocytes, with a subsequent shift towards microglia. Co-localization with oligodendrocytes reduced over the same time period, demonstrating a region-specific uptake based on the progression of the injury. We further show that systemic administration of a single dose of dendrimer-N-acetyl cysteine conjugate (D-NAC) at either sub-acute or delayed time points after injury results in sustained attenuation of the ’detrimental’ pro-inflammatory response up to 9 days after injury, while not impacting the ‘favorable’ anti-inflammatory response. The D-NAC therapy also led to improvement in myelination, suggesting reduced white matter injury. Demonstration of treatment efficacy at later time points in the postnatal period provides a greater understanding of how microglial activation and chronic inflammation can be targeted to treat neonatal brain injury. Importantly, it may also provide a longer therapeutic window.
Keywords: Dendrimer, neuroinflammation, targeted delivery, microglia, ischemia
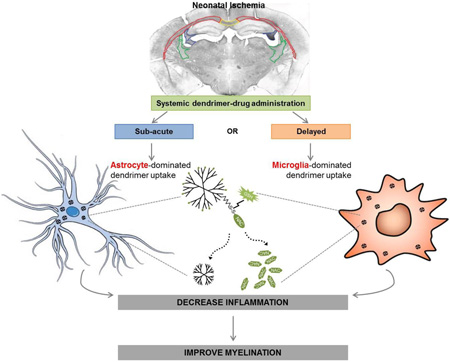
Graphical abstract
BACKGROUND
Extreme prematurity, defined as a gestational age of < 28 weeks or a birth weight of <1500g, affects up to 2% of all newborns in the United States. A high incidence of adverse neurological outcomes in this group calls for increased research in neuroprotective strategies [1, 2]. Many of these infants (20%) develop cerebral palsy (CP), and about 50% will develop cognitive, behavioral, attention, or socialization deficits of variable degree [3–7]. Epidemiological studies suggest that neuroinflammation is associated with perinatal/neonatal white matter injury, the main underlying neuropathology in this patient population, and that active inflammatory processes may prevent regeneration and/or exacerbate brain damage in the premature infant [8]. This may sensitize the brain to further injury, due to long lasting microglial activation and increased expression of inflammatory cytokines [5–8]. Strategies to target and treat neuroinflammation, mediated by microglia and astrocytes, can potentially slow disease progression, and increase the therapeutic window while enabling normal development [9, 10]. Therefore, activated microglia/macrophages and astrocytes are potent therapeutic targets. However, delivery of drugs for the treatment of diffuse brain injury in the neonate is a major challenge. Interestingly, since microglia/astrocytes are more phagocytic in this activated state [11], they may also be more amendable to selectively uptake ‘small’ nanoparticles like dendrimers, compared to other cells in the brain [12, 13].
Nanomedicine–based delivery strategies are emerging as promising candidates for the treatment of inflammatory disorders. Nanotechnologies can improve drug bioavailability and targeting, and provide sustained efficacy. Specifically, dendrimers are unique nanoparticles for targeted drug/gene delivery, including cancer and systemic inflammation [14–20]. The small size and high density of tailorable surface functional groups may provide significant advantages for therapeutic delivery to the brain [15, 21]. PAMAM dendrimers are the mostly widely studied class of dendrimers due to their commercial availability, and have been shown to cross the impaired blood-brain-tumor [18] and blood-brain barriers (BBB) in animal models [17]. In a model of intrauterine inflammation, generation-4 hydroxyl (G4-OH) PAMAM dendrimers have recently been shown to accumulate significantly in the gut and brain of fetal mice following intra-amniotic administration [22]. In the presence of cardiac ischemia, G4 PAMAM dendrimer-small molecule conjugates have shown promise in reducing ischemia-reperfusion injury and improving recovery of cardiac function [23, 24]. In the post-ischemic brain, Kim et al. have utilized PAMAM dendrimer mediated transfection of siRNA to primary neurons and glia to reduce infarct volume following a middle cerebral artery occlusion [25]. More recently, G4-OH dendrimer conjugated with N-acetyl cysteine (D-NAC) has been used in combination with dendrimer conjugated valproic acid (D-VPA) for the treatment of hypothermic circulatory arrest (HCA)-induced brain injury in a canine model [26]. Importantly, Kannan et al. have shown attenuation of the activated microglia response and improvement in the motor phenotype in a maternal inflammation-induced model of CP, following a single systemic administration of D-NAC in newborn kits with CP three days after the insult [17].
In each of these models, dendrimer uptake and localization is specific to the cells involved in ongoing injury and inflammation, at that time point. In the HCA-induced brain injury model, dendrimer accumulation was predominantly seen in dying and injured neurons, and activated microglia, when the dendrimer was administered immediately after the insult [26]. However, in the rabbit model of CP, when the dendrimer is administered three days after injury, at birth, dendrimer uptake was mostly associated with cells involved in ongoing inflammation in this disease (activated astrocytes and microglia), rather than in neurons [17]. To better design and appropriately time the administration of therapeutics, it is important to understand where the dendrimer ends up, and more specifically, what cells it localizes in. As seen in these previous models of brain injury, the cellular localization of dendrimer is dependent to some extent on the model, due to differences in disease etiology, disease progression, and also the timing of dendrimer administration in relation to the disease progression. Dendrimer distribution within the body, clearance, and brain uptake is also governed by dendrimer physicochemical properties [21]. Nanoparticle movement within the brain parenchyma and uptake by specific cells is impacted by nanoparticle size and surface functionality [27]. Comprehensive understanding of both global (whole body) and local (brain) biodistribution of a nanoparticle platform is crucial for better design and optimization of therapeutic platforms that can enhance efficacy, even in aggressive disease models [28].
In this study, we utilize a mouse model of ischemia-induced neonatal white matter injury to determine the biodistribution and brain uptake of the dendrimer at both sub-acute and delayed treatment time points. We chose this model since ischemia is thought to play a key role in neonatal white matter injury [29], and we have previously demonstrated post-ischemic neuroinflammatory response with microglial activation in this model [30]. Based on the temporal and spatial cellular localization of the dendrimer in cells involved in injury, we then performed a preliminary efficacy study to evaluate a potential therapeutic window for administering D-NAC therapy and to determine if similar efficacious results can be obtained later in the postnatal period in the presence of ongoing inflammation. This study provides a greater understanding of the therapeutic window for treatment of D-NAC in ischemic neonatal brain injury. This information will allow more rapid translation among similar models of disease, in this case neonatal white matter injury, and development of a more efficacious platform for clinical translation.
MATERIALS and METHODS
Dendrimer-Cy5 and D-NAC preparation and characterization
We have previously studied the phosphate-buffered saline (PBS) and plasma stability and drug release mechanisms of both the D-NAC conjugates used here, and other dendrimer-drug conjugates [26]. At physiological conditions (PBS 7.4, 37°C), and in plasma (37°C), the D-disulfide-NAC conjugate was stable, and did not release NAC over a 24 h period. However, at intracellular GSH concentrations (2 and 10mM), the conjugate released the drug readily within 3.5 h, indicating that use of a disulfide-linker enables rapid release of NAC from the conjugate only when it is exposed to an intracellular GSH-rich environment [31, 32].
Mouse model of ischemia-induced neonatal white matter injury
Five-day old (P5) CD-1 mouse pups underwent permanent unilateral carotid artery ligation while kept mildly hypoxic under anesthesia for 15 minutes (transcutaneous oxygen saturation between 85–90%), followed by recovery in a 36°C incubator for 30–60 minutes. These mice exhibit bilateral myelin pallor, ipsilateral ventriculomegaly, axonal injury, and astrogliosis, along with apoptosis of oligodendrocyte progenitor cells (PreOLs) and arrested maturation of PreOLs. This results in a decrease in mature oligodendrocyte numbers, as seen in patients [30, 33].
Immunohistochemistry and image analysis for biodistribution study
Newborn mice that underwent unilateral carotid ligation on P5 (ligated group) and normal healthy age-matched controls, were administered 55 mg/kg of D-Cy5 intraperitoneal (i.p.) at P6 (sub-acute) or P10 (delayed) time points. Animals were euthanized at 24 h after D-Cy5 administration, and perfused with normal saline. Brain sections were stained for microglia/macrophages (goat anti-mouse Iba1 with donkey anti-goat IgG Alexa488), astrocytes (rat-anti-GFAP with donkey anti-rat IgG Alexa488), and oligodendrocytes (anti-CC1 with donkey anti-mouse IgG Alexa488). Confocal z-stack merged images, 20µm thick, were obtained using a Zeiss LSM 710 microscope to detect co-localization.
Quantification of dendrimer uptake in the brain
Newborn mice that underwent unilateral carotid ligation on P5 (ligated group) and normal healthy age-matched controls were administered 55 mg/kg of D-Cy5 intraperitoneal (i.p.) at ‘sub-acute’ or ‘delayed’ time points. Animals were euthanized at 24 h after D-Cy5 administration, and perfused with normal saline (n=5/group/time point). Ipsilateral and contralateral hemispheres were evaluated separately by HPLC. We have developed a sensitive method for tissue quantification with detection limits as low as 100ng of D-Cy5/g of tissue (~0.001% of injected dose in brain, typically better than that for radiolabeled dendrimers). This method combines total fluorescence, fluorescence-HPLC chromatograms, and fluorescence/absorbance spectrum measurements, following appropriate tissue extraction, and prior calibration in each of the organs with known amounts of D-Cy5 [26, 34].
NAC therapy for efficacy study
Newborn mice that underwent unilateral carotid ligation on P5 (ligated group) were systemically (i.p.) administered 10 mg/kg of (1) D-NAC, (2) PBS or (3) unconjugated dendrimer (vehicle) intraperitoneal on P6 (24 h after injury, sub-acute time point) or on P10 (5 days after injury, delayed time point). Animals were euthanized at P14 and perfused with paraformaldehyde for immunohistochemistry (IHC), or perfused with normal saline (n = 6/group/time point) for mRNA expression of inflammatory cytokines. For IHC analysis in these animals, staining for Iba-1 and Myelin Basic Protein (MBP) was performed with above mentioned primary antibodies, and with biotinylated secondary antibodies. The antigen–antibody complex was visualized using an ABC ELITE kit (Vector Labs, Burlingame, CA, USA).
RNA Isolation and Real-time PCR analysis
Both untreated ligated, and age-matched control, animals were evaluated by RT-PCR on P6 and P10 for mRNA expression of inflammatory cytokines. Efficacy study animals were evaluated at P14 for mRNA expression of inflammatory cytokines. Total RNA from brain tissue was purified (AllPrep DNA/RNA/Protein Mini Kit; Qiagen), quantified (NanoDrop ND-1000 Spectrophotometer; Thermo Scientific), and integrity-verified (Agilent 2100 Bioanalyzer with Eukaryote Total RNA Nanoassay). Single-stranded complementary DNA (cDNA) was reverse-transcribed from total RNA samples with the High-Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Applied Biosystems), followed by PCR amplification with the TaqMan Universal Master Mix (Applied Biosystems). Primer sequences used are according to Table 1. Amplification conditions were the following: 30 min at 48°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and 60°C for 1 min. Samples were quantified with the ΔCt (threshold cycle, amount of target = 2−ΔΔCt) method, normalized to the internal control gene GAPDH.
Table 1.
Primer sequences for real time PCR.
| Gene | Accession Number | Forward primer | Reverse Primer |
|---|---|---|---|
| TNF-α | M20155 | CCAGTGTGGGAAGCTGTCTT | AAGCAAAAGAGGAGGCAACA |
| IL-1β | NM_008361 | AGCTTCAAATCTCGCAGCAG | TGTCCTCATCCTGGAAGGTC |
| IL-18 | NM_008360 | TTCAAAGGCAGTGCTTCACA | GTACGTTCCCTCATCCTCCA |
| IL-6 | NM_031168 | TCCAGTTGCCTTCTTGGGAC | GTGTAATTAAGCCTCCGACTTG |
| IL-12b | NM_008352 | GCATGTGTCCTCAGAAGCTAAC | CCAGTCCACCTCTACAACATAAA |
| IL-10 | NM_010548 | TTGAATTCCCTGGGTGAGAAG | TCCACTGCCTTGCTCTTATTT |
| IL-4 | NM_021283 | GAGCTATTGATGGGTCTCAAC | CGATGATCTCTCTCAAGTGATTT |
| GAPDH | GU214026 | TGTCGTGGAGTCTACTGGTGTCTTC | CGTGGTTCACACCCATCACAA |
Densitometry analysis for myelination
For quantification of myelin basic protein (MBP) immunostaining, densitometry was performed using MCID Core (InterFocus Imaging, Cambridge, UK) after calibration with optical density standards, as previously reported by Fatemi et al.. Subdivisions of the corpus callosum (CC) (right, right periventricular, medial, left, and left periventricular) were manually delineated in three sections, at the level of the anterior commissure, anterior hippocampus, and posterior CC, as well the left and right fimbriae and the left and right internal capsules. Average density values for each of these areas were calculated in each animal and used for further statistical comparisons. Hemispheric MBP density scores were determined for each animal by averaging the values of each white matter tract for each hemisphere.
Statistical analysis
All data were presented as mean ± S.E.M. Statistical analysis was performed using two-tail Student t-test for two-sample comparison and one-way ANOVA with Fisher's post-hoc analysis for multi- variant comparisons. A value of p <0.05 was considered statistically significant.
RESULTS and DISCUSSION
In vivo dendrimer biodistribution and cellular localization following systemic administration in mice with ischemia
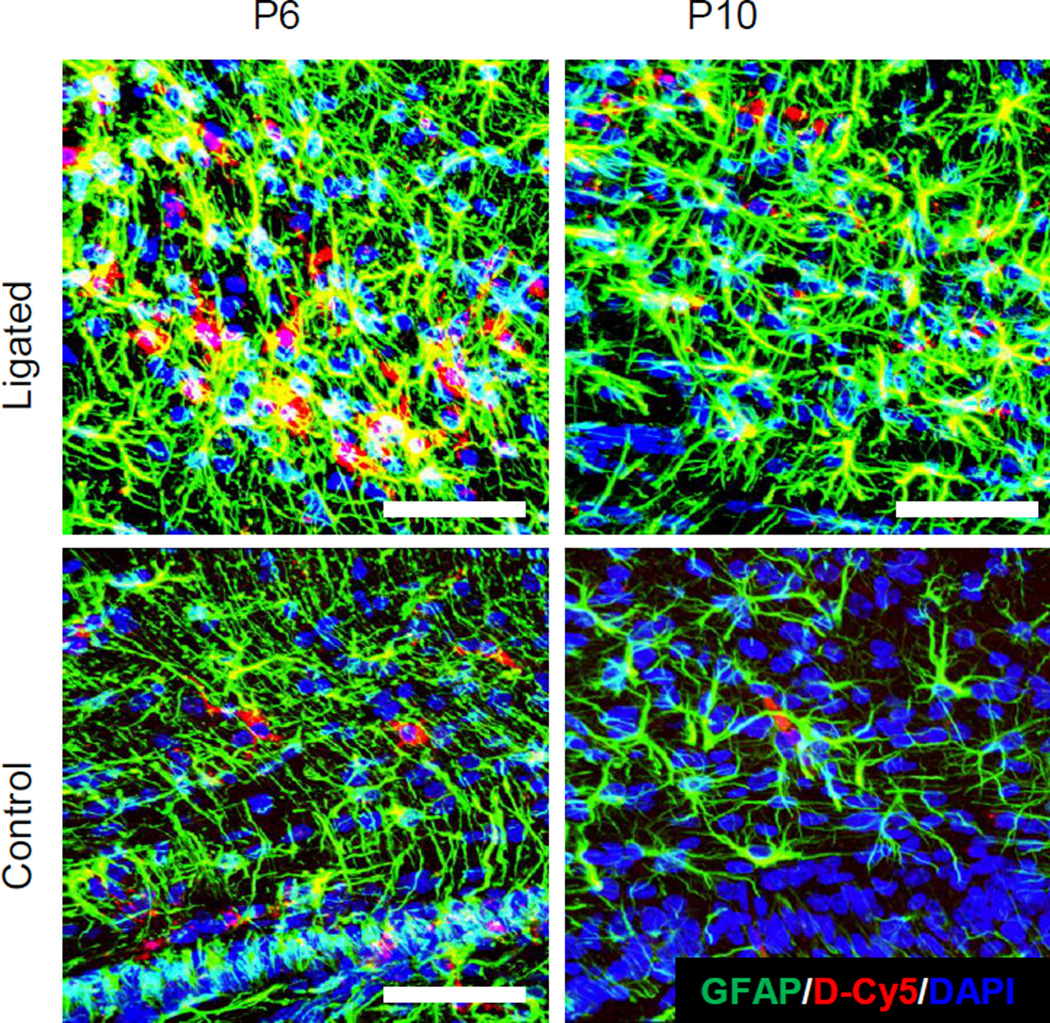
Ligated and age-matched healthy control pups were administered D-Cy5 either at P6 or P10 and brains were harvested 24 hours after administration. Dendrimer co-localized with astrocytes and microglia in ligated mice at P6 (sub-acute time point) in the supraventricular white matter, and to a lesser extent in ligated mice at P10 (delayed time point). Similar dendrimer uptake was seen in astrocytes in the mid and posterior CC at P6 and P10 in ligated pups (Figure 1). Dendrimer uptake was qualitatively greater in astrocytes at P6 compared to uptake in other cell types, including microglia and oligodendrocytes. This may be because the astrocyte response is usually an immediate response to injury [35, 36]. Astrogliosis plays a role in ischemia-induced injury in the newborn, where astrocytes have swollen processes and soma, are more phagocytic, and contribute to ongoing BBB impairment [37]. In this model, astrocytes at both P6 and P10 in ligated pups showed swollen processes, and more dense cell bodies, when compared to controls. Significant dendrimer uptake in astrocytes could be attributed to accumulation in the brain via an impaired BBB, as well as an increase in astrocyte endocytosis.
Figure 1. Dendrimer (red) co-localization at P6 and P10 in astrocytes in ligated and control pups.
Dendrimer cellular localization in astrocytes was primarily seen in the corpus callosum, and in the supraventricular white matter (above the ventricle) on the ligated hemisphere in ligated pups at P6 and P10. Astrocytes (green) were stained with anti-mouse GFAP primary and AlexaFluor 488 secondary. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (Blue). Scale bar: 50µm
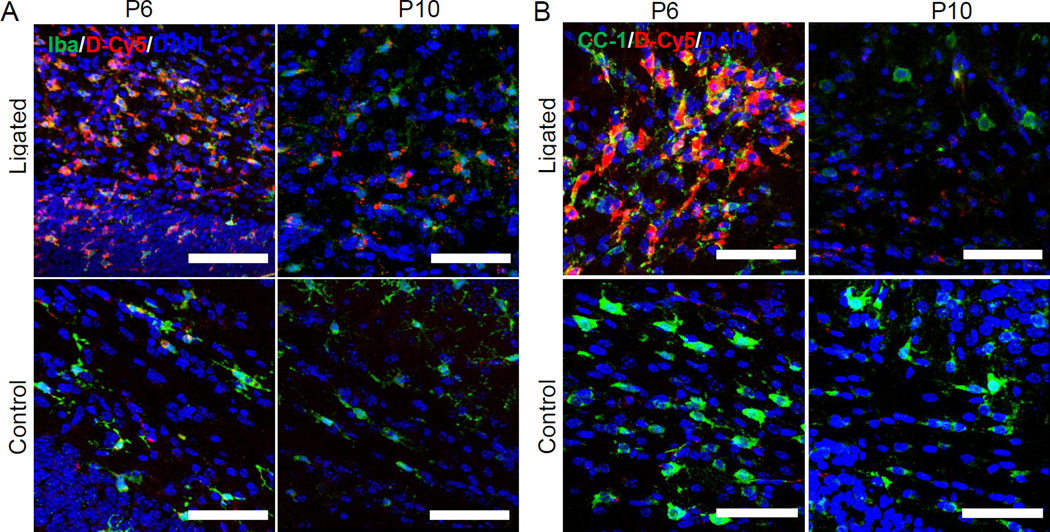
Dendrimer co-localization in microglia was seen in ligated mice at P6 in the CC (Figure 2A). Injury on P5 induces a region-specific neuroinflammatory response, leading to increased microglial activation, and recruitment and migration of microglia along the white matter, with a reduction of oligodendrocyte progenitor cells (preOLs) [30]. Following ligation, white matter areas like the CC show few microglia at P6, but by P7 there is greater microglia distribution throughout the white matter [30]. This suggests there is accelerated microglial migration 48 hours after the injury has occurred. Dendrimer co-localization in microglia at P10 was also significant, with greater localization in microglia compared to astrocytes at this time point. In this model, white matter injury is most prevalent in the mid and posterior CC, supraventricular white matter, and external capsules (EC). Microglia morphology in ligated mice in the supraventricular white matter and CC showed swollen cell bodies, and less and shorter processes, consistent with previous findings [30]. It is also possible these microglial like cells at P5 and P6 include macrophages, which decrease in number and become more microglia-like as the brain ages [38]. The variation in dendrimer uptake and localization in different areas of white matter injury at different times is consistent with the tempero-spatial inflammatory response.
Figure 2. Dendrimer (red) co-localization at P6 and P10 in microglia and oligodendrocytes in ligated and control pups.
(A) Dendrimer co-localized with microglia (green), above the ventricle on the ligated side in ligated pups, in the corpus callosum at P6 and P10. Microglia were stained with anti-mouse Iba primary and AlexaFluor 488 IgG secondary (green in Microglia panel). (B) Dendrimer co-localized with oligodendrocytes (green), above the ventricle on the ligated side in ligated pups, in the corpus callosum at P6 and P10. Oligodendrocytes were stained with anti-mouse CC1 and AlexaFluor 488 IgG (green in oligodendrocyte panel). Images were merged to observe co-localization and 20uM thick stacks were reconstructed in 3D form using Zeiss imaging software. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (Blue). Scale bar: 50µm
Overall, dendrimer co-localized with oligodendrocytes to a lesser extent than microglia and astrocytes (Figure 2B), with the greatest oligodendrocyte co-localization occurring at P6. Cellular localization in oligodendrocytes was greatest in the CC at P6, and in the supraventricular white matter on the ligated side. However, dendrimer did not significantly co-localize with oligodendrocytes or microglia at either P6 or P10 in the contralateral hemisphere of ligated mice (Figure S1). PreOLs are in greater numbers in the CC at P6, where dendrimer uptake was greatest, but due to injury, do not proliferate and migrate at later time points after injury [30]. This decrease in preOL numbers at P10 may explain the decreased co-localization of D-Cy5 in preOLs at P10.
Dendrimer uptake and localization was time- and region-dependent in this model of ischemia-induced white matter injury, as summarized in Table 2. At P6, dendrimer localization with astrocytes was greater compared to localization with microglia and oligodendrocytes. However, dendrimer localization in pre-oligodendrocytes was greater at P6 compared to P10, whereas dendrimer localization with microglia was greater at P10 than at P6. These changes in pattern and timing of distribution follow the biological response to injury, where the immediate response is astrogliosis due to excess glutamate release. As the injury progresses after P6, there is decreased astrogliosis over time and peak microglial proliferation at 48–72 hours (P7–P8), so uptake by P10 moves more towards microglia. This change in dendrimer distribution over time can be exploited to target various cells/pathological processes depending on the timing of administration in relation to the insult.
Table 2. Qualitative assessment of dendrimer biodistribution and co-localization as a function of the temporal and spatial inflammatory response in neonatal stroke.
Dendrimer distribution in the supraventricular white matter (SVWM) and corpus callosum (CC) was analyzed using confocal imaging at either a sub-acute (p6) or delayed (p10) time point in the injured hemisphere of ligated mice (Figure 1, 2, S1). Total dendrimer uptake decreases from p6 to p10 (Figure 3), so qualitative distribution in this table is not comparing dendrimer localization at p6 to dendrimer localization at p10.
| Age: Experimental Group: |
p6 | p10 | ||||
|---|---|---|---|---|---|---|
| Ligated | Control | Ligated | Control | |||
| Region: | SVWM | CC | Hemisphere | SVWM | CC | Hemisphere |
| Astrocytes | +++ | +++ | + | + | + | − |
| Microglia | ++ | ++ | + | ++ | ++ | −− |
| Oligodendrocytes | + | ++ | − | − | +/− | −−− |
The scaling system is as follows: +++ indicates greatest localization, +/− indicates unclear co-localization but dendrimer present, and −−− indicates no localization. Specified regions were assessed for n = 3 animals at each time point for each group.
Similar localization with astrocytes and microglia is seen in the contralateral hemisphere of both ligated and control mice at P6, and in ligated mice at P10 (Figure S1). Injury in this model has been shown to be bilateral, with greater injury on the ligated hemisphere. Therefore dendrimer uptake was present in both ligated and contralateral hemispheres, with a decrease in dendrimer uptake in the contralateral hemisphere of ligated mice compared to dendrimer uptake in the ipsilateral hemisphere.
Quantitative in vivo brain dendrimer uptake after injury
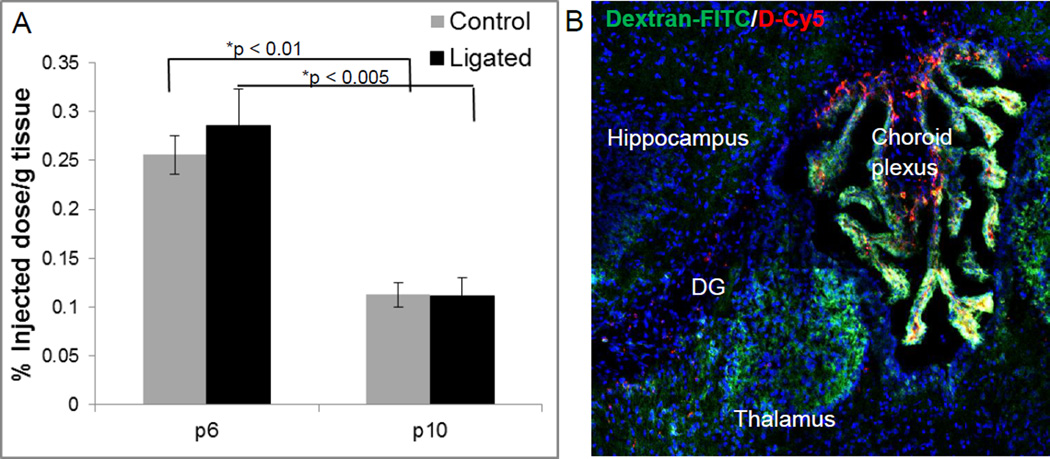
To determine the quantitative difference in uptake between ligated and control pups at sub-acute and delayed time points of administration after injury, brains were harvested and dendrimer quantified using fluorescence quantification analysis. Quantification for both the ipsilateral (ligated or right hemisphere) and contralateral side are included. Dendrimer uptake was highest in the ipsilateral hemisphere in P6 ligated and control mice, at 0.29% and 0.26% of the injected dose (ID, 0.3 mg per animal) per gram of tissue respectively (Figure 3A) and was significantly higher than the uptake at P10. Uptake in the ipsilateral and contralateral hemispheres in ligated mice was similar, showing no statistical difference at P6 or P10.
Figure 3. Quantification of dendrimer-Cy5 uptake in the brain of ligated and control pups at P6 and P10.
(A) Dendrimer-Cy5 uptake was quantified 24 hours after systemic administration using HPLC. Data is expressed as a percentage of injected dose (ID) of D-Cy5 in the ligated hemisphere per gram of tissue, with n = 5 pups per group per timepoint. Dendrimer uptake was highest in the ligated hemisphere of ligated mice at P6. *represents statistically significant (p < 0.05) data. (B) BBB impairment in thalamic nuclei and dentate gyrus near the third ventricle in P6 healthy control mice as indicated by dextran-FITC (40kDa, green) extravasation. D-Cy5 (red) accumulation is present in these regions, as well as in the choroid plexus.
In this study, leakage of dendrimer into the brain of younger healthy control mice was present, which has not been seen in our previous studies with newborn healthy control rabbits. The presence of dendrimer in the brain in healthy control mice at both P6 and P10 is surprising, but the images show a significant difference in the cellular localization in control and ligated mice (Figure 1–2). It is possible that dendrimer is localized in the blood vessel walls in control mice, and that uptake is only in areas surrounding the dorsal ventricle where extravasation of a large MW compound, dextran-FITC (40kDa, compared to a 14kDa dendrimer) was also present in these mice (Figure 3B). Some studies suggest that the BBB is still immature in the developing brain of mice at these ages, yet recent studies with dextrans demonstrate the BBB is intact, functional, and highly regulated at this age in mice [39, 40], and that immaturity does not explain uptake in the brain for some compounds, like the viral vector AAV9 [41]. However, it was also demonstrated that dextran leakage was seen in regions near the pia and choroid plexus [40], similar to our current study where dextran FITC and dendrimer leakage were present in this species of mice. In our rabbit model of CP, systemically administered dendrimer does not accumulate in the healthy brain with an intact BBB or remain in healthy tissue, and is cleared intact via the kidneys within 48 hours [34]. Yet, if dendrimer is administered via the subarachnoid route, there is dendrimer localization in microglial cells in the brain [42], suggesting that if the dendrimer does get into healthy brain tissue, normal surveying microglia cells can internalize the dendrimer. This dendrimer is much smaller in size (~4nm, 14kDa) than previously used tracers, which may also play a key role in its brain uptake. However, the microglial/astrocyte uptake appears to be much higher in the injured brain.
D-NAC suppression of inflammatory cytokines in ligated mice
Based on the significant dendrimer uptake in the brain, and specifically in regions of white matter injury, at both sub-acute and delayed time points, we sought to evaluate if we can treat the inflammation and reduce brain injury through systemic administration of dendrimer-drug conjugates at either time point. This information can lead to a better understanding of the therapeutic window for treating newborns with neonatal white matter injury. This would also be more clinically relevant since diagnosis is often later and early treatment may not be feasible. Recent studies have shown that depletion of microglia prior to an ischemic insult in a neonatal stroke rat model has been shown to be deleterious, indicating that the immediate post-injury microglial response may be protective [43]. However, chronic neuroinflammation may prevent repair and regeneration.
D-NAC was administered at either P6 or P10 to determine the effect on cytokine expression compared to non-treated (vehicle) ligated and D-NAC treated, ligated, animals. Brains were evaluated for both pro- and anti-inflammatory cytokine expression levels at p14, corresponding to eight days or four days after treatment with D-NAC, dendrimer alone or PBS. NAC was chosen as the therapeutic agent given our recent efficacious results in a rabbit model of maternal-fetal inflammation-induced CP [17]. NAC has anti-inflammatory and anti-oxidant properties, both of which play a key role in neonatal brain injury [44]. NAC is also known to block CD11b expression in microglia/macrophages, which plays a crucial role in exacerbating the neuroinflammatory process. Although NAC is already in wide clinical use for other applications in children and adults [45, 46], clinical trials for neuroinflammatory disorders have not shown adequate benefits. This may be because NAC has to be used in high doses since protein binding of the –SH groups leads to poor bioavailability [47]. Moreover, L-cysteine at high doses has been shown to have neurotoxic effects that cause neuronal death due to over activation of NMDA receptors on neurons [48].
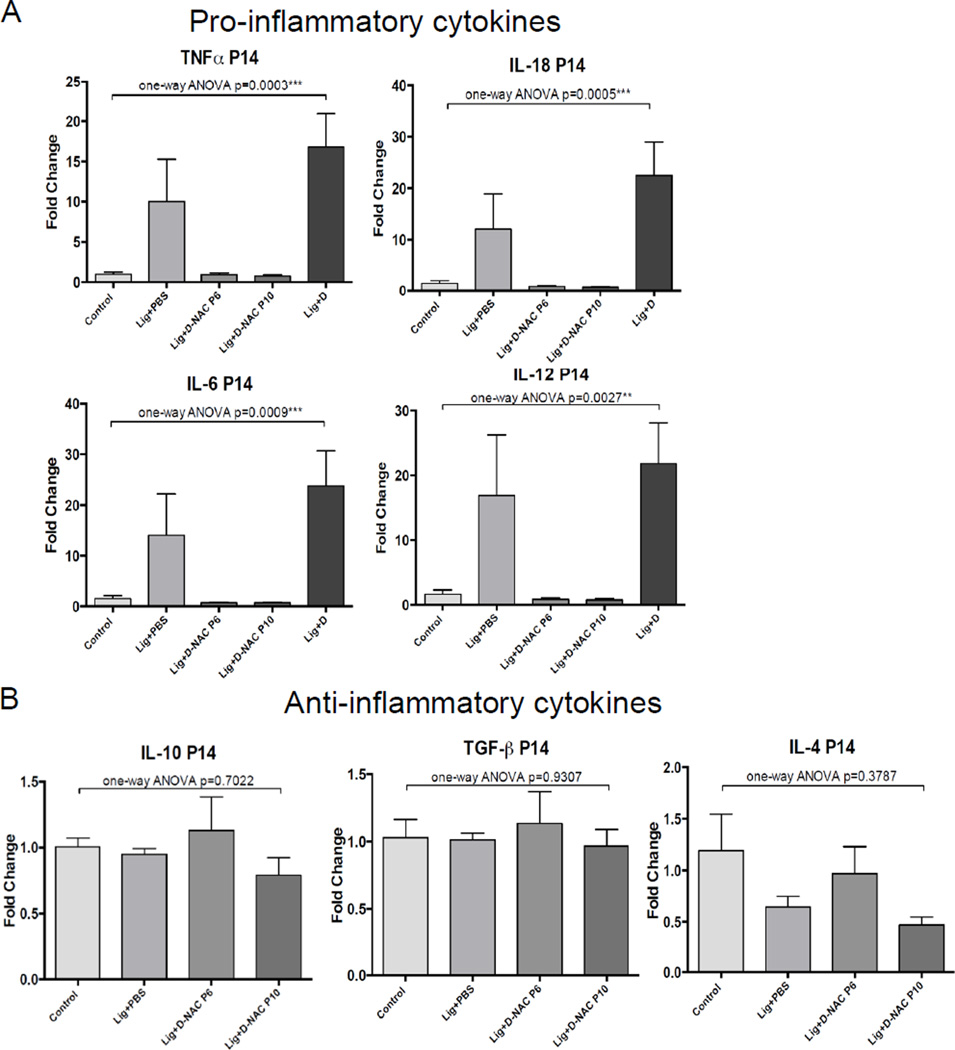
Significant inhibition of mRNA expression of pro-inflammatory cytokines was noted. Ligated pups treated with D-NAC at P6 or P10 showed a 15-fold decrease in TNF-α expression, a 25-fold decrease in IL-18 and IL-6 expression, and a 20-fold decrease in IL-12 expression compared to animals treated with unconjugated dendrimer (Figure 4A). The expression levels of these pro-inflammatory cytokines at P14 following D-NAC treatment were comparable to healthy control, age-matched pups, indicating an effect of D-NAC on both sub-acute and sustained inflammation. Inhibition of IL-1β was not significant (p=0.32, data not shown). Anti-inflammatory cytokine levels were also measured at P14 in treated and non-treated mice following either sub-acute or delayed treatment with D-NAC. The D-NAC therapy did not significantly suppress anti-inflammatory cytokines IL-10, TGF-β, or IL-4 (Figure 4B) at either P6 or P10, although there was a non-significant decrease in IL-4 expression following treatment at P10. Dendrimer uptake and localization in activated microglia and astrocytes in ligated mice was present at both sub-acute and delayed time points after injury, with a decrease in total uptake at 5 days after injury. This data suggests that dendrimer-NAC suppressed only the pro-inflammatory response, and did not impair the anti-inflammatory response at these time points. The ‘selective’ attenuation of the pro-inflammatory response, while not affecting the anti-inflammatory response, may be explained by the targeted delivery and timing of treatment, and has potentially positive consequences for therapies for ischemic injury.
Figure 4. mRNA expression of inflammation cytokines following D-NAC therapy on p6 or p10.
(A) Significant inhibition of mRNA expression of the pro-inflammatiory cytokines TNF-a, IL-6, IL-12, and IL-18 was noted in the ligated mice treated with D-NAC at P6 or P10, compared to PBS treatment. (B) Anti-inflammatory cytokines were not increased at P14 for D-NAC treated mice compared to PBS treated mice.
Increased myelination in D-NAC treated animals
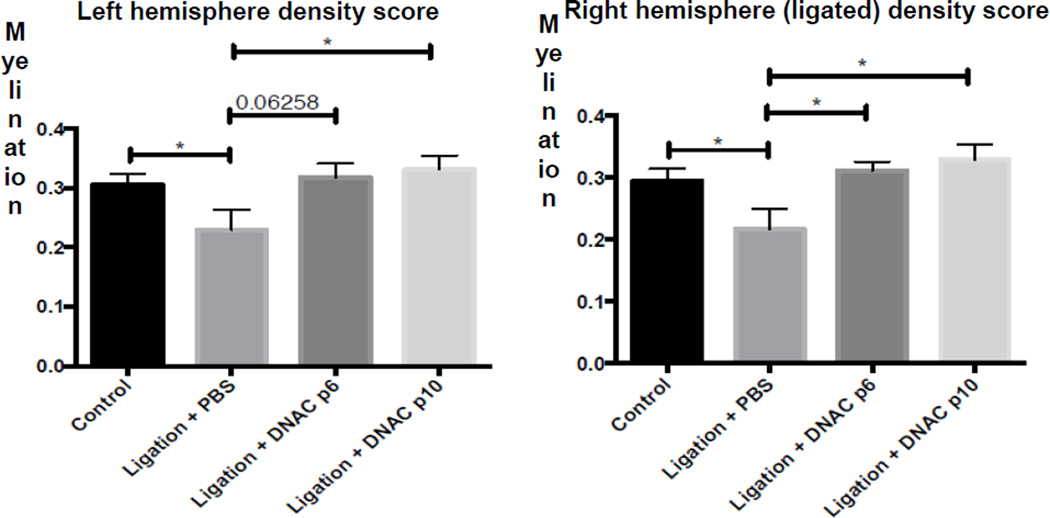
We next sought to demonstrate whether suppression of the immediate pro-inflammatory response after ischemic insult is beneficial for improvement in myelination in regions with the greatest extent of injury. Ligated mice treated with D-NAC at either P6 or P10 saw improvement in myelination in the ligated hemisphere, as well as in the contralateral hemisphere, by P14, nine days after injury (Figure 5). This improvement in myelination is indicative of functional recovery in regions of injury.
Figure 5. Myelination following D-NAC treatment at P6 or P10.
Improvement in myelination was seen by P14 in ligated mice treated with D-NAC at either P6 or P10, compared to PBS treated ligated mice at both timepoints. Improvement was seen in both the ligated (right) hemisphere and the contralateral (left) hemisphere, where injury was present.
Activated microglia/macrophages play a key role after central nervous system (CNS) injury, and can be either protective or detrimental [10]. However, the microglial/macrophage response may depend on the type of inflammation (infection versus hypoxic-ischemic) and the time course of injury (acute versus chronic). During development, microglia render a supportive role in myelinogenesis and axonogenesis, and are present predominantly in the white matter tracts, migrating to the cortex by adulthood [49]. The presence of microglia in the white matter tracts during development also increases the vulnerability of the developing brain to diverse brain insults. Pro-inflammatory microglial activation, when coupled with the inability of activated astrocytes to maintain their neuroprotective function in the presence of overwhelming inflammation, results in injury that continues long after the primary insult [50]. Furthermore, we have previously shown in this mouse model that microglial activation in the white matter showed a temporal correlation with preOL cell death and with impaired differentiation of these cells [30]. Inflammation causes depletion of glutathione in astrocytes with a loss of their normal neuroprotective role. The delivery of NAC to activated astrocytes and microglia/macrophages results in suppression of neuroinflammation, and glutathione (GSH) replenishment in astrocytes, resulting in improvement of myelination and white matter injury.
CONCLUSION
Preterm birth caused by an intrauterine insult or infection, leading to significant white matter injury, is a major risk factor for long term neurological deficits, and diseases like cerebral palsy. Since the timing of an intrauterine insult may be difficult to identify, and diagnosis may not be made until after birth, later treatment time points may be more practical for translation. Treatment at later time points in the postnatal period will also provide a longer therapeutic window. Therefore, it is important to understand the time- and cell-specific biodistribution of a therapeutic platform. In this study, we have demonstrated dendrimer uptake in cells involved in ischemic injury, and in the ongoing inflammation leading to secondary injury, which is consistent with the region-specific inflammatory response following ligation. This uptake was both time-dependent and injured region-dependent, with early dendrimer localization primarily in astrocytes, the immediate responders to injury, and later dendrimer localization primarily in microglia, which respond 48–72 hours after injury. We further show promising findings that site-specific delivery of a dendrimer-based therapeutic, D-NAC, at either sub-acute or delayed time points after injury can suppress the pro-inflammatory response and decrease microglial activation, allowing improvement in myelination. In addition, prolonged attenuation of inflammation is important to achieve symptom free survival well into adulthood. Future longer term efficacy studies, including effects on neuronal cell loss and branching, neurogenesis, myelin structure and organization, microglial/macrophage infiltration and phenotype, and immune regulation will be important to determine if this therapeutic platform results in a global improvement in the brain injury seen with neonatal ischemia. Neurobehavioral studies including evaluation of comprehensive motor and cognitive function at delayed time points after therapy, as well as into adulthood, will also be assessed in the future. This study indicates that based on the timing of administration of the dendrimer, different cells involved in the progression of the disease can be targeted. Therefore, it is important to evaluate and modify therapeutic platforms based on the pathology being targeted.
Acknowledgments
This study was partly funded by the NIH 1 R01 HD069562-01A1 (S. Kannan), 1R01EB018306-01 (R. Kannan), and NIH K08NS063956 (A. Fatemi). Dr. Nance would like to thank the Hartwell Foundation for funding for her postdoctoral fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Hartwell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gargus RA, Vohr BR, Tyson JE, High P, Higgins RD, Wrage LA, et al. Unimpaired outcomes for extremely low birth weight infants at 18 to 22 months. Pediatrics. 2009;124:112–121. doi: 10.1542/peds.2008-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson S. Cognitive and behavioural outcomes following very preterm birth. Seminars in fetal & neonatal medicine. 2007;12:363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.O'Shea TM, Joseph RM, Kuban KC, Allred EN, Ware J, Coster T, et al. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr Res. 2014;75:781–787. doi: 10.1038/pr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr. 2011;158:897–903. e1–e5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Seminars in perinatology. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Dammann O. Persistent neuro-inflammation in cerebral palsy: a therapeutic window of opportunity? Acta Paediatr. 2007;96:6–7. doi: 10.1111/j.1651-2227.2007.00097.x. [DOI] [PubMed] [Google Scholar]
- 10.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 11.Choucair N, Laporte V, Levy R, Arnold AS, Gies JP, Poindron P, et al. Phagocytic functions of microglial cells in the central nervous system and their importance in two neurodegenerative diseases: multiple sclerosis and Alzheimer's disease. Cent Eur J Biol. 2006;1:463–493. [Google Scholar]
- 12.Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, et al. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine-Uk. 2010;5:1317–1329. doi: 10.2217/nnm.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minami SS, Sun BG, Popat K, Kauppinen T, Pleiss M, Zhou YG, et al. Selective targeting of microglia by quantum dots. J Neuroinflamm. 2012;9 doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 15.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Hayder M, Poupot M, Baron M, Nigon D, Turrin CO, Caminade AM, et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002212. [DOI] [PubMed] [Google Scholar]
- 17.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, et al. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarin H, Kanevsky AS, Wu HT, Brimacombe KR, Fung SH, Sousa AA, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6 doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei T, Chen C, Liu J, Liu C, Posocco P, Liu X, et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2978–2983. doi: 10.1073/pnas.1418494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Liu C, Zhou J, Chen C, Qu F, Rossi JJ, et al. Promoting siRNA delivery via enhanced cellular uptake using an arginine-decorated amphiphilic dendrimer. Nanoscale. 2015;7:3867–3875. doi: 10.1039/c4nr04759a. [DOI] [PubMed] [Google Scholar]
- 21.Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. Journal of internal medicine. 2014 doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 22.Burd I, Zhang F, Dada T, Mishra MK, Borbiev T, Lesniak WG, et al. Fetal uptake of intra-amniotically delivered dendrimers in a mouse model of intrauterine inflammation and preterm birth. Nanomedicine-Uk. 2014 doi: 10.1016/j.nano.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Wan TC, Tosh DK, Du L, Gizewski ET, Jacobson KA, Auchampach JA. Polyamidoamine (PAMAM) dendrimer conjugate specifically activates the A3 adenosine receptor to improve post-ischemic/reperfusion function in isolated mouse hearts. BMC pharmacology. 2011;11:11. doi: 10.1186/1471-2210-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson TA, Stasko NA, Matthews JL, Cascio WE, Holmuhamedov EL, Johnson CB, et al. Reduced ischemia/reperfusion injury via glutathione-initiated nitric oxide-releasing dendrimers. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2010;22:30–36. doi: 10.1016/j.niox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS nano. 2014;8:2134–2147. doi: 10.1021/nn404872e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra19. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nance E, Zhang C, Shih TY, Xu Q, Schuster BS, Hanes J. Brain-Penetrating Nanoparticles Improve Paclitaxel Efficacy in Malignant Glioma Following Local Administration. ACS nano. 2014 doi: 10.1021/nn504210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann Neurol. 2014;75:469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falahati S, Breu M, Waickman AT, Phillips AW, Arauz EJ, Snyder S, et al. Ischemia-induced neuroinflammation is associated with disrupted development of oligodendrocyte progenitors in a model of periventricular leukomalacia. Developmental neuroscience. 2013;35:182–196. doi: 10.1159/000346682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navath RS, Kurtoglu YE, Wang B, Kannan S, Romero R, Kannan RM. Dendrimer-drug conjugates for tailored intracellular drug release based on glutathione levels. Bioconjugate chemistry. 2008;19:2446–2455. doi: 10.1021/bc800342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R, Kannan RM. Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials. 2009;30:2112–2121. doi: 10.1016/j.biomaterials.2008.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porambo M, A WP, Marx J, Ternes K, Arauz E, Pletnikov M, et al. Transplanted glial restricted precursor cells improve neurobehavioral and neuropathological outcomes in a mouse model of neonatal white matter injury despite limited cell survival. Glia. 2014 doi: 10.1002/glia.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesniak WG, Mishra MK, Jyoti A, Balakrishnan B, Zhang F, Nance E, et al. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Molecular pharmaceutics. 2013;10:4560–4571. doi: 10.1021/mp400371r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- 36.Jarlestedt K, Rousset CI, Faiz M, Wilhelmsson U, Stahlberg A, Sourkova H, et al. Attenuation of reactive gliosis does not affect infarct volume in neonatal hypoxic-ischemic brain injury in mice. PLoS One. 2010;5:e10397. doi: 10.1371/journal.pone.0010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallard C, Davidson JO, Tan S, Green CR, Bennet L, Robertson NJ, et al. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res. 2014;75:234–240. doi: 10.1038/pr.2013.188. [DOI] [PubMed] [Google Scholar]
- 38.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 39.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Frontiers in pharmacology. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders NR, Joakim EkC, Dziegielewska KM. The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9. Nat Biotechnol. 2009;27:804–805. doi: 10.1038/nbt0909-804. author reply 5. [DOI] [PubMed] [Google Scholar]
- 42.Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, et al. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine (Lond) 2010;5:1317–1329. doi: 10.2217/nnm.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. The Cochrane database of systematic reviews. 2006:CD003328. doi: 10.1002/14651858.CD003328.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Wiest DB, Chang E, Fanning D, Garner S, Cox T, Jenkins DD. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J Pediatr. 2014;165:672 e2–677 e2. doi: 10.1016/j.jpeds.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biological psychiatry. 2012;71:956–961. doi: 10.1016/j.biopsych.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. European journal of clinical pharmacology. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 48.Olney JW, Zorumski C, Price MT, Labruyere J. L-cysteine, a bicarbonate-sensitive endogenous excitotoxin. Science. 1990;248:596–599. doi: 10.1126/science.2185543. [DOI] [PubMed] [Google Scholar]
- 49.Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, et al. Development of microglia in the cerebral white matter of the human fetus and infant. The Journal of comparative neurology. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 50.Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nature clinical practice Neurology. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]