Abstract
The circadian clock controls a large variety of neuronal, endocrine, behavioral and physiological responses in mammals. This control is exerted in large part at the transcriptional level on genes expressed in a cyclic manner. A highly specialized transcriptional machinery based on clock regulatory factors organized in feedback autoregulatory loops governs a significant portion of the genome. These oscillations in gene expression are paralleled by critical events of chromatin remodeling that appear to provide plasticity to circadian regulation. Specifically, the NAD+-dependent deacetylases SIRT1 and SIRT6 have been linked to circadian control of gene expression. This, and additional accumulating evidence, shows that the circadian epigenome appears to share intimate links with cellular metabolic processes and has remarkable plasticity showing reprogramming in response to nutritional challenges. In addition to SIRT1 and SIRT6, a number of chromatin remodelers have been implicated in clock control, including the histone H3K4 tri-methyltransferase MLL1. Deciphering the molecular mechanisms that link metabolism, epigenetic control and circadian responses will provide valauble insights towards innovative strategies of therapeutic intervention.
Keywords: Epigenetics, Chromatin Remodeling, Sirtuins, NAD+, Nutrition
INTRODUCTION
Many aspects of metabolism, homeostatic balance and behavior follow the 24h daily cycle [1]. Circadian rhythms are virtually present in all life forms on our planet, including mammals, insects, plants, fungi and cyanobacteria. In higher organisms, circadian rhythms have evolved into a complex physiological and molecular system demonstrated by sleep-wake cycles, daily fluctuations in body temperature, blood pressure, cellular regeneration and behavior such as food intake and alertness levels [2]. Metabolism, nutritional intake and body homeostasis are also under circadian control, displaying rhythms in the levels of circulating hormones and metabolites, as well as enzymes within the biochemical pathways participating in their biosynthesis [1,3]. Circadian rhythms are so intimately linked to biological processes that their misregulation may lead to a number of pathologies such as obesity, metabolic syndrome, diabetes, cardiovascular diseases, inflammation, sleep disorders and some cancers [1].
The molecular bases of circadian rhythms have been explored, revealing a remarkable variety of molecular mechanisms that underlie clock function. An important system of circadian control utilizes the core clock molecular machinery that consists of transcription factors and regulators, both activators and repressors, which act in concert to drive circadian expression of an important fraction of the genome. A number of high-throughput transcriptome profiling studies have established that 15–30% of all transcripts are controlled by the clock, depending on the tissue or cell type [4–7]. Accumulating evidence has shown that this global program of gene expression is achieved through events of cyclic chromatin remodeling and epigenetic control.
LINKING CIRCADIAN TRANSCRIPTION AND CHROMATIN REMODELING
The molecular organization of the circadian system relies on a network of cellular oscillators present in virtually every cell of the organism. An intricate network of transcriptional-translational feedback loops constitutes the molecular clock [1, 8]. The basic helix-loop-helix (b-HLH)-PAS proteins CLOCK and BMAL1 are core elements of this system and function as transcriptional activators to drive the expression of many clock controlled genes (CCGs). CLOCK and BMAL1 heterodimers bind E-boxes in CCGs promoters and activate their expression. Among the CCGs there genes encoding other core clock protein repressors Period (PER1–3) and Cryptochromes (CRY1–2). PER and CRY proteins heterodimerize in the cytoplasm and translocate to the nucleus to inhibit CLOCK:BMAL1-mediated transcription. The stability of PER:CRY complexes is regulated by posttranscriptional modifications [9] and ubiquitination events [10–13]. The time-controlled clearance of the repressors primes for the next cycle of CLOCK:BMAL1-driven gene activation. This system then leads to the cyclic activation of other regulatory pathways generating interconnected transcriptional feedback loops. These provide remarkable plasticity to the circadian system, eliciting multiple daily oscillations in the transcriptome [14].
Specific cyclic chromatin transitions occur on a genome-wide scale and are associated with circadian waves of transcription [14]. Several chromatin remodelers have been found to be involved in circadian control. The protein CLOCK was found to operate as an acetyltransferase on histone H3 at K9 and K14 [15], modifications associated with a chromatin state permissive for transcription. CLOCK acts in concert with other histone acetyltransferases (HATs)[16], such as CBP (CREB binding protein), p300 and with the CBP-associated factor PCAF [17–19]. A number of histone deacetylases (HDACs) have been found to counterbalance these HATs. For example, the circadian repressor PER recruits SIN3A-HDAC1 [20], whereas the protein CRY1 associates with the complex SIN3B-HDAC1/2 [21]. The circadian regulator REV-ERBα recruits the NCoR-HDAC3 complex in a rhythmic manner to chromatin via, a process that has been linked to the control of lipids metabolism in the liver [22,23]. Thus, a variety of circadian repressive complexes appear to exist, which may elicit distinct functions at unique times of the circadian cycle. The nicotinamide adenine dinucleotide (NAD+)-dependent class III of HDACs was found to play a critical role in connecting cellular metabolism to circadian physiology. The founding member, SIRT1, gives the name to this class of enzymes, collectively known as sirtuins. There are seven sirtuins, all involved in various aspects of metabolism, inflammation and aging, and their intracellular localization is nuclear, cytoplasmic or mitochondrial. The nuclear proteins SIRT1 and SIRT6 have been shown to contribute to circadian transcription [24,25].
A number of chromatin post-translational modifications have been linked to clock function in addition to acetylation. The first evidence that a histone modification may play a role in circadian transcription was the light-inducible phosphorylation at H3-S10 in SCN neurons [26]. The activating histone methylation H3K4me3 has also been linked to clock control and it seems to be essential to permit circadian chromatin transitions that lead to activation of CCGs expression [27]. MLL1, a H3K4 histone methyltransferase (HMT), was shown to elicit CLOCK:BMAL1 recruitment to chromatin at specific circadian promoters and for the cyclic tri-methylation at H3K4 [28]. Also the repressive mark H3K27me3 is clock controlled at the Per1 promoter through a mechanism that involves the methyltransferase EZH2 [29]. Additional chromatin remodelers involved in circadian function include the demethylase JARID1a that appears to inhibit HDAC1, thereby enhancing CLOCK:BMAL1-mediated transcription [30], and the FAD (Flavin Adenine Dinucleotide) dependent demethylase LSD1 whose function is controlled by PKCα-mediated circadian phosphorylation [31].
INTIMATE INTERPLAY BETWEEN CELLULAR METABOLISM AND CIRCADIAN CLOCK
A large number of human studies and animal models provide solid evidence of the reciprocal regulation between the circadian clock and cellular and organismal homeostasis [1,32–36]. The clock regulates metabolism by controlling the expression of a large fraction of the genome. Moreover, the oscillator appears to sense the cellular energy state and consequently adapt its function accordingly.
Several levels of interplay exist between cellular metabolism and chromatin remodeling [14,37,38]. Acetylation of histones or non-histone nuclear proteins depends on the supply of acetyl-CoA in the nuclear compartment. The main carbon source in mammals is glucose which generates acetyl-CoA because of the enzyme adenosine triphosphate (ATP)-citrate lyase (ACLY). ACLY protein levels are cyclic in the liver [39], and ACLY activity controls global histone acetylation depending on glucose availability [40]. Thus, circadian changes in histone acetylation are controlled not only by specific HATs, but also by interconnected metabolic pathways and enzymes supplying nuclear acetyl-CoA. A similar regulation involves S-adenosyl methionine (SAM), the metabolite used by methyltransferases to deliver methyl groups. Changing SAM levels directly influence H3K4me3 levels in mouse pluripotent stem cells [41]. Also, treatment with 3-deazaadenosine (DAA), an inhibitor of SAH (S-adenosylhomocysteine) hydrolysis that hinders transmethylation, elongates the circadian period [42]. Further research is necessary to decipher the impact of one carbon metabolism in the circadian transcriptome.
Nicotinamide adenine dinucleotide (NAD+) is a pivotal metabolite for the circadian epigenome. NAD+ shows robust diurnal rhythms in synchronized cells and mice [43–45] and operates as cofactor for class III of HDACs, the sirtuins (see next section).
The core machinery may be directly influenced by changing metabolic states. Specifically, the DNA-binding function of NPAS2:BMAL1 and CLOCK:BMAL1 heterodimers was indicated to be influenced by the redox states of NAD(H) or NADP(H)(46). This finding implied that CLOCK:BMAL1 transcriptional activity should be sensitive to the levels of cellular redox. While a causal evidence for this regulation has not been explored, circadian oscillations in intracellular redox potentials are evolutionarily conserved [1,2]. Thus, while the ability of NPAS2 or CLOCK to sense the intracellular redox state in vivo remains to be proven, independent evidence provides interesting information. Indeed, crystallographic analyses of the CRY1-PER2 complex indicate that a disulfide bond between two cysteine residues in CRY1 weakens its interaction with PER2, whereas a reduced state of CRY1 stabilizes the complex and facilitates transcriptional repression [47]. In this scenario, CRY2 would retain specific FAD (Flavin Adenine Dinucleotide) binding activity, and FAD competes for CRY2 binding pocket with the ubiquitin ligase complex SCFFBXL3, that has been shown to control period length by regulating CRYs stability [48]. Interestingly, this finding provides a possible approach to pharmacologically adjust circadian period length by using small molecules resembling FAD [49].
Posttranslational modifications of clock proteins have been shown to modify their regulatory capaciity. For example, CLOCK, BMAL1 and PER2 can be O-linked N-acetylglucosamine (GlcNAc)-modified by the enzyme O-GlcNAc transferase (OGT), which results in a change in their activities [50,51]. Importantly, liver-specific ablation of OGT leads to dampened oscillation of Bmal1 and gluconeogenic genes. Thus, glucose levels dictate the availability of GlcNAc, being OGT a signal transducer between cellular metabolism and circadian components. Along the same lines, phosphorylation of CRY1 by the nutrient sensor kinase AMPK (AMP-activated protein kinase) connects cellular energy levels with the circadian clock by adjusting it to the changing intracellular ratio of AMP/ATP [52,53].
SIRTUINS, CIRCADIAN RHEOSTATS
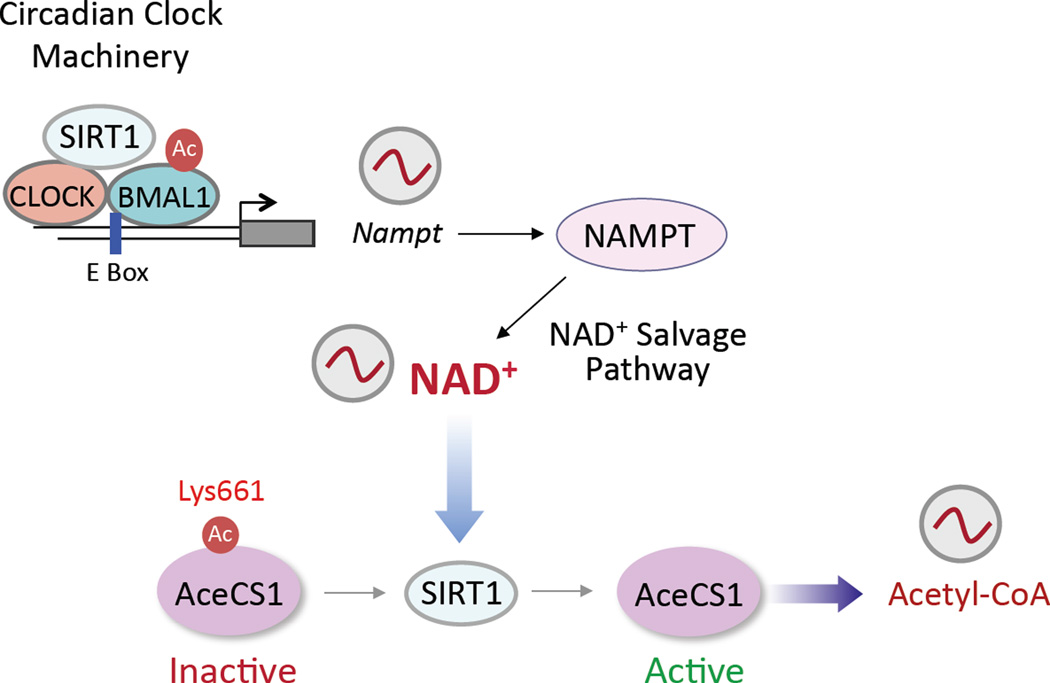
The intracellular availability in time and space of specific metabolites constitutes an intriguing level of control for their protein sensors [38]. In this respect, the circadian oscillation in NAD+ concentration represents a revealing paradigm. The NAD+ biosynthetic salvage pathway controls the conversion of nicotinamide (NAM) to β-nicotinamide mononucleotide (NMN); this step is catalyzed by rate limiting step enzyme, the nicotinamide phosphoribosyltransferase (NAMPT, also known as visfatin). The circadian machinery controls the transcription of the Nampt gene through direct binding of CLOCK:BMAL1 to E-boxes in the promoter [44,45]. NMN is converted to NAD+ by the enzymes nicotinamide mononucleotide adenylyltransferase 1–3 (NMNAT1–3) (Figure 1). Thus, a transcriptional-enzymatic feedback loop controls NAD+ biosynthesis and availability that in turn could result in circadian function of a variety of NAD+-dependent enzymes. Moreover, there is a differential regulation of NAD+ levels and NAD+ consuming enzymes in various cell compartments [54,55]. In this respect the sirtuins deserve special attention. Indeed, of the seven mammalian sirtuins, three (SIRT1, SIRT3 and SIRT6) have been functionally linked to circadian control and found to modulate cyclic outputs in response to metabolic cues.
Figure 1.
Metabolism and the circadian clock converge. A paradigm example is represented by the role of SIRT1 and other sirtuins in clock regulation. The circadian machinery controls a large fraction of the genome through the transcriptional regulation of CCGs. One of the CCGs is the gene encoding the protein NAMPT, the rate-limiting enzyme in the NAD+-salvage pathway. Cyclic transcriptional control of the Nampt gene results in the cyclic synthesis of NAD+, which in turn is consumed rhythmically by enzymes, such as SIRT1 whose deacetylase activity is consequently cyclic. One of the non-histone targets is the enzyme AceCS1, which contributes to the synthesis of Acetyl-CoA. AceCS1 is acetylated at one residue, Lys661, and its cyclic deacetylation by SIRT1 activates the enzyme, resulting in cyclic synthesis of Acetyl-CoA and thereby oscillating availability of acetyl groups required for global acetylation.
SIRT3 is a mitochondrial enzyme that displays robust changes in its deacetylase activity in response to NAD+ levels [56–58]. SIRT3 controls mitochondrial function, including fatty acid oxidation and intermediary metabolism, by directly targeting rate-limiting enzymes for mitochondrial biochemical processes [57]. As mitochondrial fatty acid oxidation and protein acetylation show circadian rhythmicity [58], the link with NAD+ availability through SIRT3 is of particular interest. Also, mitochondria from Bmal1−/− mice display reduced oxidative ability and decreased mitochondrial NAD+ levels [57]. These findings, together with the implication of SIRT1 in circadian control, raise the possibility that the sirtuins-NAD+ link with the clock may represent a critical molecular pathway to govern the process of aging.
The implication of nuclear sirtuins in clock function is multiple. SIRT1 is both nuclear and cytoplasmic whereas SIRT6 is exclusively nuclear and mostly chromatin bound, localized at transcriptionally active genomic loci. SIRT1 and SIRT6 operate through distinct mechanisms to coordinate the clock machinery in a differential manner and thereby delineate the circadian transcriptional output [25]. Because of these different mechanisms of action, in the liver these two sirtuins coordinate circadian expression of distinct groups of genes. SIRT6 exerts its function by coordinating CLOCK:BMAL1 recruitment to specific chromatin sites [25]. SIRT1, which is mostly nucleoplasmic and is recruited to chromatin only ‘on demand’, deacetylates histones and non-histone proteins. Among the non-histone targets of SIRT1 there are the clock proteins BMAL1 and PER2 [59,60]. SIRT1 is also able to deacetylate MLL1, thereby controlling its methyltransferase activity. Thus, there is control in H3K4 tri-methylation through the cyclic oscillation of NAD+ levels [61].
SIRT1-mediated deacetylation also affects circadian levels of other metabolites besides NAD+. Specifically, intracellular acetyl-CoA levels are controlled by the clock through SIRT1-controlled deacetylation of the enzyme acetyl-CoA Synthetase 1 (AceCS1)[62]. This acetylation switch controls AceCS1 activity leading to cyclic synthesis of acetyl-CoA (Figure 1), that then is likely to influence the acetylation levels of histones and non-histone proteins [62]. In contrast, SIRT6 deacetylase activity seems to be efficient in removing long chain fatty acids from lysine residues [63]. In this respect it is noteworthy that not only on NAD+, but also on fatty acids, control the activity of SIRT6 [64]. Thus, SIRT6 appears to occupy a key position in the control of fatty acids metabolism by the clock. Indeed, CLOCK:BMAL1-driven activation of genes involved in fatty acid biosynthesis is modulated by SIRT6 [25].
High-throughput analysis of the transcriptome and metabolome along the circadian cycle has revealed notable differences in the metabolic functions of SIRT1 and SIRT6. Using mice with liver-specific deletion of either SIRT1 or SIRT6, a specific role for SIRT6 was shown in dictating the synthesis and breakdown of fatty acid pathways, as well as their storage into triglycerides. SIRT6 operates at least in part through the control of alternative circadian transcriptional pathways, specifically because of the chromatin recruitment of the sterol regulatory element-binding protein 1 (SREBP1) [25]. Thus, it is through genomic partitioning which the two deacetylases contribute to a parallel segregation of cellular metabolism [25].
Finally, these findings suggest a role for genome topology in circadian control [65]. Our studies have identified the presence of circadian interactomes where co-regulated genes are physically associated in the circadian epigenome. Nuclear sirtuins may constitute a paradigm for other chromatin remodelers that could contribute in the cyclic control of the nuclear landscape. Also, specific changes in the nuclear localization of NAD+ may provide the possibility of restricting the distribution of this metabolite to “niches” of activity [38].
CONCLUSION
The ability of the circadian clock machinery for sensing the metabolic state of the cell in a time-specific manner places it in a strategic position. Indeed, a fascinating findings reviewed in this article demonstrate the direct implication of the clock in the maintenance of cellular homeostasis. The clock machinery appears to integrate environmental and metabolic signals to directly translate them in plasticity in gene expression so to favor the adaptation of the organism to specific conditions. As the circadian transcriptional landscape is highly complex, including dynamic changes in nuclear organization [38,65], it becomes critical to decipher how the nuclear landscape integrates metabolic cues and shapes the transcriptional output. It is through the analysis of the specific coordination that key chromatin remodelers have with clock transcription factors that we will gain insights on how intracellular metabolic state communicates to the clock machinery. As disruption of clock function has been linked to a variety of pathological conditions, unrevealing clock mechanisms will lead to innovative strategies towards the pharmacological treatment of metabolic syndromes, obesity, diabetes, inflammation and even cancer.
ACKNOWLEDGEMENTS
We thank all the members of the Sassone-Corsi laboratory for discussions and insights. Studies in the Center for Epigenetics and Metabolism are supported by the National Institute of Health, Merieux Research Grants and INSERM (Institut National de la Sante et Recherche Medicale, France).
Footnotes
The authors declare no conflicts of interest
CALLOUTS
The circadian epigenome appears to share intimate links with cellular metabolic processes and has remarkable plasticity showing reprogramming in response to nutritional challenges.
Circadian rhythms are so intimately linked to biological processes that their misregulation may lead to a number of pathologies such as obesity, metabolic syndrome, diabetes, cardiovascular diseases, inflammation, sleep disorders and some cancers.
The intracellular availability in time and space of specific metabolites constitutes an intriguing level of control for their protein sensors. In this respect, the circadian oscillation in NAD+ concentration represents a revealing paradigm.
Thus, it is through genomic partitioning which the two deacetylases contribute to a parallel segregation of cellular metabolism.
As the circadian transcriptional landscape is highly complex, including dynamic changes in nuclear organization it becomes critical to decipher how the nuclear landscape integrates metabolic cues and shapes the transcriptional output.
REFERENCES
- 1.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Gamble KL, Berry R, Frank SJ, Young ME. Circadian clock control of endocrine factors. Nat Rev Endocrinol. 2014;10:466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 5.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 6.Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 7.Ueda HR, Chen W, Adachi A, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 8.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Chen R, Lee Y, Yoo S, Lee C. Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc Natl Acad Sci U S A. 2009;106:21359–21364. doi: 10.1073/pnas.0906651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busino L, Bassermann F, Maiolica A, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 11.Hirano A, Yumimoto K, Tsunematsu R, et al. FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell. 2013;152:1106–1118. doi: 10.1016/j.cell.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Siepka SM, Yoo SH, Park J, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo SH, Mohawk JA, Siepka SM, et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 2013;152:1091–1105. doi: 10.1016/j.cell.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masri S, Sassone-Corsi P. Plasticity and specificity of the circadian epigenome. Nat Neurosci. 2010;13:1324–1329. doi: 10.1038/nn.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, Lee J, Kwon I, et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci. 2010;123:3547–3557. doi: 10.1242/jcs.070300. [DOI] [PubMed] [Google Scholar]
- 18.Curtis AM, Seo SB, Westgate EJ, et al. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 19.Takahata S, Ozaki T, Mimura J, Kikuchi Y, Sogawa K, Fujii-Kuriyama Y. Transactivation mechanisms of mouse clock transcription factors, mClock and mArnt3. Genes Cells. 2000;5:739–747. doi: 10.1046/j.1365-2443.2000.00363.x. [DOI] [PubMed] [Google Scholar]
- 20.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–1439. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol Cell Biol. 2004;24:6278–6287. doi: 10.1128/MCB.24.14.6278-6287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng D, Liu T, Sun Z, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Z, Feng D, Everett LJ, Bugge A, Lazar MA. Circadian epigenomic remodeling and hepatic lipogenesis: lessons from HDAC3. Cold Spring Harb Symp Quant Biol. 2011;76:49–55. doi: 10.1101/sqb.2011.76.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masri S, Rigor P, Cervantes M, et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158:659–672. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- 27.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 28.Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etchegaray JP, Yang X, DeBruyne JP, et al. The polycomb group protein EZH2 is required for mammalian circadian clock function. J Biol Chem. 2006;281:21209–21215. doi: 10.1074/jbc.M603722200. [DOI] [PubMed] [Google Scholar]
- 30.DiTacchio L, Le HD, Vollmers C, et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333:1881–1885. doi: 10.1126/science.1206022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam HJ, Boo K, Kim D, et al. Phosphorylation of LSD1 by PKCalpha is crucial for circadian rhythmicity and phase resetting. Mol Cell. 2014;53:791–805. doi: 10.1016/j.molcel.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–2629. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci U S A. 2012;109:5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasukawa T, Sugimoto M, Hida A, et al. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci U S A. 2012;109:15036–15041. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell. 2012;47:158–167. doi: 10.1016/j.molcel.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148:24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Mauvoisin D, Wang J, Jouffe C, et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci U S A. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shyh-Chang N, Locasale JW, Lyssiotis CA, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fustin JM, Doi M, Yamaguchi Y, et al. RNA-methylation dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Bellet MM, Nakahata Y, Boudjelal M, et al. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc Natl Acad Sci U S A. 2013;110:3333–3338. doi: 10.1073/pnas.1214266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 47.Schmalen I, Reischl S, Wallach T, et al. Interaction of circadian clock proteins CRY1 and PER2 is modulated by zinc binding and disulfide bond formation. Cell. 2014;157:1203–1215. doi: 10.1016/j.cell.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 48.Xing W, Busino L, Hinds TR, et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature. 2013;496:64–68. doi: 10.1038/nature11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirota T, Lee JW, StJohn PC, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaasik K, Kivimae S, Allen JJ, et al. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 2013;17:291–302. doi: 10.1016/j.cmet.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li MD, Ruan HB, Hughes ME, et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17:303–310. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol. 2013;366:163–169. doi: 10.1016/j.mce.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes AP, Price NL, Ling AJ, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Yang T, Baur JA, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebert AS, Dittenhafer-Reed KE, Yu W, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peek CB, Affinati AH, Ramsey KM, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masri S, Patel VR, Eckel-Mahan KL, et al. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci U S A. 2013;110:3339–3344. doi: 10.1073/pnas.1217632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asher G, Gatfiekd D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 60.Hirayama J, Sahar S, Grimaldi B, et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 61.Aguilar-Arnal L, Katada S, Orozco-Solis R, Sassone-Corsi P. NAD+-SIRT1 control of H3K4 trimethylation through circadian deacetylation of MLL1. Nat Struct Mol Biol. 2015;22:312–318. doi: 10.1038/nsmb.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahar S, Masubuchi S, Eckel-Mahan KL, et al. Circadian control of fatty acid elongation by SIRT1 protein-mediated deacetylation of acetyl-coenzyme A synthetase 1. J Biol Chem. 2014;289:6091–6097. doi: 10.1074/jbc.M113.537191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang H, Khan S, Wang Y, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288:31350–31356. doi: 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nature Struct Mol Biol. 2013;20:1206–1213. doi: 10.1038/nsmb.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]