Abstract
Background
Cortical stimulation (CS) combined with rehabilitative training (RT) has proven effective for enhancing post-stroke functional recovery in rats, but human clinical trials have had mixed outcomes.
Objective
To assess the efficacy of CS/RT versus RT in a non-human primate model of cortical ischemic stroke.
Methods
Squirrel monkeys learned a pellet retrieval task, then received an infarct to the distal forelimb (DFL) representation of primary motor cortex. A subdural monopolar electrode was implanted over the spared DFL representation in dorsal premotor cortex (PMD). Seven weeks post-infarct, monkeys underwent 4-6 weeks of RT (n=8) or CS/RT (n=7; 100 Hz, cathodal current) therapy. Behavioral performance was assessed before and after infarct, prior to therapy, and 1 and 12 weeks post-therapy (follow-up). The primary outcome measure was motor performance at 1 week post-therapy. Secondary outcomes included follow-up performance at 12 weeks and treatment-related changes in neurophysiological maps of spared DFL representations.
Results
While post-infarct performance deficits were found in all monkeys, both groups demonstrated similar recovery profiles, with no difference in motor recovery between the RT and CS/RT groups. Post-therapy, PMD DFL area was significantly expanded in the RT group but not the CS/RT group. A significant relationship was found between motor recovery and DFL expansion in premotor cortex.
Conclusions
Results suggest that the specific parameters utilized here were not optimal for promoting behavioral recovery in non-human primates. Though CS/RT has consistently shown efficacy in rat stroke models, the present finding has cautionary implications for translation of CS/RT therapy to clinical populations.
Keywords: implanted stimulation electrodes, primate, primary motor cortex, premotor cortex, neuronal plasticity
INTRODUCTION
Partial but incomplete functional recovery after stroke is common despite conventional therapy, resulting in chronic impairments, increased cost of care, and reduced quality of life1. As such, the need exists to develop new or enhanced approaches to improve the treatment of motor impairments in the chronic phase of stroke recovery.
The brain’s innate capacity for neural plasticity, specifically in the motor cortex, has been implicated in the acquisition of motor skills during learning and the reacquisition of motor skills following cortical ischemic injury2,3. Several therapeutic approaches designed to harness this endogenous mechanism have been investigated in recent years4-10. One such method is cortical stimulation (CS), applied either epidurally or subdurally. The general premise of CS is to pair subthreshold electrical stimulation of the intact, post-stroke motor cortex with rehabilitative training (RT), a combined treatment herein referred to as CS/RT, to enhance plasticity mechanisms responsible for mediating recovery of function. The validity of this approach to treating chronic post-stroke deficits was supported by several initial studies in rats using combined CS/RT treatment to enhance recovery on forelimb reaching tasks after cortical ischemic injury11-15 and by a series of early-phase clinical trials in human stroke survivors that demonstrated improvements on UEFM, SIS, and AMAT functional scores after CS/RT treatment16-18. However, results of a large scale trial of CS/RT in human stroke survivors showed no overall benefit for functional recovery19,20, suggesting a need for additional studies to investigate this interventional therapy.
In our own initial studies14, adult squirrel monkeys experienced an ischemic infarct to the distal forelimb (DFL) area in primary motor cortex (M1), and subsequently were implanted with a bipolar electrode on the cortical surface that spanned the spared cortex medial and rostral to the M1 infarct, which included the proximal forelimb representation in M1 and at least some of the forelimb (distal and proximal) representation in dorsal premotor cortex (PMD). Monkeys underwent several weeks of CS/RT treatment with bipolar current at a 50 Hz stimulation frequency. Behavioral performance showed significant, though incomplete, recovery, and motor maps beneath the electrode showed significant expansion of DFL representations. Performance gains persisted for up to four months. Taken together, these results indicated that CS/RT was a viable therapeutic technique after cortical stroke in non-human primates. Importantly however, this initial report was a feasibility study and did not include an RT-only control group.
In the present study, we employed a translational approach to assess the efficacy of CS/RT versus RT in a non-human primate model by adopting CS parameters found to be optimal in rat studies11,13,15. In particular, CS was found to be most effective for enhancing behavioral recovery in rats by using a monopolar electrode to deliver cathodal current at a 100 Hz stimulation frequency to motor cortex during RT (contrast with 50 Hz bipolar stimulation used in our earlier feasibility study in monkeys14). In addition, compared to our earlier study14, larger M1 infarcts were produced (an attempt to produce more severe deficits), the location of the cortical electrode was shifted rostrally to predominantly target PMD (to emphasize the role of a single, intact forelimb motor region for promoting recovery), and we have now examined treatment efficacy by comparing experimental (CS/RT) and control (RT) therapy conditions. We hypothesized that the use of optimized treatment parameters would enhance behavioral gains in the CS/RT group compared to the RT group and produce an expansion of DFL representations in the stimulated cortical tissue.
METHODS
Subjects
Fifteen adult squirrel monkeys (genus Saimiri, 10 male, 5 female; birth dates unknown, veterinary estimates ranged between 3-10 years at start of experiment) were used. Monkeys were experimentally-naïve and free of obvious physical and neurological problems at study initiation. Five squirrel monkeys (two from the cohort above along with three others) participated in additional experiments to evaluate technical aspects of the monopolar CS method; results of these studies informed the final methods design. All procedures were conducted in accordance with federal guidelines for the care and use of laboratory animals and with approval of the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Experimental Design
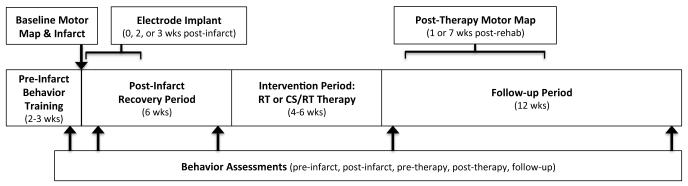
Figure 1 illustrates the experimental timeline. After determination of hand preference on a pellet reach-and-retrieval task (Kluver board)21,22, monkeys were fitted with a short-sleeved, torso-covering, nylon mesh primate jacket. Monkeys underwent training on the pellet retrieval task for two weeks, followed by a surgical procedure during which functional regions of M1, PMD, and ventral premotor cortex (PMV) were identified via intracortical microstimulation (ICMS) mapping14,22,23. In the same procedure, an ischemic infarct was produced in the M1 DFL representation14,24,25. After infarct, in either the same procedure (n=2) or in a separate procedure 2-3 weeks later (n=13), a monopolar electrode was implanted over spared DFL areas in premotor cortex. This variation in timing of electrode implantation was guided by veterinary consultation regarding the impact of the multiple survival surgery schedule on the overall health and well-being of the monkeys.
Figure 1.
Summary of experiment timeline highlighting key procedural elements. The middle series of boxes delineate major intervals (pre-infarct, post-infarct, therapy, follow-up). The top boxes, arrows, and brackets indicate the timing of surgical procedures (motor mapping, ischemic infarct, electrode implantation); brackets reflect timing variations for electrode implantation and post-therapy motor mapping (see Methods). The bottom box and arrows indicates the timing of behavior assessments reported in the manuscript.
Post-infarct motor deficits were monitored during a 6-week recovery period. This timing was chosen to model the expected clinical treatment paradigm in which human stroke survivors would enroll into CS/RT treatment programs secondary to an earlier post-stroke recovery period17,18,26; prior results suggested monkeys would have sufficient residual deficits on the pellet retrieval task at six weeks post-infarct to enable meaningful comparisons between treatment groups27. At 7 weeks post-infarct, monkeys were randomly assigned to either the RT therapy group (rehabilitative training for 4-6 weeks; n=8; 4 male, 4 female) or the CS/RT therapy group (cortical electrical stimulation concurrent with 4-6 weeks of RT; n=7; 6 male, 1 female). At the conclusion of the therapy period, task performance was monitored for 12 weeks to assess retention of performance gains. Within this follow-up period, monkeys underwent a surgical procedure at 1 week (n=5; RT=3, CS/RT=2) or 7 weeks (n=9; RT=4, CS/RT=5) post-therapy to remove the electrode and re-map DFL representations in M1, PMD, and PMV. Since only limited data was available when the present study was designed to suggest that post-therapy retention of performance gains would not be dependent on ongoing CS treatment (retention for 2 days post-CS/RT in rats12; up to 4 months in monkeys but in only 3 subjects14), the variation in post-therapy surgical timing was planned in anticipation of investigating neurophysiological changes associated with a potential relapse of behavioral performance in either group. One RT monkey died prior to the second mapping procedure (missing post-therapy map data and 12 week follow-up behavior data). A technology failure caused behavior video from two RT monkeys to be lost after experiment completion but prior to analysis of behavioral performance (behavior data missing, but mapping data present). For the 15 monkeys described here, complete behavior results were available for 12 monkeys (5 RT, 7 CS/RT) and ICMS mapping results were available for 14 monkeys (7 RT, 7 CS/RT).
Behavior Training
Skilled motor behavior was assessed using a modified Kluver board task22,23,28. In brief, food pellets (45mg, Bioserve) were placed individually into one of five wells for the monkey to retrieve and eat. The wells ranged in diameter from 25 mm (larger than the width of the monkey’s hand) to 9.5 mm (wide enough to insert 1-2 fingers). Success on this task is defined as controlled (i.e., in the grasp of the fingers) removal of the pellet from the food well. Since well depth was identical, retrieval difficulty increased with decreasing well diameter. Normal, uninjured monkeys with no prior task exposure can readily perform the largest wells with few errors, but require training to achieve proficiency with the smaller wells22,23.
Pre-infarct training was conducted in 30-minute sessions, twice per day for 10 days. For the first session, pellets were placed exclusively into the largest well. In subsequent sessions, placements were progressively shifted toward smaller wells, with all placements into the smallest well by the last several training days. Monkeys typically performed 400-500 training trials per day with this regimen. Prior to each training session, “probe” trials were conducted, which consisted of 25-50 trials distributed evenly between each well. Probe trials thus served as a consistent testing mechanism to assess overall performance on the retrieval task, and are the basis of the reported performance data in the present study.
RT sessions were similar to pre-infarct training (twice daily probe trials followed by training trials). Well progression during RT was based on achieving criterion performance at successive stages: monkeys had to achieve at least 80% of their pre-infarct performance level for the tested well for 2 consecutive days. All monkeys met this criterion within 4 days for each well. During the 6-week post-infarct recovery period and the 12-week follow-up period, task performance was assessed 1-3 times per week with a probe test consisting of 50 probe trials (10/well).
In a prior study14, we demonstrated that the time to remove a pellet from the food wells (“dwell time”) exhibited more substantial and persistent deficits after M1 infarct than the number of finger flexions required to remove pellets from the well (“flexions per retrieval”). As such, dwell time values for each well were used as the primary performance data in the present study. To facilitate comparisons between monkeys that could have a range of task skill levels after the pre-infarct training protocol, a motor performance index14,27 was calculated as follows. The average dwell time values from each test well were calculated from the final five days of pre-infarct training, then used to normalize performance for the experimental periods listed below, such that an index of 1.0 is equivalent to the 5-day pre-infarct average, 2.0 is double the pre-infarct average, etc. It is possible for individual monkeys to have a performance index less than 1.0, indicating performance better than pre-injury levels.
Five timepoints were pre-selected as the primary endpoints to assess changes in motor performance: 1) “pre-infarct” (the final 3 days of probe trials prior to ischemic injury), 2) “post-infarct” (probe trials during the first week after injury), 3) “pre-therapy” (probe trials during week 6 of the recovery period), 4) “post-therapy” (probe trials during the first week after the therapy period), and 5) “follow-up” (probe trials during the final week of the follow-up period).
Surgical Procedures
Surgical and neurophysiological mapping methods were similar to previous reports14,21-23,29. In brief, monkeys were sedated with ketamine (20 mg/kg), followed by Isoflurane anesthesia (1-2%, with 70% nitrous oxide, 30% oxygen) during surgical procedures. Using aseptic techniques, a craniotomy was performed to expose motor cortex in the hemisphere opposite the monkey’s preferred forelimb on the pellet retrieval task. The dura was excised and a plastic cylinder was secured around the cranial opening with dental acrylic and filled with sterile silicone oil to prevent tissue desiccation. Gas anesthesia was discontinued and ketamine (15-20 mg/kg/hr) supplemented with diazepam (~0.01 mg/kg/hr) was administered during the neurophysiological mapping procedure. At the conclusion of mapping, gas anesthesia was reinstated, the plastic chamber removed, and the craniotomy was closed using a layered combination of silicone sheeting (as dura replacement), gelfoam (as space filler), and dental acrylic (as skull replacement).
After completion of the first mapping procedure, and prior to surgical closing, the ischemic infarct was created (see below). All monkeys in both groups also underwent implantation of the monopolar electrode system (see below).
Neurophysiological Mapping of Motor Cortex Using ICMS
Functional maps of DFL movement representations in M1, PMD, and PMV were derived prior to infarct (baseline map) and after the intervention period (post-therapy map) using ICMS21,22. In brief, guided by a scaled digital image of the cortical surface, a sharp beveled glass micropipette (10-25μm o.d., filled with 3.5M saline) was used to deliver high frequency low amplitude electrical stimuli to layer V neurons (thirteen 200μs pulses, 3.3ms interval, 1Hz repeat, 0-30μA, 1750μm depth). The objective was to identify the extent of digit, wrist, and forearm representations (collectively, the DFL representation) in each of the three cortical regions (Figure 2). Cortical territory containing representations of other parts of the body, such as the proximal forelimb, torso, face, and hindlimb, were extensively explored to ensure all DFL locations were identified at both mapping timepoints. The total extent of cortical mapping area was typically ~95-105 mm2, based on 250-350 ICMS sites/map, using a 500μm inter-penetration distance in M1 and PMD and a 350μm inter-penetration distance in PMV.
Figure 2.
Left. Representative baseline motor map from one CS/RT monkey illustrating DFL and surrounding non-DFL representations in primary motor (M1), dorsal premotor (PMD), and ventral premotor (PMV) cortices, overlaid onto an image of the surgically exposed cortical surface. Colors represent different movement types evoked by ICMS mapping: DFL movements (red = finger or thumb, green = wrist or forearm, yellow = digit + wrist/forearm, light blue = digit + elbow/shoulder (labeled “Prox” as abbreviation for “proximal forelimb”), magenta = wrist/forearm + elbow/shoulder; the latter three colors are dual-response sites at which two movements were evoked within 2 μA of each other22,23), non-DFL movements (dark blue; e.g., elbow, shoulder, torso, face, hindlimb), and non-responsive (black). The white dashed line indicates the infarct target in this monkey, based on the location of M1 DFL responses and constraints imposed by vascular patterns. Top Right. Image of the cortical surface (from left panel) immediately after infarct creation. Surface arteries and veins within the target region have been occluded by bipolar electrocoagulation, producing a blanched appearance indicative of the absence of blood flow. Note the difference between blanched tissue and white areas due to reflective glare (dashed arrows). Bottom Right. The monopolar electrode developed by Northstar Neuroscience. The exposed portion of the cortical contact was placed on the pial surface overlying the spared PMD DFL area (black circle in left and top right panels) and secured in place during surgical closing. The return electrode was subcutaneously implanted in the mid-torso to complete the electrical circuit.
Ischemic Infarct
Bipolar electrocoagulation of surface vasculature was used to produce the ischemic infarct14,24,30. The baseline M1 DFL map was used to determine the cortical territory subjected to a permanent ischemic infarct, with the goal of targeting all of the M1 DFL area while limiting the extent of injury to other representations, notably adjacent proximal forelimb areas and nearby PMD DFL areas. All arterial and venous vessels and vessel branches within the target region were occluded, with an exception for the main “trunk” of large pass-through vessels that supplied other cortical territories. Vessels were monitored for 10 minutes for signs of reperfusion, and re-occluded as necessary. A digital image of the infarct tissue was captured to document and verify the extent of the target region (Figure 2). This infarct technique produces an injury with sharp borders that extends through all cortical layers but spares the underlying white matter24,29; as such, it produces a selective injury that is ideal for examining functional plasticity in spared tissue. Since the duration between infarct creation and final follow-up behavior testing was 22-24 weeks in each monkey, calculation of lesion volume based on histological data is unreliable29. Instead, the size of the infarct (in mm2) was calculated from the scaled image of the pre-infarct blood vessles compared to the post-infarct blood vessels (Figure 2). By superimposing ICMS maps of motor representations, this allows us to describe the infarct size in terms of the amount of loss of the M1 DFL representation14,24,25,27,31,32.
Electrode Implantation and Cortical Stimulation (CS)
All monkeys were implanted with a monopolar electrode system (developed by Northstar Neuroscience, Seattle, WA) consisting of a cortical surface electrode with a single (monopolar) 4mm diameter circular contact and a separate “return” electrode with three 4.5mm diameter circular contacts. Wire leads connected the electrodes to a portable battery-powered stimulator unit.
In the CS/RT group, the monopolar electrode was placed over the PMD DFL, with overlap onto adjacent proximal forelimb (elbow and shoulder) representations due to the size of the contact area (Figure 2). In the RT group, similar electrode placement was conducted over either PMD DFL (n=6) or PMV DFL (n=2), but no therapy stimulation was conducted in these animals. In all monkeys, the return electrode was implanted subcutaneously in the mid-to-lower back, 1-2 cm lateral to the spine, on the same side of the body as the implanted cortical hemisphere, with the contacts facing the underside of the skin. Wire leads from both the cortical and return electrodes were tunneled subcutaneously to a common exit incision in the mid-back and connected to the stimulator unit worn in a backpack-like pocket of the primate jacket. The lead connections were arranged to deliver cathodal current via the cortical electrode.
The CS-evoked movement threshold (MT) for each monkey in the CS/RT group was determined prior to RT initiation. Individual stimuli were triggered remotely (via computer and wireless controller) and evoked movements were observed while monkeys were at rest in their home cages. MT was determined by delivering a burst of cathodal pulses at 100 Hz for 1 second. Each pulse was charge balanced but asymmetrically biphasic, with an active-driven 100μs square phase followed by a passive 9900μs decaying exponential phase. Starting at a level below the expected MT, the amplitude of MT test bursts was gradually increased in 0.12 mA steps until a motor response was detected visually, then reduced in 0.06 mA steps until the response was unobservable (0.06 mA was the minimum step level possible for this stimulation system). The interval between each MT test was a minimum of 10 seconds. The lowest current that produced an observed movement was defined as the MT and an amplitude of 50% MT was used during CS therapy12-15. MT was defined twice per week during the RT period and the therapy amplitude was adjusted accordingly.
During CS/RT therapy sessions, continuous 100 Hz pulses (at 50% MT) were delivered for a total duration of 35 minutes; CS was initiated 5 minutes prior to the start of the RT session and remained on for the remainder of the session (30 minutes).
Data Analysis and Study Outcomes
Statistical analysis was performed using Prism 6 (GraphPad Software), with two-tailed hypotheses and alpha = 0.05, and reported/graphed as mean±SEM. Behavioral performance and neurophysiological mapping data were analyzed with two-way repeated measures ANOVA and Bonferroni post-hoc tests. Relationships between behavioral recovery and DFL map areas were examined with correlation analysis. CS movement thresholds were analyzed with one-way repeated measures ANOVA and Bonferroni post-hoc tests. All behavior and mapping data were normally distributed (Kolmogorov-Smirnov test).
The primary outcome measure was motor performance at 1 week post-therapy. Secondary outcome measures included motor performance at follow-up, within-group changes in behavior over the course of the experiment, and changes in DFL representations in M1, PMD, and PMV after therapy. Subgroup statistical analysis revealed no effect on outcomes related to the timing of post-infarct electrode implantation, location of cortical electrode, or post-therapy timing of ICMS mapping; thus, data was pooled and analyzed based only on RT or CS/RT group assignment.
RESULTS
Infarct Size in Baseline M1
For the RT group (n=8), the infarct affected 98.1% of the baseline M1 DFL map, intentionally sparing 0.25±0.09 mm2 of M1 DFL area (Figure 2). For the CS/RT group (n=7), the infarct affected 97.2% of the baseline M1 DFL map, sparing 0.43±0.14 mm2 of M1 DFL area. Additional non-DFL territory included in the infarct region was 8.20±0.86 mm2 in the RT group and 8.35±1.32 mm2 in the CS/RT group. There were no statistical differences between groups for any of these measurements.
Behavior Performance
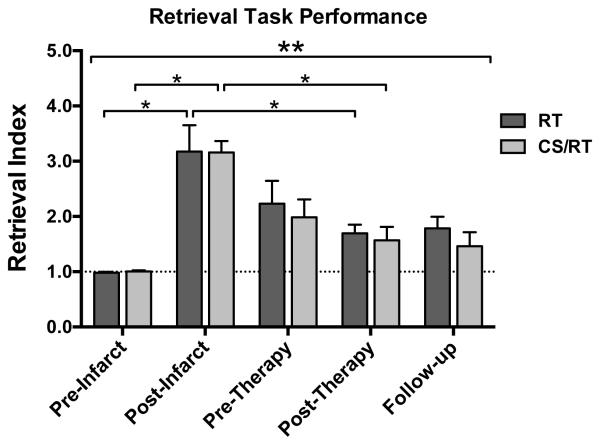
Five timepoints were selected to assess the hypothesis that CS/RT would enhance behavioral performance over RT alone (Figure 3). To facilitate comparisons between monkeys, a motor performance index score was calculated by normalizing each monkey’s pre-infarct performance scores, with pre-infarct equal to 1.0 and larger scores indicating impaired performance (see Methods and 14).
Figure 3.
Behavior performance on the pellet retrieval task for RT (dark gray) and CS/RT (light gray) groups. Both groups exhibited post-infarct impairments (3-fold increase in motor performance index score; post-infarct bars), partial spontaneous recovery by 6 weeks post-infarct (pre-therapy bars), and additional recovery during the intervention period (post-therapy bars). The CS/RT group continued to improve while the RT group regressed slightly during the 12-week follow-up period (follow-up bars). There was a significant effect of Time (ANOVA, double asterisk), and significant within-group effects (single asterisks) for post-infarct impairment (vs. pre-infarct) and post-therapy recovery (vs. post-infarct). There were no significant differences between RT and CS/RT groups at any of the five timepoints.
There was a significant effect of Time on the motor performance index (F4,44=22.66, p<0.0001), demonstrating an effect of the infarct on behavioral performance. However, there were no significant Group or Group × Time interaction effects. Importantly, there was no significant difference in the primary outcome measure comparing behavior recovery in the CS/RT group versus the RT group at 1 week post-therapy.
Each of the 15 monkeys exhibited post-infarct impairments on the retrieval task, with index scores of individual monkeys ranging from 2.05-5.35 during the first week post-infarct. Post-infarct performance for both groups was significantly impaired compared to pre-infarct (RT index score = 3.18±0.48, p<0.0001; CS/RT index score = 3.16±0.21, p<0.0001; Figure 3). Index scores improved for both groups during the 6 weeks prior to therapy initiation (pre-therapy timepoint, RT, 2.23±0.41; CS/RT, 1.98±0.32), consistent with the phenomenon of “spontaneous” recovery. After therapy (post-therapy timepoint), index scores were significantly improved in both groups compared to their post-infarct scores (RT, 1.70±0.16, p<0.002; CS/RT, 1.57±0.24, p<0.0001). At the end of the follow-up period, the RT group had regressed slightly (index score 1.79±0.21) while the CS/RT group had improved slightly (index score 1.46±0.26), though this difference was not significant (within-group versus post-therapy or between-group for RT versus CS/RT).
Changes in cortical motor representations
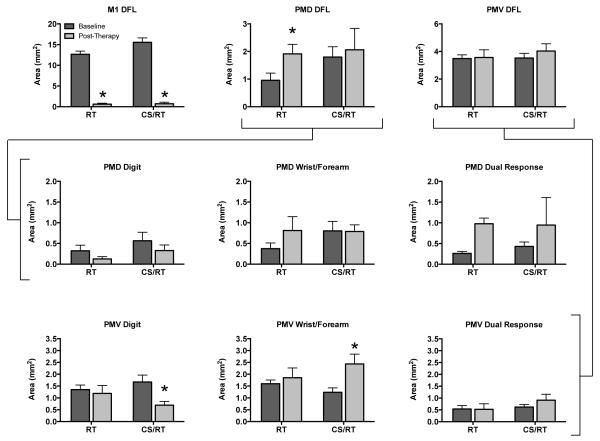
Each cortical region (M1, PMD, PMV) was compared to evaluate differential effects of RT and CS/RT on functional representations in post-infarct surviving tissue (Figures 4 and 5). Compared to the baseline map, the M1 DFL representation in the post-therapy map was significantly reduced in size in both groups (RT, −95.0%, p<0.0001; CS/RT, −95.3%, p<0.0001) (Figure 4, top row, left graph), indicating that the infarct successfully destroyed the M1 DFL map as intended, and that behavior improvements were unlikely to be related to functional recovery in M1. ICMS stimulation thresholds for DFL responses in M1 were higher in both groups between baseline and post-therapy maps (RT: 14.6±0.9 μA baseline, 21.7±3.3 μA post-therapy; CS/RT: 12.9±0.9 μA baseline, 17.0±3.8 μA post-therapy). These increases were significant overall (effect of Time, p<0.036) though neither within-group change reached significance.
Figure 4.
Area of distal forelimb (DFL) representations in M1, PMD, and PMV prior to infarct (“baseline”, dark gray) and after RT or CS/RT treatment (“post-therapy”, light gray). Top row. In M1, the ischemic infarct produced a significant and equivalent reduction in DFL area in both groups (p<0.001, left graph). PMD DFL area significantly increased in the RT group (p<0.029) but not in the CS/RT group (middle graph). There were no changes in total DFL area in PMV for either group (right graph). There were no between group differences in baseline area in PMD or PMV for any of the data shown in this figure (all 9 graphs). Middle row. Comparison of three movement types evoked by ICMS in PMD. There were no significant within-group differences in digit (left graph), wrist/forearm (middle graph), or dual-response (right graph) representations in PMD. The increase in overall DFL area in the RT group (top row) was driven by the separate non-significant increases in wrist/forearm and dual-response representations (69% of these dual-responses included wrist/forearm movements). Bottom row. Comparison of three movement types evoked by ICMS in PMV. There was a significant decrease in digit area (p<0.009, left graph) and a significant increase in wrist/forearm area (p<0.021, middle graph) for the CS/RT group in PMV. These changes offset each other in the overall size of the PMV DFL map in this group (top row). There were no significant changes in the RT group in PMV.
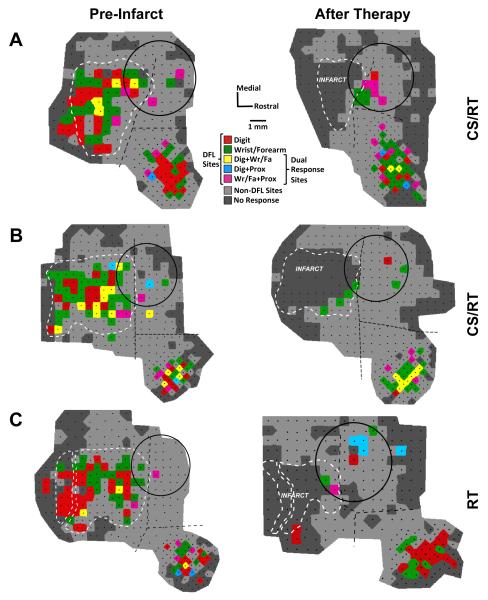
Figure 5.
Representational maps from two monkeys in the CS/RT group (panels A and B) and one monkey in the RT group (panel C). Black dots indicate the location of ICMS stimulation sites in each map, and DFL sites are indicated by one of five colors (as in Figure 2). Non-DFL sites (proximal forelimb, face, etc.) are shaded light gray (rather than dark blue, as in Figure 2) to improve visibility of the DFL regions. Borders between M1, PMD, and PMV (dashed black lines) were defined in pre-infarct (baseline) maps based on ICMS data and vascular landmarks. Notably, some PMD DFL sites are often adjacent to the M1 DFL area, with as little as 500 μm separation. In post-therapy maps, the vascular landmarks from the baseline map were used to delineate the borders. Tissue necrosis of the infarct caused deformation of intact cortical tissue and surface vasculature, which is reflected by the shift in border positions in the post-therapy maps. In monkey C, the two infarct outlines indicate that a large pass-through artery was spared from occlusion (see Methods). All three subjects had near-complete loss of DFL area in M1, with a limited number of ICMS-responsive sites at the rostral edge of the infarct (consistent with prior reports and likely reflecting uneven tissue distortion between the cortical surface and deeper layers14). Movements were evoked by ICMS throughout PMD and PMV after therapy, indicating that CS did not adversely affect the physiological responsiveness of the cortical tissue. For all three subjects, the monopolar electrode was implanted 2 weeks post-infarct (shown on the pre-infarct map only for reference), and post-therapy maps were derived 7 weeks post-therapy. Image scaling for figure display is responsible for the apparent change in size of the electrode between maps (all electrode circles are 4 mm in diameter).
In PMD (the target of CS in the CS/RT group), the post-therapy DFL representation increased significantly in the RT group (+100.2%, p<0.029) but not in the CS/RT group (+14.5%) (Figure 4, top row, middle graph). ICMS thresholds for PMD DFL responses were not significantly different for either group (RT: 18.1±3.1 μA baseline, 15.9±2.6 μA post-therapy; CS/RT: 17.9±1.6 μA baseline, 18.4±2.4 μA post-therapy). In PMV, the post-therapy DFL representation increased in both groups (RT, +2.4%; CS/RT, +14.3%) (Figure 4, top row, right graph) but neither increase was significant. PMV DFL ICMS thresholds were not significantly different for either group (RT: 19.1±2.0 μA baseline, 19.5±1.9 μA post-therapy; CS/RT: 16.9±0.6 μA baseline, 16.9±0.9 μA post-therapy). Combining both premotor regions (PMD+PMV), DFL area increased in both groups (RT, +29.8%; CS/RT, +14.4%) but again neither increase was significant.
DFL representations in PMD and PMV were further explored by comparing changes in area for digit, wrist/forearm, and all dual-response movements evoked by ICMS. In PMD (Figure 4, middle row), there were no significant changes for any of the three movement types for either group. Although no individual change was significant, the increase in PMD DFL described earlier for the RT group was primarily driven by increases in wrist/forearm (+0.44 mm2, +118%) and dual-response (+0.72 mm2, +274%) movements, with 69% of the dual-response area including a wrist/forearm component. In PMV (Figure 4, bottom row), the CS/RT group had a significant decrease in digit area (−0.98 mm2, −59%, p<0.009) and a significant increase in wrist/forearm area (+1.19 mm2, +96%, p<0.021). These PMV changes in the CS/RT group offset each other in the overall DFL area analysis.
Baseline and post-therapy ICMS maps from two subjects in the CS/RT group and one subject in the RT group are illustrated in Figure 5. One CS/RT subject (Figure 5A) had large DFL expansion in both PMD (+1.33 mm2, +177%) and PMV (+1.44 mm2, +34%) while the other CS/RT subject (Figure 5B) had a reduction of DFL in PMD (−0.26 mm2, −27%) and a smaller expansion in PMV (+0.56 mm2, +18%). It is notable that CS (delivered to both subjects) did not affect tissue responsiveness to ICMS, indicating that group differences in PMD DFL were not due to a physiological dysfunction in the stimulated cortex. The RT subject (Figure 5C) had a large DFL expansion in PMD (+1.28 mm2, +582%) and a smaller DFL expansion in PMV (+0.56 mm2, +16%).
Relationship between behavior and map changes
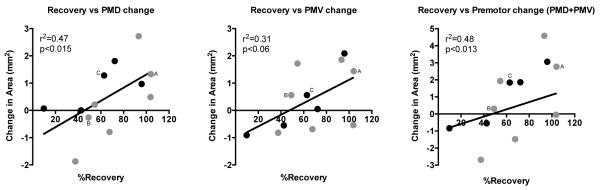
Although significant recovery occurred in both groups, recovery levels of individual monkeys ranged from 10% to 104% of their post-infarct impairment level. Similarly, both groups had expanded premotor DFL maps, but changes in individual monkeys ranged from −47% to +66% (for the combined PMD+PMV area). Correlation analysis found that behavioral recovery was positively related to expanded DFL areas in PMD (r=0.682, p<0.015), PMV (r=0.558, p<0.06), and combined PMD+PMV (r=0.693, p<0.013) (Figure 6). This suggests that, independent of the application of CS during RT, changes in behavioral performance were associated with plasticity of motor representations in premotor cortex.
Figure 6.
Scatterplot of behavior improvement (%Recovery) versus change in DFL representational area in PMD (left graph), PMV (middle graph), and the combined premotor cortex area (PMD+PMV; right graph). There was a strong association between better behavioral recovery and larger DFL areas in the intact premotor cortex. Monkeys illustrated in Figure 5 are indicated on each of the scatterplots (“A”, “B”, and “C”); note that monkey A (CS/RT subject) had good recovery and larger DFL maps, monkey B (CS/RT subject) had poorer recovery and smaller DFL maps, and monkey C (RT subject) was intermediate between A and B. %Recovery (x-axis) is defined as the amount of performance improvement (post-infarct minus post-therapy) divided by the total deficit (post-infarct minus pre-infarct). Change in area (y-axis) is defined as post-therapy DFL map area minus baseline DFL map area; thus negative values indicate a reduction in DFL area in post-therapy maps. Data is plotted for the 12 monkeys (5 RT, black circles; 7 CS/RT, gray circles) for which complete behavior and mapping results were available (see Methods). Linear curve fit added to graphs for visual clarity after statistics were obtained by correlation analysis.
CS-Evoked Movements and Movement Thresholds (MT)
CS reliably evoked movements in all seven CS/RT subjects during each MT determination session. Evoked movements most often involved movements of the head, neck, and torso, typically characterized as “torso twitches” or “head ducking”, with very few instances of evoked forelimb movements. Movements did not appear to be visibly synchronized to the 100 Hz stimulation frequency, but rather were unitary events in response to each MT pulse burst.
The average MT in the CS/RT group was 2.98±0.12 mA one week prior to therapy, 2.90±0.15 mA during the first week of therapy, and 3.05±0.05 mA during the final week of therapy. There were no significant differences in MT values over time, nor were there any significant correlations between MT and behavioral performance or between MT and DFL areas in motor maps.
DISCUSSION
This report is the first controlled efficacy study in non-human primates to evaluate the effects of CS/RT as a therapeutic treatment for chronic motor deficits after ischemic stroke. The results indicate that 100 Hz monopolar cathodal-current CS/RT produced no statistically significant benefit for behavioral recovery during the therapy period compared to RT alone for either the primary outcome measure (between group post-therapy performance) or secondary behavioral outcome measures (between group follow-up performance or within group performance over time). ICMS motor mapping revealed a larger DFL area expansion in PMD for the RT group compared to the CS/RT group. There was a significant correlation between expanded premotor cortex DFL maps and improved behavioral performance after treatment.
The lack of behavioral benefit in the CS/RT group compared to the RT group was unexpected and contrary to repeated findings in rats11-13,15,33-36, our own initial study in monkeys14, and the results of early clinical trials16-18. The CS/RT method had previously been shown to be robust, in that improvements were evident using both bipolar and monopolar stimulation, and using a range of stimulation frequencies. However, CS/RT in rats has been shown to be less effective under certain conditions, notably in animals with severe post-injury behavioral impairments33, in animals with electrode placements that spanned smaller regions of spared peri-infarct tissue34, and in animals in which CS/RT intervention was initiated after a substantial (i.e. 3 month) post-infarct delay36. These contingencies do not appear to have been factors in the present study, in that a) there was no clear relationship between the level of post-infarct impairment and the amount of post-therapy recovery (r2=0.053, p<0.45; data not shown), b) the region under the implanted CS electrode was comprised almost entirely of intact cortical tissue, and c) initiation of CS/RT was 7 weeks post-infarct (though an effect of this delay cannot be entirely ruled out). The present results are consistent with the results of a larger clinical trial (Everest trial) of CS/RT therapy in which primary measures of upper extremity motor function showed no difference between CS/RT and RT at the end of therapy19,20,26. Subgroup analysis of the Everest data suggests that another key factor affecting CS/RT efficacy is the integrity of descending pathways between motor cortex and the spinal cord19,20. This factor is not likely to be relevant to the present results, as the integrity of descending pathways in the monkeys was demonstrated by the responsiveness of premotor cortex to ICMS mapping (DFL and non-DFL regions) and to MT determination procedures.
Non-human primates, including squirrel monkeys, are well suited to examine translational aspects of putative rehabilitative therapies, as they share many neurological complexities with humans. Compared to rats, squirrel monkeys possess multiple distinct motor cortical areas, greater cortical thickness and neuropil volume, and a greater ratio of white matter to gray matter37,38, though their brains are less gyrencephalic than humans and contain substantially fewer direct corticomotoneuronal connections in the spinal cord39,40. While it is unclear if any one factor carries substantially more weight than others, it can be assumed that a non-human primate model will better predict the outcome of the therapy in humans than one based soley on rodent results.
It is noteworthy that epidural cathodal stimulation over the affected region of motor cortex has been found more effective than anodal stimulation in rodents for enhancing post-stroke recovery11,13, whereas human non-invasive (i.e., extra-cranial) stimulation methods such as tDCS (transcranial direct current stimulation) often produce superior results using anodal stimulation over the target region8,41,42. Epidural and extracranial anodal stimulation requires lower current amplitudes to produce cortical excitation, whereas cathodal stimulation within cortical tissue via ICMS requires lower amplitudes than anodal-ICMS43. Cathodal current was chosen in the present study based on results from rodent studies that utilized the same Northstar Neuroscience stimulator system and whose outcomes favored cathodal over anodal11,13. However, in light of the present results, the inter-species neurological differences, and the differences in electrode proximity to the cortical tissue between invasive and non-invasive stimulation techniques, it remains unclear whether cathodal or anodal would be the optimal polarity in our model of post-stroke CS/RT therapy.
Before dismissing CS/RT as a viable therapy in human and non-human primates, it is important to note that a recent subgroup analysis of the Everest trial data demonstrated that CS/RT resulted in continued functional improvement up to 24 weeks post-treatment, whereas RT alone resulted in substantial regression of motor function from 12 to 24 weeks post-treatment19. In the present monkey study, there was a slight reduction in performance in the RT group and a slight continuation of improvement in the CS/RT group by the end of the follow-up period (Figure 3). Monkeys were tracked for 12 weeks, suggesting that a longer follow-up period may have paralleled the recent Everest results. That is, CS/RT may be effective, but its benefits may not be completely evident until long after treatment has been completed. Additionally, there are a variety of design factors that could influence the effectiveness of CS/RT, including the post-infarct timing of therapy initiation, the intensity of the rehabilitation experience, and the target location selected for CS application. A complete examination of these parameters was beyond the scope of the present study, but should be considered in future investigations.
The premise of CS/RT therapy is that CS will enhance RT-associated recovery mechanisms, but in the absence of differential effect of CS/RT versus RT on behavioral recovery, it is reasonable to ask whether the RT protocol was effective at all. Frost et al. (2003) examined spontaneous recovery of motor performance on the pellet retrieval task for 12 weeks after an M1 DFL infarct. Recovery during weeks 7-12 post-infarct was 33% relative to baseline performance27. In the present study, RT was conducted during weeks 7-12 post-infarct for the RT and CS/RT groups, and recovery was 42% for both groups (Figure 3). While only a 9% difference relative to their respective performance at week 6 post-infarct, this was a 27% improvement in recovery for both RT-treated groups compared to spontaneous recovery. The infarcts produced by Frost et al. were smaller (81% of M1 DFL) than in the present study (98% of M1 DFL), so it is reasonable to conclude that spontaneous recovery would be somewhat reduced after a larger infarct and that RT would yield correspondingly larger improvements. Thus, while CS/RT did not enhance recovery compared to RT alone, it is clear that the rehabilitation protocol used in both groups did produce modest gains in performance.
In contrast to prior CS/RT therapy studies13,14,34, expansion of spared DFL representations was greater in the RT group rather than the CS/RT group. Expansion of cortical representational areas is generally considered to be a correlate of acquired (and recovered) motor skills23,25,27,28,44-50, reflecting a combination of changes in synaptic strength, synaptogenesis, and sprouting of new axonal connections between neurons within the motor cortex. In light of the equivalent behavioral recovery in both groups, there are several, possibly concurrent, explanations for the mapping results found in the present study. First, although map expansion is usually posited to reflect the underlying changes in neural circuitry that support the newly acquired skills, it is improbable that such large scale functional expansion would persist indefinitely, as this would imply a “ceiling effect” on the ability to learn additional new skills in the future. Thus, CS may have accelerated the consolidation process for the neural circuits that supported the re-acquired skills, resulting in less PMD map expansion in the CS/RT group. Alternatively, CS may have interfered with the normal synaptic and axonal plasticity mechanisms that support map expansion within the region directly affected by CS (perhaps as a function of current polarity). If so, recovery-related plasticity still could have occurred in other structures within the motor system in the form of terminal sprouting in the afferent targets of PMD, such as red nucleus, spinal cord, or another cortical area such as PMV. In this regard, it is interesting that PMV in the CS/RT group showed significant changes in digit (decreased) and wrist/forearm (increased) representations, whereas PMV representations did not change in the RT group. Finally, although task outcomes were equivalent, it is possible that CS could have influenced the specific compensatory strategies used by the monkeys, such that RT group favored the involvement of PMD whereas the CS/RT group favored PMV involvement. PMD and PMV are considered to have different roles in the control of limb movement, with PMD being more involved in reaching into interpersonal space (e.g., moving the hand to the food well) and PMV being more involved in hand-to-body interactions (e.g., bringing a food pellet to the mouth). Expanded wrist/forearm map areas in both groups suggest that wrist and forearm movements were critical to task success after the infarct, but we did not examine movement kinematics to determine whether there were differences in the reaching versus retrieval phases of task performance between the two groups.
It is unfortunate that the random distribution of animals into RT and CS/RT groups resulted in a visibly notable, though statistically insignificant, difference in the size of the baseline DFL maps in PMD (Figure 4, top row, middle graph), but it raises a question about whether the non-expansion of DFL maps in the CS/RT group was due to a size limit on the PMD maps. The two smallest baseline PMD maps were in the RT group (0.24 and 0.22 mm2), and the largest baseline PMD map was in the CS/RT group (3.61 mm2); these three maps account for most of the apparent difference in average baseline seen in Figure 4. However, of the 12 monkeys in Figure 6, these three monkeys also had the first (3.61 to 6.33 mm2), second (0.24 to 2.05 mm2), and fourth (0.22 to 1.50 mm2) largest increases in PMD area, suggesting that a limit on map size is unlikely to account for group differences in map area. As Figures 4 and 6 indicate, the change in PMD DFL area in the CS/RT group was more variable than in the RT group. Figure 6 also indicates that 3 of 7 CS/RT monkeys had >90% recovery on the behavior task, whereas only 1 of 5 RT monkeys had >90% recovery, hinting that CS/RT may have been effective in a subgroup of the monkeys. It is unclear what factor(s) might account for this possible subgroup outcome in the CS/RT monkeys.
An interesting difference between the monopolar stimulation experiments conducted in rats versus monkeys was the location of the return electrode relative to the central nervous system. In rats, the return electrode was placed near the occipital skull11,13,33,35,36,51,52, such that the current path between electrode poles was largely confined to the cranial chamber (cerebrum and cerebellum), with a direction of charge flow that was approximately transverse to the radial orientation of the corticospinal neurons within the target cortical tissue. In monkeys however, mid-torso implantation was necessitated by the size of the return electrode. This placement produced a current path that potentially included the entire motor axis (cerebrum through spinal cord), with a direction largely parallel to the orientation of the corticospinal neurons. It is unclear, and beyond the scope of the present study to examine, to what extent these differences in current paths may have affected the response of the nervous system to CS, though modelling studies suggest that the effect of anodal or cathodal polarity on neuronal activity is dependent on neuron orientation relative to the electrode contact53. Anecdotally, the evoked responses during MT determination using bipolar stimulation in our prior non-human primate study produced precise movements of discreet body parts (e.g., elbow flexion or toe extension) in a visible rhythm consistent with the 50 Hz stimulation frequency14. In contrast, responses evoked in monkeys by monopolar stimulation were non-synchronized, non-discreet movements of axial body elements (head, neck, torso), implying a physiological difference in motor system activation using bipolar versus monopolar stimulation in non-human primates. Human trials, including Everest, used only bipolar stimulation, in which both electrode poles overlay the target cortical tissue16-20. However, the gyri and sulci of the human brain likely would have produced a mixture of parallel and transverse current flows with respect to the corticospinal neurons regardless of whether a bipolar or monopolar contact arrangement was used. Furthermore, it is unknown how return electrode location might have affected clinical outcomes.
In conclusion, monopolar CS/RT in monkeys failed to promote enhanced behavioral recovery after cortical ischemic stroke during the therapy period compared to RT alone. However, there may have been some secondary benefit for improved skill retention during follow-up testing. It is unclear from the present results whether monopolar CS/RT is simply ineffective in non-human primates, though it seems more likely that the translation of CS methodology from rat to monkey was inadequate. Further studies would be needed to more conclusively address this issue.
Acknowledgments
The authors thank Bob Cross for behavior testing and Drs. David Guggenmos and David McNeal for assistance with collection of mapping data.
Funding
NIH [grant numbers NS48126, NS30853, and HD02528] and Northstar Neuroscience, Inc. (Seattle, WA, USA).
Footnotes
Disclosures
A portion of these results has previously appeared in abstract form at the 2007 Society for Neuroscience Annual Meeting.
The authors have no financial interests to disclose.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 4.Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurol. 2007;6(3):279–286. doi: 10.1016/S1474-4422(07)70056-X. [DOI] [PubMed] [Google Scholar]
- 5.Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010820.pub2. CD010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessel MJ, Zimerman M, Hummel FC. Non-invasive brain stimulation: an interventional tool for enhancing behavioral training after stroke. Front Hum Neurosci. 2015;9:265. doi: 10.3389/fnhum.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 8.Liew SL, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci. 2014;8:51. doi: 10.3389/fnint.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodaparast N, Hays SA, Sloan AM, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28(7):698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200(2):356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 12.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25(8):780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 13.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25(8):789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 14.Plautz EJ, Barbay S, Frost SB, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 15.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25(8):794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 16.Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003;25(8):815–818. doi: 10.1179/016164103771953907. [DOI] [PubMed] [Google Scholar]
- 17.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58(3):464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108(4):707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- 19.Levy RM, Harvey RL, Kissela BM, et al. Epidural Electrical Stimulation for Stroke Rehabilitation: Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968315575613. [DOI] [PubMed] [Google Scholar]
- 20.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40(5):1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12(8):2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74(1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 23.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 25.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 26.Harvey RL, Winstein CJ, Everest Trial Group Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23(1):32–44. doi: 10.1177/1545968308317532. [DOI] [PubMed] [Google Scholar]
- 27.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89(6):3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 28.Milliken GW, Plautz EJ, Nudo RJ. Distal forelimb representations in primary motor cortex are redistributed after forelimb restriction: a longitudinal study in adult squirrel monkeys. J Neurophysiol. 2013;109(5):1268–1282. doi: 10.1152/jn.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nudo RJ, Larson D, Plautz EJ, Friel KM, Barbay S, Frost SB. A squirrel monkey model of poststroke motor recovery. ILAR J. 2003;44(2):161–174. doi: 10.1093/ilar.44.2.161. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–266. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- 31.Barbay S, Plautz EJ, Friel KM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169(1):106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dancause N, Barbay S, Frost SB, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96(6):3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- 33.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212(1):14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boychuk JA, Adkins DL, Kleim JA. Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil Neural Repair. 2011;25(1):88–97. doi: 10.1177/1545968310385126. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J, Liu L, Xue X, et al. Cortical electrical stimulation promotes neuronal plasticity in the peri-ischemic cortex and contralesional anterior horn of cervical spinal cord in a rat model of focal cerebral ischemia. Brain Res. 2013;1504:25–34. doi: 10.1016/j.brainres.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 36.O'Bryant AJ, Adkins DL, Sitko AA, Combs HL, Nordquist SK, Jones TA. Enduring Poststroke Motor Functional Improvements by a Well-Timed Combination of Motor Rehabilitative Training and Cortical Stimulation in Rats. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314562112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol. 2004;281(1):1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97(10):5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci. 1993;13(12):5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tigges J, Nakagawa S, Tigges M. Efferents of area 4 in a South American monkey (Saimiri). I. Terminations in the spinal cord. Brain Res. 1979;171(1):1–10. doi: 10.1016/0006-8993(79)90727-3. [DOI] [PubMed] [Google Scholar]
- 41.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37(12):742–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114(4):600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- 43.Young NA, Vuong J, Flynn C, Teskey GC. Optimal parameters for microstimulation derived forelimb movement thresholds and motor maps in rats and mice. J Neurosci Methods. 2011;196(1):60–69. doi: 10.1016/j.jneumeth.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 44.Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11(5):471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- 45.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16(6):638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res. 2001;123(2):133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 48.Tennant KA, Adkins DL, Scalco MD, et al. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012;98(3):291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68(3):793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 50.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250(1):5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Q, Zhang Q, Zhao X, et al. Cortical electrical stimulation alone enhances functional recovery and dendritic structures after focal cerebral ischemia in rats. Brain Res. 2010;1311:148–157. doi: 10.1016/j.brainres.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Cheng X, Li T, Zhou H, et al. Cortical electrical stimulation with varied low frequencies promotes functional recovery and brain remodeling in a rat model of ischemia. Brain Res Bull. 2012;89(3-4):124–132. doi: 10.1016/j.brainresbull.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Manola L, Holsheimer J, Veltink P, Buitenweg JR. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118(2):464–474. doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]