Abstract
Objectives
Increased vascular permeability is a hallmark of sepsis and acute respiratory distress syndrome. Angiopoietin (Ang2) induces vascular leak, and excess Ang2 generation is associated with patient mortality from these diseases. However, mechanisms dampening Ang2 generation during injury remain unclear. Interestingly, microRNA-150 levels were decreased in septic patients. MicoRNAs (miR) regulate signaling networks by silencing mRNAs containing complementary sequences. Thus, we hypothesized that miR-150 suppresses Ang2 generation and thereby resolves vascular injury.
Approach and Results
Wild-type or miR-150−/− mice or endothelial cells were exposed to lipopolysaccharide or sepsis and Ang2 levels, adherens junction re-annealing, endothelial barrier function and mortality were determined. While Ang2 transiently increased during lipopolysaccharide-induced injury in wild-type endothelial cells and lungs, miR-150 expression was elevated only during recovery from injury. Deletion of miR-150 caused a persistent increase in Ang2 levels and impaired adherens junctions re-annealing after injury, resulting thereby in an irreversible increase in vascular permeability. Also, miR-150−/− mice died rapidly after sepsis. Rescuing miR-150 expression in endothelial cells prevented Ang2 generation, thereby restoring vascular barrier function in miR-150−/− mice. miR-150 terminated Ang2 generation by targeting the transcription factor, early growth response 2. Thus, early growth response 2 or Ang2 depletion in miR-150−/− endothelial cells restored junctional re-annealing and reinstated barrier function. Importantly, upregulating miR-150 expression by injecting a chemically synthesized miR-150 mimic into WT-mice vasculature decreased EGR2 and Ang2 levels and hence mortality from sepsis.
Conclusions
miR-150 is a novel suppressor of Ang2 generation with a key role in resolving vascular injury and reducing mortality resulting from sepsis.
Keywords: miR-150, adherens junctions, angiopoietin 2, sepsis, lung injury, endothelium
Introduction
The vascular endothelium is the innermost layer of the vessel wall, and performs the vital task of providing nutrients to the underlying tissue while dynamically regulating the transvascular flux of proteins and inflammatory cells.1 Adherens junctions (AJs), formed through homotypic adhesions of the extracellular domain of vascular endothelial (VE)-cadherin across contiguous endothelial cells, maintain vascular barrier function.2 LPS or endotoxin, a component of the outer membrane of Gram-negative bacteria, induces vascular leak by disrupting adherens junctions (AJs).3–5 AJs can re-anneal following injury, thus restoring barrier function. 6, 7 However, impairment of AJ re-annealing results in uncontrolled accumulation of protein-rich fluid and inflammatory cells in the underlying tissue, thereby perturbing tissue-fluid homeostasis, which is a hallmark of chronic vascular inflammatory diseases such as sepsis and acute respiratory distress syndrome (ARDS).8, 9
Angiopoietin (Ang1 and 2), which ligate the endothelial Tie2 receptor, have a well-recognized role in regulating the stability of developing vessels.10, 11 Ang1 phosphorylates the Tie2 receptor and promotes endothelial survival, migration and barrier formation in the mature endothelium.10, 11 In contrast, Ang2 blocks Tie2 phosphorylation and induces vascular leak by disrupting AJs through activation of actin-myosin induced stress fiber formation.12–14 Interestingly, circulating levels of Ang2 have been shown to be consistently higher in patients who die of ARDS or sepsis than the patients who survive these diseases.9, 15–18 Ang2 has therefore emerged as a predictor of patient’s mortality from ARDS and sepsis. Thus, extensive studies are underway to find approaches which can suppress Ang2 generation for rescuing endothelial barrier function. In this regard, Ang1 infusion seems to be a therapeutic approach to counteract Ang2 disruption of the endothelial barrier.19 However, Ang1 has also been shown to induce pulmonary hypertension, thereby limiting its use in rescuing vascular barrier function.20–22
miRNAs are small (19–23 nucleotides) non-coding RNAs known to downregulate protein expression by targeting specific mRNAs for translational repression or degradation, resulting in altered expression of the target genes.23, 24 In sharp contrast to Ang2, miR-150 levels were shown to be decreased in plasma from septic patients.25, 26 miR-150 has been shown to regulate endothelial cell differentiation and vasculogenesis.27 Thus, we focused on investigating whether miR-150 plays an obligatory role in re-annealing AJs and in resolving vascular barrier function by suppressing Ang2 generation. We show here that miR-150 is induced in ECs during recovery from injury. Loss of miR-150 impaired re-annealing of AJs leading to an irreversible increase in endothelial permeability. We show that miR-150 restores barrier function by suppressing Ang2 generation and downstream signaling through targeting the transcription factor EGR2. Additionally, we show that delivery of a miR-150 mimic into the vasculature of WT mice, pre-treated with a lethal dose of LPS, reduces EGR2 and Ang2 and promotes survival during sepsis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
MiR-150 Re-anneals Adherens Junctions and Restores Endothelial Barrier Function
Because re-annealing of AJs is required for the restoration of basal endothelial barrier function after vascular injury1, 2, 7, 28, we addressed the possibility that miR-150 re-anneals AJs. LPS disrupted AJs leading to interendothelial gap formation in both WT and miR-150−/− ECs at 6 h (Figure 1A). However, after 24 h, AJs in ECs from WT mice had re-annealed, sealing the inter-endothelial gaps (Figure 1A). Strikingly, AJs failed to re-anneal in ECs from miR-150−/− mice, leaving numerous inter-endothelial gaps (Figure 1A). We consistently found that LPS induced similar increases in transendothelial albumin flux after 6 h in both WT and miR-150−/− ECs (Figure 1B). Transendothelial albumin flux recovered to basal levels 24 h after LPS challenge in the WT ECs but remained persistently high in the miR-150−/− ECs (Figure 1B). Loss of miR-150 had no effect on the expression of AJ components, i.e., VE-cadherin and β–catenin (data not shown). These observations demonstrate that miR-150 expression in ECs is required for re-annealing AJs and thereby promoting endothelial barrier recovery after injury.
Figure 1.
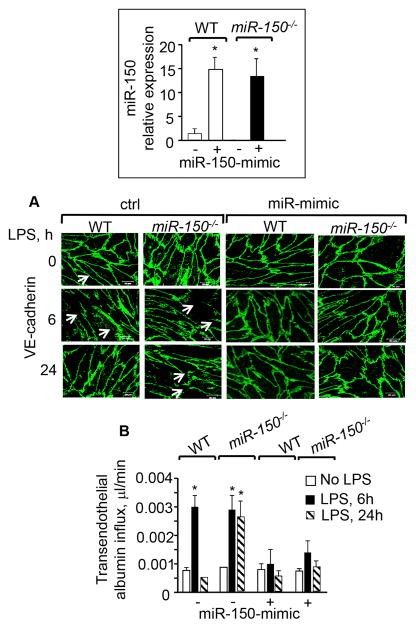
miR-150 re-anneals adherens junctions and restores endothelial barrier function. WT or miR-150−/− ECs plated on coverslips (A) or trans-well dishes (B) were stimulated with 1μg/ml LPS for the indicated time points. A, Re-annealing of AJs and inter-endothelial gap closure were determined by staining ECs with anti-VE-cadherin antibody. Representative image is shown in left while right panel shows quantitation of the interendothelial gaps (n=3–4). B, Transendothelial flux of albumin following LPS challenge (n=4). Inset, miR150 expression quantified taking U6 as the internal control (n=4; ND, not determined). All data are mean±SEM. n is the number of experiments. One way ANOVA and post-hoc t-test were used to compare data between groups. *P<0.05.
MiR-150 Mimic Strengthens Adherens Junctions and Prevents Endothelial Barrier Disruption
We assessed the effect of upregulating miR-150 levels on AJs annealing and barrier function by transducing WT and miR-150−/− ECs with a miR-150 mimic. The miR-150 mimic increased miR-150 expression by ~14 fold in both WT and miR-150−/− ECs (Figure 2, inset). Interestingly, miR-150 mimic prevented AJs disruption by LPS and as a result LPS failed to increase endothelial permeability in WT ECs and miR-150 null ECs (Figures 2A–2B).
Figure 2.
miR-150-mimic rescues LPS induced loss of endothelial barrier function. WT or miR150−/− ECs were transfected with miR-150-mimic or control mimic (ctrl) for 48 h. miR-150 expression was quantified taking U6 as the internal control (Inset). Transfected cells were stimulated with LPS (1 μg/ml) for the indicated time points and AJs re-annealing (A) or transendothelial albumin flux (B) were determined. n=3. All data are mean ± SEM. n is the number of experiments. One way ANOVA and post-hoc t-test were used to compare data between groups. * P<0.05.
MiR-150 Resolves Vascular Injury and Promotes Survival of Mice During Sepsis
As proof of the concept that miR-150 annealing of AJs promotes resolution of vascular injury in vivo, we employed a mouse model in which LPS induces lung vascular injury that peaks at 4 h but resolves in next 24–48 h.3, 7, 29 We observed that LPS increased the lung wet-to-dry weight ratio and trans-endothelial albumin extravasation into lung parenchyma of both WT and miR-150−/− mice at 4 h (Figure 3A). However, in contrast to WT mice, an increase in lung vascular permeability persisted in the miR-150−/− mice (Figure 3A).
Figure 3.
Loss of miR150−/− impairs resolution of vascular injury and increases sepsis mortality. A, Lung wet-dry ratio (left) and transendothelial albumin influx (EBAE) from lungs or plasma (right) were quantified after exposing indicated mice to nebulized (1 mg/ml) LPS (n=4–5). B, Mice were challenged with LPS (i.p, 40 mg/kg body weight) (left) or CLP was induced (right). n=10. C–D, WT-mice were exposed to nebulized (1 mg/ml) LPS and lungs were harvested to quantify alteration in miR-150–5p (left) or pri-miR-150 (right) (n=4–5). miR-150 expression is quantified taking U6 as an internal control while GAPDH was used as an internal control for quantifying pri-miR-150 expression. All bar graphs shows mean ± SEM. n is the number of mice/group. One way ANOVA and post-hoc t-test were used to compare data between groups (A and C–D). *P<0.05. In B, mice survival in from sepsis was assessed using log-rank test.
We next induced sepsis using a high dose of LPS or cecal ligation and puncture (CLP) in WT and miR-150 null mice to assess whether miR-150 reduces mortality from sepsis. We observed that both LPS and CLP produced an accelerated and increased overall mortality in miR-150−/− mice relative to WT mice (Figure 3B).
Mature miRNAs are generated through a series of steps with initial being transcription of pri-miR followed by processing of this transcript by enzymes including Drosha and Dicer to yield a double-stranded ~22 nt product comprised of the mature miRNA guide strand and the miRNA* passenger strand. 23, 24 Thus, we examined the expression of mature miR-150-5p (referred hereafter as miR-150), miR-150-3p* (passenger strand) and pri-miR-150 in lungs and ECs after LPS challenge. We found that miR-150 expression remained unaltered at the peak of lung injury (i.e., at 4 h) but increased by ~2 fold at 24 and 48 h (Figure 3C), coinciding with the resolution phase of lung injury (Figures 2A). However, the expression of miR-150-3p was not altered (1.0± 0.0 at 0 h; 0.89± 0.06 at 4 h and 0.86 ± 0.11 at 24 h respectively). LPS increased the expression of pri-miR-150 at 4 h by 1.5 fold which further increased by 3-fold at 24 h indicating LPS induces miR-150 expression transcriptionally (Figure 3D).
Endothelial MiR-150 is Required for Resolution of Lung Vascular Injury
We delivered liposomes containing GFP-tagged pre-miR-150 cDNA under the control of the VE-cadherin promoter into WT and miR-150−/− mouse vasculature3, 7 to address if restoration of miR-150 expression in ECs is sufficient to resolve the increase in lung vascular permeability produced by LPS. Mice injected with liposomes containing GFP-tagged empty vector under the control of the VE-cadherin promoter were used as controls. Using the endothelial marker, CD31, along with FITC-gating of CD45- cells, FACS analysis showed that ~94% of the ECs were GFP+ in lungs of mice receiving GFP or GFP-pre-miR-150 constructs while GFP+ cells were barely detectable in WT lung homogenates (negative control), thus confirming EC-specific delivery of constructs (Figure 4A). Furthermore, Pre-miR-150 cDNA but not vector restored miR-150 expression in the lungs of miR-150−/− mice to levels similar to those seen in the lungs of WT mice under basal conditions (Figure 4B). Importantly, restoration of miR-150 in the ECs of miR-150−/− mice resolved lung vascular injury (Figure 4C).
Figure 4.
Rescuing miR-150 expression in endothelium of miR-150−/− mice resolves lung-vascular injury. Lungs from WT or miR-150−/− mice transducing GFP-tagged empty vector or pre-miR-150 cDNA under the control of VE-cadherin promoter were harvested, homogenized and stained with anti-CD31/anti-CD45 antibodies to confirm miR-150 expression in endothelium (A). Representative FACS plots are shown from experiments which were repeated at least three times. (B) miR-150 expression in lungs of WT mice (negative control) or miR-150 null mice receiving GFP-vector or pre-miR-150 cDNA. miR-150 expression was quantified taking U6 as the internal control. n=3–4. C, Lung-wet dry ratio were determined at indicated times. n = 3–4. n is number of mice. All bar graph shows mean ± SEM. One way ANOVA and post-hoc t-test were used to compare data between groups. *P < 0.05.
MiR-150 Reanneals Adherens Junctions by Suppressing Ang2 Generation and Signaling
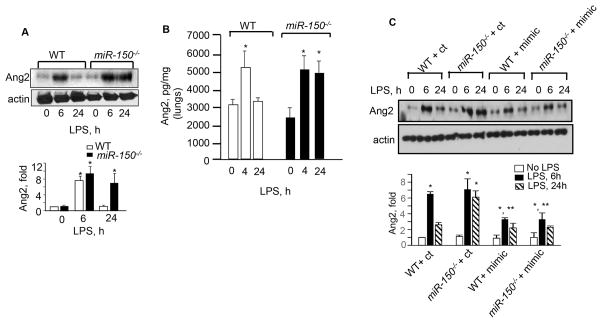
Unlike miR-150, Ang2 levels have been shown to be higher in plasma of sepsis or ARDS patients.9, 12, 15, 17, 18, 30 Thus, we addressed the possibility that miR-150 functioned by suppressing Ang2 generation and downstream signaling. We found that basal levels of Ang2 did not differ between WT and miR-150 null ECs or lungs (Figures 5A–5B). As expected, LPS increased the expression of Ang2 in WT ECs and lungs during injury i.e. at 4h (Figure 5A–5B), but expression returned to basal levels within 24 h (Figure 5A–5B). In contrast, miR-150−/− ECs or lungs displayed persistent Ang2 generation following LPS treatment even after 24 h (Figure 5A–5B). Transduction of the miR-150 mimic in WT and miR-150−/− ECs suppressed Ang2 generation, corroborating the above findings that miR-150 terminates Ang2 generation (Figure 5C).
Figure 5.
miR-150 suppresses Ang2 generation. Ang2 expression in WT or miR-150−/− ECs (A) or lungs (B) following exposure to LPS. Ang2 was quantified using Ang2 antibody taking actin as a loading control in panel A (n =3) whereas panel B shows Ang2 levels in lungs determined using ELISA assay (n=4–5). C, WT or miR-150−/− ECs were transfected with either control mimic (ct) or miR-150 mimic (mimic). Ang2 expression was determined using anti-Ang2 antibody taking actin as a loading control (n= 3). All data represents mean ± SEM. In all immunoblots, band density was quantified taking actin as the control. In panels, A and C n is the number of experiments while in panel B n is the number of mice. One way ANOVA and post-hoc t-test were used to compare data between groups. *P < 0.05.
Ang2 disrupts AJs through actin-myosin induced stress fiber formation. 9 Thus, we depleted Ang2 using siRNA (Supplemental figure 1, inset) and found that Ang2 depletion rescued re-annealing of AJs, MLC dephosphorylation and barrier function in miR-150 null ECs similar to WT cells (Supplemental Figures 1A–1C). Also, inhibition of MLC-phosphorylation, using the Rho kinase inhibitor Y-27632 31, in miR-150−/− mice resolved LPS-induced lung edema in miR-150−/− mice to levels similar to those seen in WT mice (Supplemental Figures 1D).
MiR-150 Suppresses Ang2 by Targeting EGR2
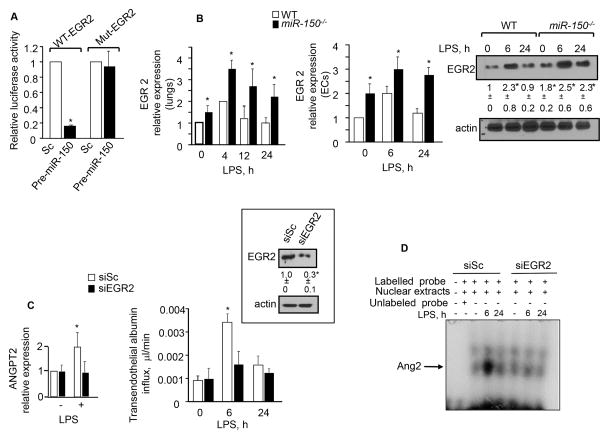
Multiple transcription factors, including NFAT, FOXO1, FOXC2, NFκB, TAL1, LYL1, LMO2, H1F2α are thought to regulate ANG2 expression in various cell types including endothelial cells 32–34. We used miR-walk, an online database for predicting miRNA targets35 to assess if miR-150 targets Ang2 or the Ang2 inducing transcription factors listed above. However, neither Ang2 nor any of the above mentioned transcription factors were found to be major targets of miR-150. Prediction analysis indicated the early growth response protein 2 (EGR2) to be the major target of miR150. EGR2 has a single conserved miR-150 binding site in its 3′UTR sequence with a seed length of 10. Analysis of the Ang2 promoter sequence also revealed the presence of a putative binding site for EGR2 at positions −59 to−-51. Thus, we determined EGR2 luciferase reporter activity in HPAECs co-transducing WT or mutated EGR2 luciferase reporter (Mut-EGR2) and pre-miR-150 cDNAs. miR-150 significantly decreased the WT-EGR2 luciferase activity (Figure 6A). However, this response was absent in cells transducing Mut-EGR2 (Figure 6A). These findings demonstrate that miR-150 directly targets EGR2. Consistently, EGR2 expression was significantly downregulated in ECs expressing the miR-150-mimic (Supplemental Figure 2A). qPCR analysis also showed that EGR2 expression was higher in the lungs of miR-150−/− mice compared to the lungs of WT mice under basal conditions (Figure 6B, left). EGR2 expression was transiently upregulated in the lungs of WT mice but remained persistently elevated in the lungs of miR-150−/− mice (Figure 6B, left). A similar alteration in EGR2 RNA and protein levels were seen in WT and miR-150−/− ECs after LPS challenge (Figure 6B, middle and right panels).
Figure 6. miR-150 targets EGR2 to control Ang2 generation.
A, Luciferase activity in HPAECs co-transfected with scrambled or pre-miR-150 cDNA along with WT or mutant EGR2 3′UTR luciferase reporter (n=3 experiments). B, EGR2 mRNA (left and middle panel) or protein (right panel) expression following exposure of lungs (left, n=3–4 mice) or cells to 1 mg/ml LPS (n=3 experiments). RNA was normalized against GAPDH while immunoblot with anti-actin antibody was used as a loading control. C, Ang2 expression (left) and transendothelial albumin flux (right) in HPAECs depleted of EGR2 after 1 μg/ml LPS challenge. EGR2 depletion was assessed in HPAECs after 48 h transfection with scrambled (siSc) or EGR2 siRNA (SiEGR2) (n=3 experiments). D, EMSA of control or EGR2 depleted HPAECs after LPS challenge using 32P-labeled Ang2 oligonucleotide (n=3 experiments). E, Liposomes conjugated with either miR-150-mimic or control mimic were delivered into vasculature of mice (E, top). Forty five min later these mice were challenged with a lethal dose of LPS (40 mg/kg) and mice survival was monitored over the next 72 h. n= 8 in each group. B–C, EGR2, and Ang2 expression in mice receiving miR-150 mimic versus negative control. n=3–4. All data represent mean ± SEM. In all immunoblots, band density was quantified taking actin as the control. Survival was assessed by log rank test in panel E while in all other panels one way ANOVA and post-hoc t-test were used to compare data between groups. *P<0.05.
We next immunostained HPAECs (with or without prior LPS treatment) with anti-EGR2 antibody. We. We first determined the expression of miR-150 in human pulmonary arterial endothelial cells (HPAECs) following exposure to LPS. LPS induced miR-150 expression at 12 h which persisted up to 24 h (Supplemental Figure 2B). We found that EGR2 was primarily localized in the cytosol in unstimulated HPAECs. Treatment with LPS increased EGR2 expression at 6 h and importantly, higher levels of EGR2 were found in the nucleus. EGR2 levels returned to near basal levels 24 h after LPS challenge (Supplemental Figure 2C). To examine whether EGR2 expression is causally related to Ang2 generation that disrupts barrier function, we depleted EGR2 by ~70% using siRNA (Figure 6C, inset). We observed that LPS failed to induce Ang2 expression (Figures 6) and MLC phosphorylation, disruption of AJs and barrier function (Supplemental Figure 2D). EMSA assay showed that LPS increased DNA-Ang2 binding at 6h in control cells, which returned to baseline at 24h (Figure 6D). However, LPS failed to induce these responses in cells depleted of EGR2.
MiR-150 Mimic Reduces Mice Mortality from Sepsis
Systemic and local delivery of miRNA mimics have been shown to have therapeutic effects in mice. 36 We set out to determine whether miR-150 can be therapeutically exploited in the setting of sepsis by targeting Ang2 generation. We delivered a single-dose of miR-150 mimic or a control mimic conjugated within liposomes into the vasculature of WT mice followed by i.p. injection of a lethal dose of LPS (Figure 6E). qPCR analysis of the lung tissue showed that the miR-150 mimic caused a 3.2±0.25 fold increase in miR-150 expression above the control value. Compared to the control mimic, the miR-150 mimic significantly reduced EGR2 and Ang2 expression in lungs, and increased survival by 50% at 20 h and by 25% at 60 h (Figures 6E–6F). Together, these findings demonstrate that enhancing miR-150 levels during sepsis may be a promising approach for suppressing Ang2 expression and hence prevention of sepsis-induced lethality.
Discussion
We have identified a novel role of miR-150 in endothelial cells in re-annealing AJs and thereby promoting barrier recovery after injury induced by LPS. We showed that miR-150 suppresses Ang2 generation by targeting the transcription factor, EGR2, and thereby limits EC contraction and AJ disruption following LPS challenge. Loss of miR-150 therefore led to unrestrained Ang2 generation and downstream signaling, thus impairing resolution of vascular injury. Also, miR-150 null mice had a higher mortality rate after sepsis than WT mice while vascular delivery of a miR-150 mimic opposed these effects. We propose miR-150 as a new therapeutic tool for suppressing Ang2 generation and for promoting resolution of endothelial barrier dysfunction after vascular injury in conditions such as sepsis and lung injury.
A persistent increase in endothelial permeability is a hallmark of several chronic inflammatory vascular diseases, including sepsis and ARDS. 1, 2, 8 AJs play a key role in maintaining endothelial barrier function. 1, 2 Permeability increasing mediators such as LPS disassemble AJs but several studies showed that AJs can reform. 3–7, 37 miRNAs can suppress or augment cellular signaling in several cell types, including endothelial cells based on their ability to target mRNA.24, 38 Surprisingly, only a few studies have addressed the role of miR in regulating AJs function. For example, Young et al showed that miR-27a targets VE-cadherin and thereby induces vascular leak during ischemia and reperfusion injury.38 In this study, we sought to identify a miR that can facilitate reformation of AJs following endothelial injury. miR-150 was shown to be a LPS-responsive miR. 39 Moreover, miR-150 expression was shown to be reduced in septic patients. 25, 26 These findings prompted us to investigate the role of miR-150 in re-annealing AJs. We showed that the expression of mature miR-150-5p but not miR-150-3p was induced during recovery from endothelial injury post LPS challenge. Mature miRNA’s are generated through binding of transcription factor to the promoter region upstream of a precursor (Pri-mRNA) sequence leading to induction of Prim-miR-150. Consistently, we showed that LPS increased Pri-miR-150 albeit earlier than mature miR-150 expression indicating that, following injury, a parallel sequence of events takes place to transcriptionally induce miR-150. However, the identity of the transcription factor that induces miR-150 remains to be unraveled. We also showed that loss of miR-150 did not alter AJs organization or barrier function under basal conditions but markedly impaired AJs re-annealing after LPS challenge leading to persistent lung vascular injury. miR-150 null mice also die rapidly after sepsis. We conclude from these findings that miR-150 serves as a key endogenous mechanism to re-anneal AJs, restoring thereby endothelial barrier function.
Ang2, a naturally occurring antagonist of Ang1, blocks the Tie2 receptor and induces vascular leak by disrupting AJs. 12–14, 40, 41 Despite the evidence that unrestrained generation of Ang2 underlies the high mortality associated with ARDS or sepsis, 9, 15–18 means to dampen Ang2 levels during vascular injury remain unknown. Interestingly, we showed that, whereas Ang2 levels were reduced during recovery from endothelial injury, miR-150 was found to have been induced. Loss of mir-150 abolished this relationship since the Ang2 level remained elevated in miR-150 null ECs and lungs. Conversely, depletion of Ang2 in miR-150 rescued AJ re-annealing and barrier function, demonstrating that miR-150 functioned by suppressing Ang2 generation. Ang2 disrupts AJs by stimulating actin-myosin induced cell contractility mediated through RhoA-Rho kinase signaling. 1, 2, 9, 42 Consistently, we showed that depletion of Ang2 in miR-150 null ECs suppressed MLC phosphorylation and rescued barrier function. Furthermore, inhibition of MLC phosphorylation using Rho kinase inhibitor Y27632 resolved edema in miR-150 null lungs. Thus, our findings for the first time identify mir-150 as a suppressor of Ang2 generation and signaling.
ECs are known to secrete paracrine factors including miRs into the circulation through exosomes.43, 44 We showed that restoration of miR-150 expression in the endothelium of miR-150−/− mice via a VE-cadherin-promoter driven vector resolved vascular injury in miR-150−/− mouse lungs. We also showed that vascular delivery of synthetic miR-mimic in the setting of sepsis reduced mouse mortality. Thus, our findings suggest that EC generation of miR-150 may be crucial for maintaining circulating miR-150 and thereby Ang2 levels, and that impairment of miR-150 generation from ECs may be a contributing factor to the high mortality of patients suffering from sepsis as reported. 25, 26, 45
Because miR can suppress both RNA and protein by targeting 3′UTR sequences in genes,24 we searched for evidence that miR-150 directly targets Ang2. However, our findings showed that loss of miR-150 does not increase basal Ang2 levels, but rather suppresses Ang2 generation coinciding with recovery of barrier function. Thus, we focused on identifying transcription factors (TFs) that have been shown to induce Ang2. 32, 34, 46, 47 For example, LPS was shown to enhance Ang2 expression through activation of transcription factors NF-κB and AP-1.46 Other studies showed the role of the nuclear factor of activated T cells (NFAT), FOXO or FOXC2 in inducing Ang2 expression. 32, 34, 47 However, none of these TFs were predicted to be ubiquitous targets of miR-150. The prediction search shortlisted EGR2 as a specific target of miR-150, and interestingly, we found that the Ang2 promoter has a 9 bp GC-rich EGR binding element (GCGT/GGGGCG).48 EGR2 belongs to the EGR family of zinc finger transcription factors, which can be induced by various extracellular signals such as growth factors and cytokines through non-redundant cell-specific intracellular signaling pathways. 48–50 We showed that miR-150 targets EGR2 and that EGR2 directly binds the Ang2 promoter sequence. Consistently, we showed that in WT lungs EGR2 expression increased transiently but remained persistently elevated in the lungs of miR-150−/− mice. Induction of miR-150 or depletion of EGR2 suppressed Ang2 generation and downstream signaling, thereby accelerating resolution of lung injury in miR-150−/− mice. We showed that basal expression of EGR2 is greater in miR-150−/− mice than in WT mice. However miR-150−/− mice did not show any increase in basal Ang2 levels in ECs or apparent phenotypic changes in the lung. We showed that EGR2 is mainly localized to the cytosol under basal conditions, but treatment with LPS increased EGR2 expression and translocation to the nucleus in human ECs. Since this translocation to the nucleus may be coupled to transcription factor activation 51, our findings suggest that, in addition to EGR2 induction, nuclear transport by extracellular signals may be required for EGR2 activity. Hence, our results demonstrate that miR-150, by targeting EGR2 activity, prevents long-lasting Ang2 generation and thereby resolves vascular injury. In this regard, our findings suggest a new way to control Ang2 generation and downstream signaling through the induction of miR-150 expression.
miRNA mimics are being currently used in several disorders, such as cancer. 36 We have also shown that a single-dose of miR-150-mimic delivered in the vasculature of WT mice during sepsis reduced EGR2 and Ang2 expression and their mortality from sepsis, indicating the therapeutic relevance of our findings.
In conclusion, we have demonstrated a key role of miR-150 in re-annealing AJs and thereby resolving vascular injury by downregulating the generation of Ang2 and barrier disruptive signaling. We showed that miR-150 serves this function by targeting EGR2. Consistently, a single-dose of miR-150 mimic liposomes decreased Ang2 levels and mortality from sepsis in WT mice. While Ang1 infusion can counteract Ang2 effects it was shown to induce pulmonary hypertension. 20–22, 52 Thus, our findings identify miR-150 as a new therapeutic strategy for suppressing Ang2 generation and for rescuing endothelial barrier function after vascular injury.
Supplementary Material
Significance.
Excessive angiopoietin (Ang2) levels in the setting of vascular injury is known to be linked with patient mortality from acute respiratory distress syndrome (ARDS) and sepsis. Here we report that microRNA (miR)-150, which regulates signaling networks by silencing complementary nucleotide mRNA sequences, suppressed Ang2 generation in endothelial cells (ECs) and prevented endotoxin (LPS)-induced lung vascular injury in mice. Targeting miR-150 therefore is a potential therapeutic strategy for treating diseases associated with unrestrained Ang2 generation, including ARDS.
Acknowledgments
We thank Dr. Youyang Zhao from Department of Pharmacology at the University of Illinois for his kind gift of VE-cadherin promoter.
Funding Sources
This work was supported by National Institute of Health grants HL71794, HL84153, HL060678.
Nonstandard Abbreviations and Acronyms
- EC
Endothelial cell
- AJs
Adherens junctions
- miR
microRNA
- Ang2
Angiopeoitin 2
- Ang1
Angiopoietin 1
- EGR2
Early growth response 2
- HPAECs
Human pulmonary arterial endothelial cells
- LPS
Lipopolysaccharide
- CLP
Cecal ligation and puncture
Footnotes
Disclosure
None
References
- 1.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 2.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 3.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. The Journal of experimental medicine. 2012;209:1953–68. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–45. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. American journal of physiology Lung cellular and molecular physiology. 2014;306:L497–507. doi: 10.1152/ajplung.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu P, Usatyuk PV, Lele A, Harijith A, Gregorio CC, Garcia JG, Salgia R, Natarajan V. c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury. American journal of physiology Lung cellular and molecular physiology. 2015;308:L1025–38. doi: 10.1152/ajplung.00306.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knezevic N, Tauseef M, Thennes T, Mehta D. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. The Journal of experimental medicine. 2009;206:2761–77. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell and tissue research. 2003;314:61–8. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 11.Gale NW, Thurston G, Davis S, Wiegand SJ, Holash J, Rudge JS, Yancopoulos GD. Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harbor symposia on quantitative biology. 2002;67:267–73. doi: 10.1101/sqb.2002.67.267. [DOI] [PubMed] [Google Scholar]
- 12.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nature medicine. 2006;12:1286–93. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, Papapetropoulos A. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. The Journal of pharmacology and experimental therapeutics. 2005;314:738–44. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 14.Mammoto T, Jiang E, Jiang A, Lu Y, Juan AM, Chen J, Mammoto A. Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression. PLoS One. 2013;8:e73407. doi: 10.1371/journal.pone.0073407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Critical care medicine. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Jesmin S, Gando S, Yanagida Y, Mizugaki A, Sultana SN, Zaedi S, Yokota H. The role of angiogenic factors and their soluble receptors in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) associated with critical illness. Journal of inflammation. 2013;10:6. doi: 10.1186/1476-9255-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond JM, Porteous MK, Cantu E, Meyer NJ, Shah RJ, Lederer DJ, Kawut SM, Lee J, Bellamy SL, Palmer SM, Lama VN, Bhorade SM, Crespo M, Demissie E, Wille K, Orens J, Shah PD, Weinacker A, Weill D, Arcasoy S, Wilkes DS, Ware LB, Christie JD Lung Transplant Outcomes G. Elevated plasma angiopoietin-2 levels and primary graft dysfunction after lung transplantation. PLoS One. 2012;7:e51932. doi: 10.1371/journal.pone.0051932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, Aplenc R, Abramova H, Atochina-Vasserman EN, Beers MF, Calfee CS, Cohen MJ, Pittet JF, Christiani DC, O’Keefe GE, Ware LB, May AK, Wurfel MM, Hakonarson H, Christie JD. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. American journal of respiratory and critical care medicine. 2011;183:1344–53. doi: 10.1164/rccm.201005-0701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature medicine. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 20.Chu D, Sullivan CC, Du L, Cho AJ, Kido M, Wolf PL, Weitzman MD, Jamieson SW, Thistlethwaite PA. A new animal model for pulmonary hypertension based on the overexpression of a single gene, angiopoietin-1. The Annals of thoracic surgery. 2004;77:449–56. doi: 10.1016/S0003-4975(03)01350-X. discussion 456–7. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci U S A. 2003;100:12331–6. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thistlethwaite PA, Lee SH, Du LL, Wolf PL, Sullivan C, Pradhan S, Deutsch R, Jamieson SW. Human angiopoietin gene expression is a marker for severity of pulmonary hypertension in patients undergoing pulmonary thromboendarterectomy. The Journal of thoracic and cardiovascular surgery. 2001;122:65–73. doi: 10.1067/mtc.2001.113753. [DOI] [PubMed] [Google Scholar]
- 23.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Ichimura A, Ruike Y, Terasawa K, Tsujimoto G. miRNAs and regulation of cell signaling. FEBS J. 2011;278:1610–8. doi: 10.1111/j.1742-4658.2011.08087.x. [DOI] [PubMed] [Google Scholar]
- 25.Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, Nicoloso MS, Barbarotto E, Popa M, Stanciulea O, Fernandez MH, Tulbure D, Bueso-Ramos CE, Negrini M, Calin GA. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Scholten D, Frey N, Koch A, Trautwein C, Tacke F, Luedde T. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PLoS One. 2013;8:e54612. doi: 10.1371/journal.pone.0054612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Z, Wen G, Wang G, Pu X, Ye S, Xu Q, Wang W, Xiao Q. MicroRNA-200C and −150 play an important role in endothelial cell differentiation and vasculogenesis by targeting transcription repressor ZEB1. Stem cells. 2013;31:1749–62. doi: 10.1002/stem.1448. [DOI] [PubMed] [Google Scholar]
- 28.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circulation research. 2008;103:1164–72. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–43. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, Sukhatme VP. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–61. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, Ichiki T, Glasgow WC, Rao GN. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2009;29:809–15. doi: 10.1161/ATVBAHA.109.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minami T, Jiang S, Schadler K, Suehiro J, Osawa T, Oike Y, Miura M, Naito M, Kodama T, Ryeom S. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep. 2013;4:709–23. doi: 10.1016/j.celrep.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deleuze V, El-Hajj R, Chalhoub E, Dohet C, Pinet V, Couttet P, Mathieu D. Angiopoietin-2 is a direct transcriptional target of TAL1, LYL1 and LMO2 in endothelial cells. PLoS One. 2012;7:e40484. doi: 10.1371/journal.pone.0040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–92. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839–47. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Taylor MA, Schiemann WP. Therapeutic Opportunities for Targeting microRNAs in Cancer. Mol Cell Ther. 2014;2:1–13. doi: 10.1186/2052-8426-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. The Journal of biological chemistry. 2006;281:2296–305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 38.Young JA, Ting KK, Li J, Moller T, Dunn L, Lu Y, Moses J, Prado-Lourenco L, Khachigian LM, Ng M, Gregory PA, Goodall GJ, Tsykin A, Lichtenstein I, Hahn CN, Tran N, Shackel N, Kench JG, McCaughan G, Vadas MA, Gamble JR. Regulation of vascular leak and recovery from ischemic injury by general and VE-cadherin-restricted miRNA antagonists of miR-27. Blood. 2013;122:2911–9. doi: 10.1182/blood-2012-12-473017. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt WM, Spiel AO, Jilma B, Wolzt M, Muller M. In vivo profile of the human leukocyte microRNA response to endotoxemia. Biochem Biophys Res Commun. 2009;380:437–41. doi: 10.1016/j.bbrc.2008.12.190. [DOI] [PubMed] [Google Scholar]
- 40.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 41.Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos N, McDonald DM, Thurston G, Yancopoulos GD, Rudge JS. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15491–6. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–10. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- 43.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 44.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circulation research. 2015;117:870–83. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–59. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Menden H, Welak S, Cossette S, Ramchandran R, Sampath V. Lipopolysaccharide (LPS)-mediated angiopoietin-2-dependent autocrine angiogenesis is regulated by NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial cells. The Journal of biological chemistry. 2015;290:5449–61. doi: 10.1074/jbc.M114.600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue Y, Cao R, Nilsson D, Chen S, Westergren R, Hedlund EM, Martijn C, Rondahl L, Krauli P, Walum E, Enerback S, Cao Y. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci U S A. 2008;105:10167–72. doi: 10.1073/pnas.0802486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang F, Ooka K, Bhattacharyya S, Wei J, Wu M, Du P, Lin S, Del Galdo F, Feghali-Bostwick CA, Varga J. The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-beta that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Pathol. 2011;178:2077–90. doi: 10.1016/j.ajpath.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. The Journal of biological chemistry. 2013;288:20488–98. doi: 10.1074/jbc.M112.447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poirier R, Cheval H, Mailhes C, Garel S, Charnay P, Davis S, Laroche S. Distinct functions of egr gene family members in cognitive processes. Front Neurosci. 2008;2:47–55. doi: 10.3389/neuro.01.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dillon RL, Brown ST, Ling C, Shioda T, Muller WJ. An EGR2/CITED1 transcription factor complex and the 14-3-3sigma tumor suppressor are involved in regulating ErbB2 expression in a transgenic-mouse model of human breast cancer. Molecular and cellular biology. 2007;27:8648–57. doi: 10.1128/MCB.00866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, Dumont DJ, Stewart DJ. Cell-based angiopoietin-1 gene therapy for acute lung injury. American journal of respiratory and critical care medicine. 2007;175:1014–26. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.