Abstract
Oxidative stress plays an important role in the progression of HIV-1 infection. Nicotine can either protect neurons from neurodegeneration or induce oxidative stress, depending on its dose and degree of oxidative stress impairment. However, the relationship between nicotine and oxidative stress in the HIV-1-infected individuals remains largely unknown. The purpose of this study was to determine the effect of nicotine on expression of genes related to the glutathione (GSH)-centered antioxidant system and oxidative stress in the nucleus accumbens (NAc) and ventral tegmental area (VTA) of HIV-1 transgenic (HIV-1Tg) and F344 control rats. Adult HIV-1Tg and F344 rats received nicotine (0.4 mg/kg; base; s.c.) or saline injections once per day for 27 days. At the end of treatment, various brain regions including the NAc and VTA were collected from each rat. Following total RNA extraction and cDNA synthesis of each sample, quantitative RT-PCR analysis was performed for 43 oxidative stress-related genes. Compared with F344 control rats, HIV-1Tg rats showed a significant down-regulation of genes involved in ATPase and cyctochrome oxidase at the mRNA level in both regions. Further, we found a significant down-regulation of Gstm5 in the NAc and up-regulation of Cox1, Cox3 and Gsta6 in the VTA of HIV-1Tg rats. HIV-1Tg rats showed brain region-specific responses to chronic nicotine treatment. This response resulted in a change in the expression of genes involved in antioxidant mechanisms including the down-regulation of genes such as Atp5h, Calml1, Gpx7, Gstm5, Gsr and Gsta6 and up-regulation of Sod1 in the NAc, as well as down-regulation of genes like Cox5a, Gpx4, Gpx6, Gpx7, Gstm5, and Sod1 expression in the VTA of HIV-1Tg rats. Together, we conclude that chronic nicotine treatment has a dual effect on the antioxidant defense system and oxidative stress-induced apoptosis signaling in HIV-1Tg rats. These findings suggest that nicotine has a negative effect on response to oxidative stress and antioxidant processes in HIV-1 Tg rat brain, especially in the VTA.
Keywords: HIV, nicotine, NAc, VTA, oxidative stress, antioxidants
1. Introduction
The central nervous system (CNS) is one of the major targets of the human immunodeficiency virus (HIV-1) and exhibits high vulnerability to HIV-1-mediated pathogenesis (Churchill et al, 2014). HIV-1 proteins can directly cross the blood-brain barrier (BBB) and exert effects on the brain in early stages of HIV-1 infection (Kaul et al, 2005; Kimura et al, 1993a). HIV-1 proteins affect the activation of monocytic cells (e.g., microglia, macrophages and astrocytes), leading to the increased production of neuroactive and neuroimmune products in the brain (Kimura et al, 1993b). Several lines of evidence suggest that an increased inflammatory response to viral proteins can facilitate the activation of oxidative stress and apoptosis signaling cascades (Kaul et al, 2005; Kimura et al, 1993b). In addition, HIV-1 proteins induce mitochondrial dysfunction by directly interacting with mitochondrial membrane proteins, leading to high levels of oxidative stress in neurons (Ladha et al, 2005). Alterations in mitochondrial function and oxidative stress have been suggested as key mediators in developing HIV-1-associated neurocognitive disorders (HAND) during late stage of HIV-1-infected patients (Perez-Matute et al, 2013).
Nicotine is an agonist of nicotinic acetylcholine receptors (nAChRs) and a major psychoactive component in tobacco smoke (Levin et al, 2014). However, research on the neuroprotective and neurotoxic effects of nicotine in the CNS has been inconclusive (Nie et al, 2013; Nordberg et al, 2002; Picciotto and Zoli, 2008b; Zeng et al, 2001). For example, nicotine has been shown to produce significant improvement in the attention span and memory performance in both human and animal studies (Knott et al, 2012; Levin et al, 2014) and has been implicated in neuroprotective effects against β-amyloid-mediated neurotoxicity as well as the enhanced neuroplasticity in transgenic model for Alzheimer’s disease in mice (Nie et al, 2013). These effects are likely mediated by up-regulation of nAChR expression and decrease in the accumulation of β-amyloid peptide leading to a decrease in neurotoxicity (Kihara et al, 1997; Nordberg et al, 2002; Picciotto and Zoli, 2008b; Zeng et al, 2001). On the other hand, nicotine has been shown to modulate expression of multiple immune-related pathways in SH-SY5Y cells (Cui et al, 2012). The effect of nicotine on immune function was also demonstrated in mitochondrial signaling pathways by inhibiting chemotherapy-induced apoptosis in lung cancer (Zhang et al, 2009). There is also considerable evidence from in vivo and in vitro studies, showing that nicotine increases oxidative stress and consequently neuronal cell death (Guan et al, 2002; Quik et al, 2007; Soto-Otero et al, 2002). Under oxidative stress conditions, neurons increase their antioxidant defense mechanisms by enhancing the production and release of free radical scavengers. This alternation enhances the survival of neurons through the activation of glutathione (GSH) and antioxidant enzymes like catalase and superoxide dismutase (SOD).
Further, nicotine has also been shown to disrupt the antioxidant defense mechanisms by decreasing levels of GSH and SOD in rats and altering the balance between antioxidant capacity and oxidative stress-induced free radicals in the brain (Guan et al, 2002; Soto-Otero et al, 2002; Wang et al, 2009; Yildiz et al, 1998). The interaction between nicotine and oxidative stress mechanisms is also detected in the lipid peroxidation mechanism which is initiated by free radical reactions from mitochondria. Nicotine is reported to stimulate lipid peroxidation by increasing the malondialdehyde (MDA) and lactate dehydrogenase in CHO cells (Yildiz et al, 1998). The decrease in the antioxidant defense system and increase in the production of reactive oxygen species have been implicated in many forms of neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Subramaniam and Chesselet, 2013; Uttara et al, 2009).
Epidemiological studies have revealed an inverse correlation between smoking and the incidences of AD and PD (Picciotto and Zoli, 2008a; Zhang et al, 2009). Although studies have clearly shown the importance of mitochondria in the biological response to nicotine in these disease states, the relationship between nicotine and oxidative stress in these diseases, especially in HIV-1-infected individuals, remains largely unknown. To better understand the possible neuroprotective and neurotoxic effects of nicotine in HIV-1 patients, we compared the effect of nicotine on the expression of genes related to the (GSH)-centered antioxidant system and oxidative stress in dopaminergic midbrain regions of HIV-1Tg and F344 control rats.
2. Materials and methods
2.1 Animals
Adult male HIV-1Tg and F344 control rats were purchased from Harlan Inc. (Indianapolis, IN). All rats were maintained under controlled lighting (12:12 Light/Dark) and temperature (20°C) with ad libitum access to food and water. All animal experiments reported in this study were approved by the Animal Care and Use Committee of University of Virginia.
2.2 Nicotine treatment
Nicotine hydrogen bitartarate (Sigma Aldrich Co., St. Louis, Mo) was dissolved in 0.9% w/v NaCl solution (saline) and neutralized to PH 7.2~7.0 with NaOH. Rats from each strain were divided randomly into two groups (n = 6 per group) Control (i.e., HIV-1Tg_ or F344_saline control) and nicotine-treatment group (i.e., HIV-1Tg_ and F344_nicotine-treated groups). Either nicotine (0.4 mg/kg; base) or saline was administered once a day at 9:00AM by subcutaneous injection for 27 days. This drug treatment paradigm was based on previous reported behavioral studies (Matta et al, 2007; Menon, 2001a) and our own reported study where we showed that the 27 days of 0.4 mg/kg dose of nicotine given subcutaneously to HIV-1Tg rats lead to significant changes of gene expression in the brain (Nesil et al., 20015, in press). Furthermore, previous studies have also shown that more than 20 days of nicotine injection leads to oxidative stress and tissue injury (Sener et al, 2004; Sener et al, 2005).
2.3 Tissue collection
Animals were sacrificed one day after the last injection and brains were immediately removed after decapitation. Using a rat brain matrix, brain slices of approximately 1-mm were taken from each brain. Tissues from the ventral tegmental area (VTA) and nucleus accumbens (NAc) were collected bilaterally from each brain using a 3.00-mm Harris Micro-Punch (GE Healthcare Life Sciences, Piscataway, NJ, USA) according to the rat brain atlas (Paxinos and Watson, 1998). Punched tissues were stored at −80°C until use.
2.4 Quantitative RT-PCR array analysis
Total RNA was isolated from punched brain tissues using the Trizol reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. The quantity and purity of the extracted total RNA were measured at the optical densities of 260 and 280 nm with NanoDrop 2000c (Thermo Fisher Scientific, Waltham, MA). The primers for each gene of interest were designed using Primer Express (v. 3.0) software (Applied Biosystems, Carlsbad, CA, USA) and spanned introns to avoid amplifying genomic DNA, with a melting temperature (Tm) from 59°C to 61°C. The amplicon sequences were subjected to a BLAST search to ensure the specificity of the primers for the target genes of interest. All the primers were tested for their specificity by checking the cycle number and the dissociation curve before they were included in the qRT-PCR array. The primer sequencers of all examined genes in this study are listed in Table 1.
Table 1.
Primer sequences used for quantitative RT-PCR in this study
| Gene Symbol |
Gene Name | Forward Primer (5'-3') | Reverse Primer (5'-3') |
|---|---|---|---|
| Atp6v1f | V-type proton ATPase subunit F |
AGACACTTTCAGGCAGTTTCTAA ACA |
CCGAACCATCTCTGCGATGT |
| Abcd2 | ATP-binding cassette sub- family D member 2 |
GATGGCCCTAAGCAGGCTATG | CAGTATAACCCGCTAGTTCAGT GATC |
| Atp5h | ATP-binding cassette sub- family D member 2 |
CCATCGATTGGGTATCTTTTGTG | TTATTCCAGGACTTCAGAGCAT TTC |
| Abcc8 | ATP-binding cassette sub- family C member 8 |
ATCTCCCCAGGACAGAAGATTG | CGGAAAAAGGCGAGAGAGAA |
| Ndufa8 | NADH dehydrogenase | GGAGGGCAAGCTGGTCAAC | AAAGGCTCCGCACAGTGACT |
| Ndufb2 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 2 |
TCTTTGGAGATTTTGGCATGACT | TCATCTGTCCACTTCGATGGAT |
| Ndufa2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2 |
CGTGAGATTCGCATTCACTTATG | ACCGTTGCTGGATGAAATCTCT |
| Ndufb11 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 11 |
CCTGCCTGATTACAGGATGCA | TTCTCGGTATTTCACAAGTCTC TCA |
| Ndufs4 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, mitochondrial |
AGGCAAAGGGCAGTAGCTACAG | CAGCTTCCATGTCGATGTGTTC |
| Cox1 | Cytochrome c oxidase subunit I (mitochondrion) |
GAGCAGTATTCGCCATCATAGCT | TTTTGCTCATGTGTCATTTAGG GTAT |
| Cox2 | Cytochrome c oxidase subunit II (mitochondrion) |
CCGTCCCTTCACTAGGGTTAAAA | TGATGTCACTGTAGCTTGGTTT AGG |
| Cox3 | Cytochrome c oxidase subunit 3 |
ACGAGATATCATCCGTGAAGGAA | TTCCGTATCGGAGGCCTTT |
| Cox5a | Cytochrome c oxidase subunit 5A, mitochondrial |
TGGGCCTTGACAAAGTGTAAACT | TTCCAGGCAACTGTTTCAATCA |
| Cox6a | Cytochrome c oxidase subunit 6A1, mitochondrial |
TGAAGTCGCGACACGAAGAG | GGGAAGGGCTTAGTCCTGATG |
| Cox8b | Cytochrome c oxidase subunit 8B, mitochondrial |
CTCTTCCAAGCCAGCCAAAT | GCCAGCGATTATGACTGACATC |
| Sod1 | Superoxide dismutase [Cu- Zn] |
CACTGCAGGACCTCATTTTAATCC | GTCTCCAACATGCCTCTCTTCA T |
| Sod2 | Superoxide dismutase [Mn], mitochondrial |
CGCTGGCCAAGGGAGAT | CCCCGCCATTGAACTTCA |
| Sod3 | Extracellular superoxide dismutase [Cu-Zn] |
GGCAGCTCAGAGGCTCTTTCT | CTGAGGTTCCACACCTGACAAG |
| Gpx1 | Glutathione peroxidase 1 | CTCGGTTTCCCGTGCAAT | TCGGACATACTTGAGGGAATTC A |
| Gpx4 | Glutathione peroxidase 4 | ACGAGTTCCTGGGCTTGTGT | TGCGAATTCGTGCATGGA |
| Gpx5 | Glutathione peroxidase 5 | TGGTGAAAAAGAGCAGGAAATCT | CCCAGAATGTATGTTTGCTTGT G |
| Gpx6 | Glutathione peroxidase 6 | CCCGTACCCAGTAGGATGTTTTA | TTGCAGGCTTTGATCATATGGA |
| Gpx7 | Glutathione peroxidase 7 precursor |
GCTGGTGTCGCTGGAGAAGT | AGCCACATTCGCTAGCTACATT C |
| Gstk1 | Glutathione S-transferase kappa 1 |
AACACCTCTGGAATATCAAGCTG AA |
GTGGTTGGTTTCCACTGTCTTTC |
| Gstm5 | Glutathione S-transferase Mu 5 |
TCACCCAGAGTAACGCCATCT | CTTCTTCGGTGTCACCACACA |
| Gsta6 | Glutathione S-transferase A6 | TCAACTGGAGGACACCTTGGA | CCCCGGGCCTCATCA |
| Gsr | Glutathione reductase | TGAGCCGCCTGAACAACA | TTGCGTAGCCGTGGATGAC |
| Perp | P53 apoptosis effector related to PMP-22 |
TGCCAGAGCCTCATGGAATAC | GAAGCAGATGACCAGGATGAT G |
| Bmf | Bcl-2-modifying factor | ACAGATCGCCAGAAAGCTTCA | CTGCTGGTGTTGTTGCATATGA |
| Bcl-2 | Apoptosis regulator Bcl-2 | GGAGGCTGGGATGCCTTTG | CTGAGCAGCGTCTTCAGAGACA |
| Bax | Apoptosis regulator BAX | CTCAAGGCCCTGTGCACTAAA | CCCGGAGGAAGTCCAGTGT |
| Bad | Bcl2 antagonist of cell death | CGGGACAGGCAGCCAATA | CGACTCCGGGTCTCCATAGTC |
| Bcl2L1 | Bcl-2-like protein 1 | GCAGTCAGCCAGAACCCTATCT | GTCCAAAACACCTGCTCACTCA |
| Bcl2L11 | Bcl-2-like protein 11 | GGATCGGAGACGAGTTCAATG | CCTCTCGGTAATCGTTTGCAA |
| Casp1 | Caspase-1 | CCTCAAGTTTTGCCCTTTAGAAAT | TGAAAGTCTGTGCTGCAGATAA TG |
| Casp2 | Caspase-2 | CCATGCACTCCTGAGTTTTACCA | CGAGGCTGAGACTGCAACCT |
| Casp3 | Caspase-3 | AGGCCGACTTCCTGTATGCTT | TGACCCGTCCCTTGAATTTC |
| Casp4 | Caspase 4 | TCAGGCAGCCACCATGGT | AGAGTGTCCACTGATTCTTCTG GTT |
| Casp6 | Caspase 6 | GAAAACCCAAGATATTCATCATC CA |
TCCACCACGTCCAGAGGAA |
| Casp7 | Caspase-7 | CATCTCATCCCTTCTCTGGAAGA | GCGGTCCCGGGTGGTA |
| Casp8 | Caspase-8 | CGAACGATCAAGCACAGAGAGA | CTGGCGAGTCCCACATGTC |
| Casp9 | Caspase-9 | GAGAGACATGCAGATATGGCATA CA |
CAGAAGTTCACGTTGTTGATGA TG |
| Camk2b | Calcium/calmodulin- dependent protein kinase type II subunit beta isoform 2 |
CATCACCAGCCCCAAAGG | TGGCATCTTCGTCCTCTATGG |
First-strand cDNAs were synthesized from two µg of total RNA using a Reverse Transcription System Kit (Promega, Madison, WI). The qRT-PCR analysis was conducted as described previously (Wei et al, 2011). Briefly, RT product was amplified in a volume of 10 µl containing 5 µl 2×Power SYBR Green PCR Master Mix (Applied Biosystems, CA, USA), equally mixed sense and antisense primers (with a final concentration 250 nM each), and 2.5 µl diluted cDNA in a 384-well plate using the 7900HT Fast Real Time PCR system (Applied Biosystems) (Cui et al, 2012; Wang et al, 2011). The PCR condition was as follows: 950C for 10 min, followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. A cycle threshold (Ct ) was assigned in the beginning of logarithmic phase of amplification and difference in Ct values of the control and treated groups were used to determine the relative expression. Melting curve analysis was applied to characterize the specificity of the amplicons.
2.5 Statistical analysis
Expression levels of all genes of interest were first normalized to the expression of housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and then analyzed using a comparative Ct method (Winer et al, 1999; Zhao et al, 2014). The relative gene expression level was compared between F344 and HIV-1Tg rats using the Student’s t test. Significant alteration in mRNA expression level was defined as a fold change >20% with a p value <0.05.
3. Results
3.1 Strain differences in the expression of genes related to oxidative stress and mitochondrial function
There is a large body of evidence revealing that HIV-1 proteins induce mitochondrial dysfunction as a result of increased oxidative stress which has been implicated in several types of neuropathogenesis of HIV-1 infection in the brain (Churchill et al, 2014; Perez-Matute et al, 2013). Considering the effects of HIV-1 proteins on the mitochondrial function and oxidative stress, we examined the expression profiles of 43 genes involved in electron transport chain including ubiquinone oxidoreductase (i.e., mitonchondrial complex I), succinate dehydrogenase (mitonchondrial complex II), cytochrome bc1 complex (mitonchondrial complex III), cytochrome c oxidase (mitonchondrial complex IV), apoptosis, antioxidant activity, and ATP transporters in the NAc and VTA regions of HIV-1Tg and F344 control rats. Our statistical analysis of mRNA expression data in the HIV-1Tg and F344 rats at the cut-off ratio of > 20% mRNA fold change revealed that 9 genes in the NAc (Table 2) and 12 genes in the VTA (Table 3) showed significant gene expression differences in HIV-1Tg rats compared to F344 rats. In the NAc, expression levels of the genes in mitochondrial complex I (Ndufa2; P = 0.0004; Ndufb2; P = 0.009), complex IV (Cox5a; P = 0.018; Cox6a; P = 0.007) and ATPase in complex V (Abcd2, Atp5h and Atp6v1f; P = 0.001–0.05) were significantly down-regulated by viral proteins. We also found a significant down–regulation of the expression of antioxidant enzyme glutathione s-transferase subunit Gstm5 in the NAc of HIV-1Tg rats.
Table 2.
Differentially expressed genes in the NAc between HIV-1Tg and F344 rats
| Gene Symbol | F344 (Mean ± SEM) |
HIV-1Tg (Mean ± SEM) |
Ratio (HIV-1Tg/F344) |
P-value |
|---|---|---|---|---|
| Calml1 | 0.972 ± 0.099 | 1.417 ± 0.457 | 1.458 | 0.042 |
| Abcd2 | 0.017 ± 0.002 | 0.013 ± 0.002 | 0.762 | 0.017 |
| Atp5h | 0.204 ± 0.028 | 0.167 ± 0.010 | 0.818 | 0.013 |
| Atp6v1f | 0.156 ± 0.012 | 0.122 ± 0.012 | 0.777 | 0.0005 |
| Cox5a | 0.077 ± 0.004 | 0.056 ± 0.016 | 0.723 | 0.018 |
| Cox6a | 0.443 ± 0.060 | 0.342 ± 0.026 | 0.772 | 0.007 |
| Ndufa2 | 0.046 ± 0.002 | 0.035 ± 0.004 | 0.754 | 0.0004 |
| Ndufb2 | 0.027 ± 0.005 | 0.020 ± 0.001 | 0.738 | 0.009 |
| Gstm5 | 0.035 ± 0.004 | 0.025 ± 0.003 | 0.709 | 0.002 |
Table 3.
Differentially expressed genes in the VTA between HIV-1Tg and F344 Rats
| Gene Symbol | F344 (Mean ± SEM) |
HIV-1Tg (Mean ± SEM) |
Ratio (HIV-1Tg/F344) |
P-value |
|---|---|---|---|---|
| Cox1 | 21.371 ± 1.912 | 27.430 ± 5.076 | 1.284 | 0.021 |
| Cox3 | 14.918 ± 1.319 | 20.230 ± 4.55 | 1.356 | 0.021 |
| Gsta6 | 0.008 ± 0.0006 | 0.011 ± 0.002 | 1.286 | 0.045 |
| Perp | 0.003 ± 0.0006 | 0.005 ± 0.001 | 1.545 | 0.033 |
| Abcd2 | 0.012 ± 0.002 | 0.008 ± 0.004 | 0.653 | 0.044 |
| Atp5h | 0.174 ± 0.028 | 0.127 ± 0.026 | 0.730 | 0.013 |
| Atp6v1f | 0.153 ± 0.017 | 0.123 ± 0.020 | 0.809 | 0.022 |
| Ndufa2 | 0.064 ± 0.017 | 0.042 ± 0.016 | 0.651 | 0.042 |
| Ndufa8 | 0.137 ± 0.016 | 0.106 ± 0.028 | 0.778 | 0.044 |
| Ndufb2 | 0.029 ± 0.009 | 0.019 ± 0.0040 | 0.640 | 0.025 |
| Cox8b | 0.00002 ± 0.000005 | 0.00002 ± 0.000001 | 0.524 | 0.021 |
| Casp9 | 0.006 ±0.001 | 0.005 ± 0.0007 | 0.750 | 0.002 |
The calcium signals and activation of Ca+2/calmodulin-dependent kinase signaling lead to mitochondrial reactive oxygen species (ROS) generation and are critical in oxidative stress-induced neuronal apoptosis. The impact of mitochondrial dysfunction on intracellular calcium levels has been reported in neurodegenerative diseases (Subramaniam and Chesselet, 2013). Our results indicated that expression of calcium binding protein calmodulin 1 (Calm1) in the VTA, which functions as calcium binding protein and activates calmodulin-dependent kinases, was 45.8% higher HIV-1Tg rats than that in F344 rats (P = 0.042; Table 2).
In the VTA (Table 3), HIV-1 proteins significantly down-regulated the expression of genes in the mitochondrial complex I (Ndufa2, Ndufb2 and Ndufa8; P < 0.05 for all of them) and complex V (Abcd2, Atp5h and Atp6v1f; P < 0.05 for all of them). We found a biphasic expression pattern of cyctochrome oxidase in mitochondrial comlex IV encoding genes where expression of Cox1 and Cox3 mRNA were increased by 28.4% and 35.6% (P = 0.021 for both genes), respectively. The expression of Cox8b was decreased by 48% in mRNA expression (P = 0.021) in VTA. In addition, genes associated with antioxidant defence (Gsta6; P = 0.045) and the p53-dependent apoptosis pathway (Perp; P = 0.033) showed a significant increase of mRNA expression in the VTA of HIV-1Tg rats (Table 3).
3.2 The effects of HIV-1 proteins and nicotine on the mRNA expression in the NAc and VTA of HIV-1Tg rats
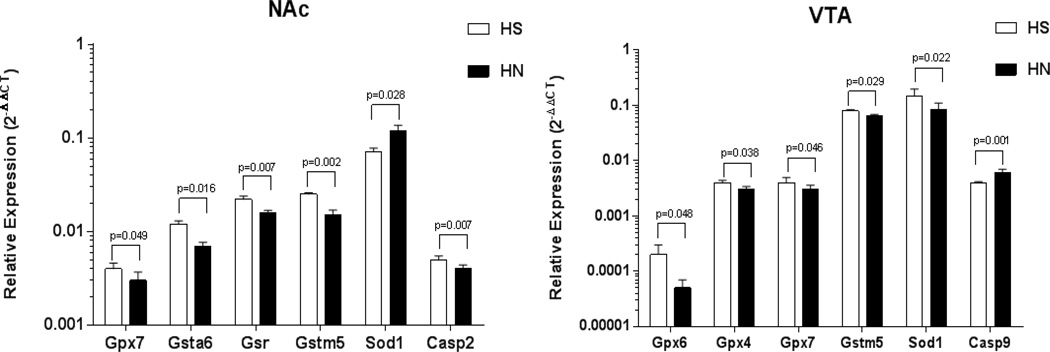
The role of oxidative stress in midbrain dopaminergic cell loss has been indicated to be one of the major causes of neurodegeneration during HIV-1 infection (Nath et al, 2000). Since nicotine has a dual effect on induction of oxidative stress and neuroprotection, we next examined whether nicotine can potentiate mitochondrial dysfunction-mediated oxidative stress caused by viral proteins in the NAc and VTA of HIV-1Tg rats. Chronic nicotine treatment significantly modified the mRNA expression levels in the HIV-1Tg rats; this effect was more pronounced in the GSH-centered antioxidant system-related genes in the NAc and VTA. As shown in Table 4 and Figure 1, nicotine significantly down-regulated the expression of Gsta6, Gpx7, Gstm5 and Gsr by 25%-34% (P = 0.002–0.049), but up-regulated the expression of superoxide dismutase encoding gene Sod1 by 69% (P = 0.02) in the NAc of HIV-1Tg rats (Table 4; top panel). Further, chronic nicotine treatment decreased the expression of genes involved in mitochondrial complex V (Atp5h; P = 0.004) and in Ca+2/calmodulin-dependent kinase signaling (Calm1; P = 0.005).
Table 4.
Significantly regulated genes by nicotine in the NAc and VTA of HIV-1Tg rats
| Brain Region |
Gene symbol |
HIV-1Tg_Saline (Mean ± SEM) |
HIV-1Tg_Nicotine (Mean ± SEM) |
Nicotine/Saline Ratio |
P-value |
|---|---|---|---|---|---|
| NAc | Atp5h | 0.167 ± 0.004 | 0.119 ± 0.013 | 0.715 | 0.004 |
| Calml1 | 1.417 ± 0.457 | 0.644 ± 0.109 | 0.454 | 0.005 | |
| Casp2 | 0.005 ± 0.0005 | 0.004 ± 0.0004 | 0.846 | 0.007 | |
| Casp7 | 0.002 ± 0.0003 | 0.002 ± 0.0001 | 0.809 | 0.017 | |
| Gsta6 | 0.012 ± 0.001 | 0.007 ± 0.0007 | 0.609 | 0.016 | |
| Gpx7 | 0.004 ± 0.0006 | 0.003 ± 0.0007 | 0.757 | 0.049 | |
| Gsr | 0.022 ± 0.002 | 0.016 ± 0.0008 | 0.712 | 0.007 | |
| Gstm5 | 0.025 ± 0.001 | 0.015 ± 0.002 | 0.594 | 0.002 | |
| Sod1 | 0.071 ± 0.007 | 0.119 ± 0.018 | 1.687 | 0.028 | |
| VTA | Bcl2L11 | 0.0006 ± 0.00003 | 0.0008 ± 3.39E-05 | 1.267 | 0.009 |
| Casp9 | 0.004 ± 0.0002 | 0.006 ± 0.0001 | 1.281 | 0.001 | |
| Casp1 | 0.0006 ± 5.81E-05 | 0.0004 ± 4.52E-05 | 0.661 | 0.034 | |
| Cox5a | 0.067 ± 0.017 | 0.048 ± 0.004 | 0.721 | 0.046 | |
| Gpx4 | 0.004 ± 0.0004 | 0.003 ± 0.0002 | 0.729 | 0.038 | |
| Gpx6 | 0.0002 ± 0.0001 | 0.00005 ± 0.00002 | 0.267 | 0.048 | |
| Gpx7 | 0.004 ± 0.001 | 0.003 ± 0.0006 | 0.714 | 0.046 | |
| Gstm5 | 0.080 ± 0.003 | 0.065 ± 0.004 | 0.813 | 0.029 | |
| Sod1 | 0.147 ± 0.050 | 0.085 ± 0.026 | 0.576 | 0.022 |
Figure 1.
Brain region-specific effects of nicotine on the expression of genes in the glutathione-centered antioxidant defense system and apoptosis signaling in the NAc (left panel) and VTA (right panel) of HIV-1Tg rats. The relative quantity is presented in a log10 scale. Bars indicate mean ± SEM. Statistical comparisons were made using two-tailed, unpaired Student’s t-test between saline control and nicotine-treated groups. HS = Saline-treated HIV-1Tg rats; HN = Nicotine-treated HIV-1Tg rats (n = 4–6 per group).
In the VTA, nicotine significantly modulated the expression of genes in the apoptosis signaling, antioxidant defence system, and mitochondrial complex IV in the HIV-1Tg rats. The expression levels of the genes in the GSH-centered antioxidant system (Gpx4, Gpx6, Gpx7, Gstm5) and superoxide dismutase encoding Sod1 were significantly down-regulated by 18.7%-73.3% (P = 0.02–0.04) in the nicotine-treated HIV-1Tg rats compared to the saline-trated HIV-1Tg rats (Figure 1). Similiarly, expression level of Cox5a in complex IV was significantly down-regulated by nicotine in the VTA of HIV-1Tg rats (27.9%; P = 0.038). Different from the observed effect in the NAc, chronic nicotine treatment significantly increased the expression of the genes involved in apoptosis signaling, including pro-apoptotic gene (Casp9; 28.1%; P = 0.001) and apotosis facilitator (Bcl2L11; 26.7%; P = 0.009) (Table 4; bottom panel).
3.3 Nicotinic modulaton of oxidative stress and mitochondrial function related gene expression in F344 rats
Previous studies have shown that nicotine has neuroprotective effects in the CNS during neurodegeneration (Quik et al, 2007). To determine whether nicotine induces oxidative stress or has a positive effect on the antioxidant system under normal physiological conditions, we compared the same set of genes involved in oxidative stress, antioxidant defense mechanism, apoptosis signaling and mitochondrial function between saline and nicotine treatment groups in F344 rats. Nicotine significantly increased the mRNA expression of the gene encoding superoxide dismutase antioxidant enzyme (Sod1; 93.5%; P = 0.034), but decreased the glutathione peroxidase 1 (Gpx1) mRNA expression (31.9%; P = 0.005) in the NAc (Table 5).
Table 5.
Significantly regulated genes by nicotine in the NAc and VTA of F344 rats
| Brain Region |
Gene Symbol |
F344_Saline (Mean ± SEM) |
F344_Nicotine (Mean ± SEM) |
Nicotine/Saline Ratio |
P-value |
|---|---|---|---|---|---|
| NAc | Sod1 | 0.080 ± 0.009 | 0.154 ± 0.025 | 1.935 | 0.034 |
| Atp6v1f | 0.156 ± 0.005 | 0.135 ± 0.005 | 0.864 | 0.015 | |
| Gpx1 | 0.014 ± 0.0008 | 0.009 ± 0.0009 | 0.681 | 0.005 | |
| VTA | Casp8 | 0.0004 ± 3.51E-05 | 0.0005 ± 3.78E-05 | 1.421 | 0.031 |
| Cox8b | 2.14E-05 ± 2.13E-06 | 0.0001 ± 4.07E-05 | 5.233 | 0.023 | |
| Gsta6 | 0.008 ± 0.0006 | 0.013 ± 0.003 | 1.595 | 0.008 | |
| Gpx4 | 0.003 ± 0.0003 | 0.004 ± 0.0002 | 1.455 | 0.004 | |
| Gpx5 | 0.00005 ± 0.00001 | 0.0001 ± 0.00004 | 2.5 | 0.011 | |
| Gpx7 | 0.004 ± 0.0009 | 0.005 ± 0.0006 | 1.328 | 0.021 | |
| Gstk1 | 0.019 ± 0.002 | 0.024 ± 0.001 | 1.270 | 0.022 | |
| Perp | 0.003 ± 0.0006 | 0.004 ± 0.0004 | 1.242 | 0.035 | |
| Sod2 | 0.085 ± 0.005 | 0.065 ± 0.004 | 0.766 | 0.015 |
In contrast to the observed effects of nicotine in the HIV-1Tg rats, genes encoding the GSH-centered antioxidant system members (Gpx4, Gpx5, Gpx7, Gstk1 and Gsta6) were significantly up-regulated (27%-150%; P = 0.02–0.008) by nicotine in the VTA of F344 rats. However, antioxidant enzyme superoxide dismutase encoding gene Sod2 was significantly down-regulated (23.4%; P = 0.01) by nicotine treatment. Our results also indicated a significant increase in the expression of Casp8 (42.1%; P = 0.031) in the apoptosis signaling pathway and of apoptosis facilitator Perp gene (24.2%; P = 0.035) by nicotine in the VTA of F344 rats (Table 5).
4. Discussion
We demonstrated the effects of HIV-1 proteins and nicotine on the expression of genes associated with mitochondrial function and the antioxidant defense system in the VTA and NAc of HIV-1Tg and F344 rats. Results showed that HIV-1 proteins disrupted mitochondrial function through mitochondrial complexes I-V and showed a brain region-specific effect on the expression of glutathione-centered antioxidant enzymes in the NAc and VTA. We also found that a moderate dose of nicotine partially attenuated the effects of HIV-1 proteins in the NAc and potentiated the effects of viral proteins on oxidative stress and apoptosis signaling in the VTA. Further, chronic nicotine treatment showed brain region-specific effects on the expression of genes associated with the antioxidant defense system. A significant increase was observed in the expression of genes in the GSH-antioxidant system in the VTA and expression of superoxide dismutase in the NAc of F344 control rats.
Alterations in mitochondrial function play a major role in the development and progression of neurodegenerative disorders, including HAND (Lee et al, 2010; Stack et al, 2008). HIV-1 proteins induce oxidative stress by decreasing the activity of mitochondrial complexes I-V and increasing the production of ROS in the brain (Aksenov et al, 2001; Ladha et al, 2005; Mollace et al, 2001). Previous studies have shown that HIV-1 proteins (e.g., gp120 and Tat) preferentially decrease mitochondrial function and increase the neurotoxicity in the midbrain dopaminergic neurons (Aksenov et al, 2001). The current study showed that HIV-1 proteins induced mitochondrial dysfunction in the NAc and VTA by altering the expression profiles of genes in mitochondrial complexes I-V. The genes involved in complex I showed lower expression levels in the NAc (Ndfun2a) and VTA (Ndfub2, Ndfub8 and Ndfub2a) of HIV-1Tg rats. Impaired complex I activity has been shown in HIV-1-induced apoptosis in T-cells (Tripathy and Mitra, 2010). The decrease in the expression of complex I subunit Ndufa6 has been reported to have a role in the mitochondrial dysfunction during HIV-1 infection (Ladha et al, 2005). Among the mitochondrial respiratory chain complexes, alterations in the activity of complex IV have an important role in mediating the effects of HIV-1 proteins on oxidative stress. Miro et al. (Miro et al, 2004) showed that complex IV activity was decreased by 19% in the mononuclear cells of HIV-1-infected patients. In contrast, Tripathy et al. (Tripathy and Mitra, 2010) reported that an up-regulation of Cox2, a subunit of complex IV, with a concomitant increase in HIV-1 induced apoptosis in T-cells. In our analysis of the gene expression in HIV-1Tg rats, the complex IV subunits Cox5a and Cox6a in the NAc and Cox8a in the VTA displayed low expression levels in the HIV-Tg rats compared to F344 rats.
Mitochondrial ATP-synthase (complex V) activity has a pivotal role in energy metabolism of neurons (Chaban et al, 2014). Decreased activity in complexes I-IV and increased oxidative stress are defined as important factors in the impairment of mitochondrial ATP-synthase activity (Mollace et al, 2001; Navarro and Boveris, 2010). There is a strong relationship between deprivation of energy metabolism and ROS production; however the interaction between excessive ROS production and the decline of mitochondrial ATP synthesis in the midbrain has not been fully investigated during HIV-1 infection. In the current study, our results indicate a possible dysfunction in the complex V due to the reduced expression levels of ATPase subunits in the midbrain regions of HIV-1Tg rats. The expression levels of Abcd2, Atp5h and ATP6v1f genes were significantly down-regulated by HIV-1 proteins in the NAc and VTA. These results indicate that HIV-1 proteins can alter energy metabolism through both complexes I and IV and increase ROS production in the midbrain.
The interaction between ROS and the antioxidant defense system has been shown in the molecular mechanisms of HIV-1-associated neurocognitive disorders (Mollace et al, 2001; Price et al, 2005). Depletion of the antioxidant defense system leads to an accumulation of ROS in the mitochondria (Mollace et al, 2001; Price et al, 2005). This alteration increases the formation of apoptosome complex. This formation activates caspase-9-mediated apoptotic signaling cascades during neurodegeneration (Fratiglioni and Wang, 2000). HIV-1 proteins trigger the apoptotic signaling cascades by decreasing the levels of antioxidant enzymes with uncompensated ROS production (Louboutin et al, 2012). Our results partially confirm the aforementioned findings and show that HIV-1 proteins decreased the expression of antioxidant enzymes in the NAc (Gstm5) and up-regulated the Gsta6 in the VTA. Our results also showed the effect of HIV-1 proteins on the expression of genes in apoptotic signaling cascades in both VTA and NAc. Compared to F344 rats, HIV-1Tg rats showed higher expression of Perp, which is linked to P53-dependent apoptosis signaling, and lower expression levels of pro-apoptotic Caspase-9. The loss of mitochondrial calcium buffering capacity triggers calmodulin-dependent apoptosis signaling pathways in the brain. Also, we showed that HIV-1 proteins increased the calmodulin expression in the NAc, suggesting the interaction of mitochondrial dysfunction and calcium in the loss of dopaminergic neurons.
Previous studies have revealed the dual effects of nicotine on oxidative stress and neurodegenerative disorders (Picciotto and Zoli, 2008a; Wang et al, 2009) . It has been reported that the dose, route of treatment and the degree of oxidative stress determine the action of nicotine in the brain during neurodegeneration. A low dose of nicotine showed neuroprotective effects depending on activation kinetics of nAChR subunits and localization of the pathogenesis in the brain (Picciotto and Zoli, 2008a). On the other hand, a high dose of nicotine showed neurotoxic effects, which trigger oxidative stress in the CNS resulting in neuronal loss in the midbrain and cortex (Gallo et al, 2010; Ravikumar et al, 2004). By examining the effect of a moderate dose of nicotine (0.4 mg/kg, base, s.c) after 27 days of chronic treatment on the expression of genes in the mitochondrial complexes, antioxidant defense system and apoptosis signaling in the HIV-1Tg rats, we found that nicotine under this moderate dose, had a biphasic effect, i.e., exhibiting antioxidant activities in the NAc and neurotoxic effects in the VTA. Chronic nicotine treatment up-regulated Sod1 expression and down-regulated the genes in the glutathione-centered antioxidant defense system in the NAc of HIV-1Tg rats. In the VTA, genes encoding the subunits of antioxidant enzymes were down-regulated by nicotine in the HIV-1Tg rats. The balance between oxidant species and removal of oxidants by the antioxidant enzymes determines the ROS-mediated apoptosis signaling in neurons. Multiple in vitro and in vivo studies have demonstrated the neuroprotective effect of Sod1 overexpression in neurodegeneration including HAND (Boven et al, 1999; Louboutin et al, 2012). Results demonstrated that increased levels of Sod1 induced a decrease in ROS levels that lead to an enhanced resistance to caspase-mediated apoptosis in neurons. In contrast to the previous findings (Picciotto and Zoli, 2008b; VanCott et al, 1997), our results showed that a moderate dose of chronic nicotine treatment exhibited neurotoxic effects in the VTA of HIV-1Tg rats.
The discrepancies in the protective effects of nicotine might be related to differences in the oxidative stress levels and actions of HIV-1 proteins in the VTA. There is increasing evidence suggesting the involvement of free radicals and iron (Fe)-induced oxidative stress in the pathogenesis of PD, AD and HIV-1 associated neurodegeneration (Tolnay et al, 1997). The increased levels of Fe and degeneration of nigrostriatal dopaminergic neurons have been determined to be a future characteristic of PD (Marshall et al, 2011). Moreover, the effect of HIV-1 proteins on Fe levels was demonstrated in primary human fetal adrenocortical (HFA) cells (Kaul et al, 2005). Results showed that viral proteins increased the concentration of ferritin (important role in the storage of Fe) and oxidative stress. Our results also showed that nicotine could alter the Fe (II) and H2O2 levels by increasing the formation of 6-hydroxydopamine (6-OHDA) which is formed through the oxidation of dopamine by Fe (II) and H202 in Fenton’s reaction (Kimura et al, 1993b; Linert et al, 1999). Given this evidence, the effect of nicotine and HIV-1 proteins in the molecular mechanisms of oxidative stress signaling, we propose that nicotine potentiates the effect of HIV-1 proteins by stimulating dopaminergic neurons in the VTA. This stimulation may increase dopamine production and release from the VTA which contributes to excessive production of Fenton reagents and depletes antioxidant enzyme activity.
Taken together, our results show that a moderate dose of chronic nicotine treatment partially attenuates the effect of HIV-1 proteins in the NAc by up-regulating Sod1 expression levels. However, nicotine potentiates the neurotoxic effects of HIV-1proteins and stimulates oxidative stress in the VTA of HIV-1 Tg rats.
We also evaluated nicotine’s effects on mitochondrial function and oxidative stress induction related genes under normal physiological conditions by exposing F344 rats to chronic nicotine treatment. Our results showed that nicotine altered the expression of antioxidant defense mechanism related genes in a brain region-specific manner. A moderate dose of chronic nicotine treatment decreased the expression of glutathione-centered antioxidant enzyme Gpx1 and increased the Sod1 expression in the NAc of F344 rats. In contrast, nicotine increased the expression levels of glutathione-center antioxidant defense system enzymes (Gsta6, Gpx4, Gpx5, Gpx7 and Gstk1) and decreased Sod1 expression levels in the VTA. In fact, nicotine enhanced the expression of genes involved in p53-apoptosis signaling (Perp) and caspase signaling (Casp8) pathways in the VTA of F344 rats. These results indicate that nicotine showed brain region-specific alterations in the antioxidant enzyme expression and induction of oxidative stress signaling pathways. The antioxidant and neuroprotective properties of nicotine have been demonstrated by reported studies, which showed that the neurotoxic and neuroprotective actions of nicotine in the health state depend on the difference in experimental models and treatment dose of nicotine (Kimura et al, 1993b; Picciotto and Zoli, 2008b). Guan et al. (Picciotto and Zoli, 2008b) showed that a low dose of nicotine (10 µM) provided a protective effect against ROS production, whereas a high concentration of nicotine (10 mM) induced oxidative-stress mediated apoptosis in PC12 cells. In contrast, Linert et al. (Linert et al, 1999) showed that nicotine had no effect on the formation of ROS or neuronal damage in the neocortex, hippocampus and neostriatum of rats. The same group also showed the beneficial effects of nicotine in neocortical primary cells and indicated the possible effect of nicotine in formation of 6-OHDA through interaction between dopamine and Fenton’s reagents. Soto-Otero et al. (Dandekar et al, 2005) reported that nicotine increased the production of hydrogen peroxide by 6-OHDA autoxidation that resulted in an increase in the neurotoxicity. Chronic nicotine exposure increases the dopamine release from VTA to NAc by regulating nicotinic acetylcholine receptors from VTA. Excessive levels of synaptic dopamine are eliminated by monoamine oxidase (MAO) which is associated with the formation of cellular oxidants (hydrogen peroxide) and oxidative stress induction. Increased production of hydrogen peroxide is scavenged by GSH-centered antioxidant enzymes that protect neurons against oxidative stress (Menon, 2001b). Therefore, brain regions with high concentrations of dopamine are more vulnerable to oxidative stress due to dopamine turnover and subsequent oxidation processes. Consistent with the literature, our results indicate that nicotine has a selective effect on oxidative stress mechanisms in the mesolimbic system. Nicotine increases the antioxidant defense mechanisms by regulating the expression of Sod1 in the NAc and GSHs in the VTA. However, a moderate dose of nicotine decreases the expression of Sod2 that results in an enhancement in the expression of genes related to apoptosis signaling (Casp8 and Perp) in the VTA.
5. Conclusion
Our study focused on the characterization of synergistic effects between HIV-1 proteins and nicotine on mitochondrial function, oxidative stress and apoptosis signaling in the dopaminergic midbrain regions. Our results indicate a novel interaction between nicotine and HIV-1 proteins in the mesolimbic brain regions where a moderate dose of chronic nicotine treatment partially attenuated the HIV-1 proteins, induced oxidative-stress mediated apoptosis by increasing the Sod1 expression, and decreasing the caspase signaling in the NAc. However, nicotine potentiated the effect of HIV-1 proteins in the VTA by decreasing the expression of genes in the antioxidant defense system and by increasing the levels of genes associated with apoptosis signaling. Further, we showed that nicotine exhibits a dual effect on the antioxidant defense system and oxidative stress mediated apoptosis signaling in a brain region-specific manner in F344 rats. These findings suggest that nicotine enhances the detrimental effects of HIV-1 proteins in the VTA and partially attenuates this effect in the NAc. Our findings need further investigation to elucidate the mechanisms of the possible interaction between nicotine and HIV-1 proteins in the dopaminergic pathways in response to psychostimulant drugs of abuse.
Acknowledgements
The project was supported, in part, by US National Institutes of Health grants DA-013783 to MDL, DA-016149 to SLC, and DA-026356 to SLC and MDL.
Footnotes
Conflict of Interest
The authors declare no conflict of interest with the study or preparation of the manuscript.
References
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochimica Et Biophysica Acta-Bioenergetics. 2014;1837:418–426. doi: 10.1016/j.bbabio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR. HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol. 2014 doi: 10.1007/s13365-014-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WY, Wang J, Wei J, Cao J, Chang SL, Gu J, Li MD. Modulation of innate immune-related pathways in nicotine-treated SH-SY5Y cells. Amino Acids. 2012;43:1157–1169. doi: 10.1007/s00726-011-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar DH, Kumar M, Ladha JS, Ganesh KN, Mitra D. A quantitative method for normalization of transfection efficiency using enhanced green fluorescent protein. Anal Biochem. 2005;342:341–344. doi: 10.1016/j.ab.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies. Behavioural Brain Research. 2000;113:117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- Gallo C, Renzi P, Loizzo S, Loizzo A, Piacente S, Festa M, Caputo M, Tecce MF, Capasso A. Potential therapeutic effects of vitamin e and C on placental oxidative stress induced by nicotine: an in vitro evidence. Open Biochem J. 2010;4:77–82. doi: 10.2174/1874091X01004010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Nordberg A, Mousavi M, Rinne JO, Hellstrom-Lindahl E. Selective changes in the levels of nicotinic acetylcholine receptor protein and of corresponding mRNA species in the brains of patients with Parkinson's disease. Brain Res. 2002;956:358–366. doi: 10.1016/s0006-8993(02)03571-0. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;(12 Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, Maeda T, Akaike A. Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann Neurol. 1997;42:159–163. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kameoka M, Ikuta K. Amplification of superoxide anion generation in phagocytic cells by HIV-1 infection. FEBS Lett. 1993a;326:232–236. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kameoka M, Ikuta K. Amplification of Superoxide Anion Generation in Phagocytic-Cells by Hiv-1 Infection. Febs Letters. 1993b;326:232–236. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- Knott V, Shah D, Millar A, McIntosh J, Fisher D, Blais C, Ilivitsky V. Nicotine, Auditory Sensory Memory, and sustained Attention in a Human Ketamine Model of Schizophrenia: Moderating Influence of a Hallucinatory Trait. Front Pharmacol. 2012;3:172. doi: 10.3389/fphar.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladha JS, Tripathy MK, Mitra D. Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 2005;12:1417–1428. doi: 10.1038/sj.cdd.4401668. [DOI] [PubMed] [Google Scholar]
- Lee HM, Hallberg LM, Greeley GH, Jr, Englander EW. Differential inhibition of mitochondrial respiratory complexes by inhalation of combustion smoke and carbon monoxide, in vivo, in the rat brain. Inhal Toxicol. 2010;22:770–777. doi: 10.3109/08958371003770315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Hao I, Burke DA, Cauley M, Hall BJ, Rezvani AH. Effects of tobacco smoke constituents, anabasine and anatabine, on memory and attention in female rats. J Psychopharmacol. 2014;28:915–922. doi: 10.1177/0269881114543721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochim Biophys Acta. 1999;1454:143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BA, van Bockstaele EJ, Strayer DS. Gene delivery of antioxidant enzymes inhibits human immunodeficiency virus type 1 gp120-induced expression of caspases. Neuroscience. 2012;214:68–77. doi: 10.1016/j.neuroscience.2012.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MM, Kirk GD, Caporaso NE, McCormack MC, Merlo CA, Hague JC, Mehta SH, Engels EA. Tobacco use and nicotine dependence among HIV-infected and uninfected injection drug users. Addictive Behaviors. 2011;36:61–67. doi: 10.1016/j.addbeh.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Menon NK. Neuropathy associated with Norplant. J Fam Plann Reprod Health Care. 2001a;27:241. [PubMed] [Google Scholar]
- Menon NK. Single-handed practice--the reality. Br J Gen Pract. 2001b;51:1014–1015. [PMC free article] [PubMed] [Google Scholar]
- Miro O, Lopez S, Martinez E, Pedrol E, Milinkovic A, Deig E, Garrabou G, Casademont J, Gatell JM, Cardellach F. Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clinical Infectious Diseases. 2004;39:710–716. doi: 10.1086/423176. [DOI] [PubMed] [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction in aging, neurodegeneration, and Parkinson's disease. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Wang Z, Zhao W, Lu J, Zhang C, Lok K, Wang Y, Shen H, Xu Z, Yin M. New nicotinic analogue ZY-1 enhances cognitive functions in a transgenic mice model of Alzheimer's disease. Neurosci Lett. 2013;537:29–34. doi: 10.1016/j.neulet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellstrom-Lindahl E, Lee M, Johnson M, Mousavi M, Hall R, Perry E, Bednar I, Court J. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer's disease (APPsw) J Neurochem. 2002;81:655–658. doi: 10.1046/j.1471-4159.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. edn. New York: Academic Press; 1998. [Google Scholar]
- Perez-Matute P, Perez-Martinez L, Blanco JR, Oteo JA. Role of mitochondria in HIV infection and associated metabolic disorders: focus on nonalcoholic fatty liver disease and lipodystrophy syndrome. Oxid Med Cell Longev. 2013;2013:493413. doi: 10.1155/2013/493413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008a;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson’s disease. Frontiers in Bioscience-Landmark. 2008b;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, O’Leary K. Nicotinic receptors as CNS targets for Parkinson’s disease. Biochem Pharmacol. 2007;74:1224–1234. doi: 10.1016/j.bcp.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar R, Flora G, Geddes JW, Hennig B, Toborek M. Nicotine attenuates oxidative stress, activation of redox-regulated transcription factors and induction of proinflammatory genes in compressive spinal cord trauma. Brain Res Mol Brain Res. 2004;124:188–198. doi: 10.1016/j.molbrainres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Sener G, Kapucu C, Paskaloglu K, Ayanoglu-Dulger G, Arbak S, Ersoy Y, Alican I. Melatonin reverses urinary system and aorta damage in the rat due to chronic nicotine administration. J Pharm Pharmacol. 2004;56:359–366. doi: 10.1211/0022357022818. [DOI] [PubMed] [Google Scholar]
- Sener G, Ozer Sehirli A, Ipci Y, Cetinel S, Cikler E, Gedik N, Alican I. Taurine treatment protects against chronic nicotine-induced oxidative changes. Fundam Clin Pharmacol. 2005;19:155–164. doi: 10.1111/j.1472-8206.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64:125–135. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- Stack EC, Ferro JL, Kim J, Del Signore SJ, Goodrich S, Matson S, Hunt BB, Cormier K, Smith K, Matson WR, Ryu H, Ferrante RJ. Therapeutic attenuation of mitochondrial dysfunction and oxidative stress in neurotoxin models of Parkinson’s disease. Biochim Biophys Acta. 2008;1782:151–162. doi: 10.1016/j.bbadis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A. Argyrophilic grain disease: widespread hyperphosphorylation of tau protein in limbic neurons. Acta Neuropathol. 1997;93:477–484. doi: 10.1007/s004010050642. [DOI] [PubMed] [Google Scholar]
- Tripathy MK, Mitra D. Differential modulation of mitochondrial OXPHOS system during HIV-1 induced T-cell apoptosis: up regulation of Complex-IV subunit COX-II and its possible implications. Apoptosis. 2010;15:28–40. doi: 10.1007/s10495-009-0408-9. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanCott TC, Mascola JR, Kaminski RW, Kalyanaraman V, Hallberg PL, Burnett PR, Ulrich JT, Rechtman DJ, Birx DL. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cui W, Wei J, Sun D, Gutala R, Gu J, Li MD. Genome-wide expression analysis reveals diverse effects of acute nicotine exposure on neuronal function-related genes and pathways. Front Psychiatry. 2011;2:5. doi: 10.3389/fpsyt.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kim JM, Donovan DM, Becker KG, Li MD. Significant modulation of mitochondrial electron transport system by nicotine in various rat brain regions. Mitochondrion. 2009;9:186–195. doi: 10.1016/j.mito.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14:91–106. doi: 10.1017/S1461145710000416. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Yildiz D, Ercal N, Armstrong DW. Nicotine enantiomers and oxidative stress. Toxicology. 1998;130:155–165. doi: 10.1016/s0300-483x(98)00105-x. [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhang Y, Peng L, Shao H, Menon NK, Yang J, Salomon AR, Freidland RP, Zagorski MG. Nicotine and amyloid formation. Biol Psychiatry. 2001;49:248–257. doi: 10.1016/s0006-3223(00)01111-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–146. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SF, Cui WY, Cao JR, Luo C, Fan LJ, Li MD. Impact of Maternal Nicotine Exposure on Expression of Myelin-Related Genes in Zebrafish Larvae. Zebrafish. 2014;11:10–16. doi: 10.1089/zeb.2013.0889. [DOI] [PubMed] [Google Scholar]