Abstract
Most studies that have examined neuropsychological impairments associated with human immunodeficiency virus (HIV) have focused on males, yet females represent one of the largest groups of newly infected patients. Further, few studies have examined neuropsychological performance and neuroimaging outcomes among females compared to males in the modern era of highly active anti-retroviral therapy (HAART). The present study investigated neuropsychological performance and brain volumetrics among HIV+ males (n = 93) and females (n = 44) on stable HAART compared to HIV seronegative (HIV−) males (n = 42) and females (n = 49). An effect of HIV, but not gender, was observed for neuropsychological performance and neuroimaging measures. Additionally, no significant differences in neuropsychological performance or brain volumetrics were seen between HIV+ males and females. No significant interaction was observed between HIV and gender on either neuropsychological or neuroimaging indices. Our results suggest that both HIV+ males and females treated with HAART experience similar outcomes in terms of brain integrity.
Keywords: HIV, Gender, Cognition, Volumetrics, Neuropsychology
Introduction
Human immunodeficiency virus (HIV) infects the central nervous system (CNS) and disrupts both gray (GM) and white matter (WM) regions of the brain (Becker et al. 2011; Kumar et al. 2011; Thompson et al. 2005). Common neuropsychological abnormalities exhibited by HIV-infected (HIV+) patients include deficits in memory, attention, executive function, information processing speed, and psychomotor speed (Reger et al. 2002; Grant et al. 1999; Woods et al. 2009). These neuropsychological abnormalities correlate with indices of brain integrity on magnetic resonance imaging (MRI) including alterations in WM microstructure (Pfefferbaum et al. 2006, 2007; Thurnher et al. 2005), gross atrophy (Archibald et al. 2004; Jernigan et al. 1993), and cortical and callosal thinning (Thompson et al. 2005; Jernigan et al. 1993; Goodkin et al. 1997; Mason et al. 1998; Paul et al. 2002; Tate et al. 2010). Despite treatment with highly active antiretroviral therapy (HAART), HIV-associated cognitive difficulties can be observed and lead to reduced overall quality of life in this population (Woods et al 2009; Heaton et al. 2004).
Research conducted on cognitive difficulties related to HIV has primarily focused on males, especially men who have sex with men (Robertson et al. 2004). However, females represent a significant percentage of HIV infections, and account for over 25% of new HIV/AIDS diagnoses in the United States (CDC; 2013). Globally, females constitute more than half of the HIV/AIDS population and the prevalence of HIV is two-fold greater for younger females than males (15–24 years old; Joint United Nations Programme on HIV/AIDS, 2013). Collectively these data highlight the importance of research that focuses on the health outcomes among females infected with HIV.
HIV+ females may be particularly susceptible to cognitive impairment due to interactions between host and viral factors (Wojna et al. 2006). For example, depression is more prevalent in HIV+ females than males (Pereira and Canavarro 2011) and is associated with greater immune compromise and disease progression (Hestad et al. 2009). Additionally, females are at a higher risk of experiencing early life stress (ELS) than males (Spies et al. 2012). Common ELS events that have been shown to be prevalent in HIV+ individuals, particularly HIV+ females, include neglect and physical, emotional, and sexual abuse (Clark et al. 2012). Previous ELS events have been linked to significant memory impairment and poor processing speed in HIV+ females (Spies et al. 2012; Clark et al. 2012). Given the increased risk of traumatic events and depression among females compared to males it is possible that HIV+ females have an increased risk of neuropsychological abnormalities.
Studies examining the relationship between gender and cognition in HIV+ populations have produced mixed results with some studies reporting significant effects and others reporting no impact of gender on brain integrity in HIV. Importantly, with one exception, most of these investigations were conducted prior to revised HAART guidelines. Recent advances in our understanding of HAART dosing and potential neurotoxic effects (Caniglia et al. 2014; Willig et al. 2008; Kauf et al. 2008; Robertson et al. 2012), as well as enhanced HIV clinical management (Vellozzi et al. 2009) have improved HIV clinical care outcomes. In a recent study evaluating the cognitive profile of HIV+ females, HIV status had significant effects on verbal learning and memory and psychomotor tasks (Maki et al. 2015). However, additional variables including age, years of education, reading level, and race were more strongly associated with neuropsychological profile than serostatus. Despite the large sample size and design of the study, it is necessary to extend these findings by directly comparing the neuropsychological and neuroimaging profile of both males and females with similar demographics, HIV treatment, and comorbidities. The aim of the current study was to investigate whether gender was associated with an increased risk for poor brain integrity (both cognitive performance and brain volumetrics) among HIV+ individuals.
Methods
Participants
A total of 228 individuals were included in this study. Participants were classified into four groups consisting of (1) 44 HIV+ females; (2) 49 HIV uninfected (HIV−) females; (3) 93 HIV+ males; and (4) 42 HIV− males (Table 1). Participants were recruited from the Washington University School of Medicine (WUSM) Infectious Disease Clinic in Saint Louis, the WUSM AIDS Clinical Trial Group (ACTG) community medical providers and local organizations. All HIV+ participants were infected for greater than 4 months and were on stable HAART for at least 3 months. Exclusion criteria for all participants included fewer than 9 years of education, history of loss of consciousness > 30 minutes, claustrophobia, seizures, developmental delays, metal implants in the head, or any other contraindication for MRI. Participants with a recent history of alcohol or substance abuse/dependence or Beck Depression Inventory-II (BDI-II; Beck and Steer1996) total score > 29 were excluded. All participants provided informed consent and were financially compensated for their participation. The WUSM Institutional Review Board (IRB) approved this study.
Table 1.
Demographic Characteristics
| HIV+ Males (n = 93) | HIV Males (n = 42) | HIV+ Females (n = 44) | HIV− Females (n = 49) | |
|---|---|---|---|---|
| Age (years) | 41.7 (1.8) | 35.0 (3.2) | 44.0 (2.5) | 34.0 (2.8) a |
| Education (years) | 13.5 (0.3) | 13.6 (0.4) | 13.3 (0.3) | 14.1 (0.3) |
| Race (% African American) | 77.7% | 57.7% b | 75.0% | 70.6% |
| Depression (BDI-II) | 9.5 (0.8) | 5.8(1.5) | 11.6 (1.2) c | 6.2 (1.1) |
| Duration of HIV diagnosis (months) | 123 (11) | 97 (12) | ||
| Mean CD4 (cells/μl) (IQR) | 553 (364–719) | NA | 668 (402–888) | NA |
| Mean Nadir CD4 (cells/μl) (IQR) | 229 (24–346) | NA | 283 (67–430) | NA |
| Mean Log plasma viral load (IQR) | 3.75 (3.0–3.04) | NA | 3.67 (3.0–3.28) | NA |
| % undetectable | 77% | 84% |
BDI-II=Beck Depression Inventory-II, IQR=interquartile range
Values represent the mean values and standard error for each group. Significance (p) was determined using independent samples t-tests and chi-square analyses (group x gender). Education was measured as highest year of school completed.
HIV− females were significantly younger than HIV+ males (p <0.05) and HIV+ females (p< 0.05)
HIV− males had a significantly lower percentage of African Americans than HIV+ male, HIV+ female, and HIV− female groups (p<0.05).
HIV+ females had significantly higher BDI-II scores than HIV− males (p< 0.05) and HIV− females (p <0.01).
Screening Measures
All HIV− controls were administered a rapid oral HIV buccal test to confirm negative result at the time of enrollment. All participants underwent a urine drug screen and females were administered a pregnancy test.
HIV clinical factors
A neuromedical examination was performed on all participants at the time of enrollment. Lab values including plasma CD4 T-cell count, and plasma HIV RNA levels were collected within 3 months of enrollment for all HIV+ participants. The majority of HIV+ males (72/93) and females (37/44) had undetectable plasma viral loads (< 50 copies/mL). Nadir plasma CD4 T-cell counts were obtained by patient self-report and confirmed by medical records when available.
Beck Depression Inventory
Current depression symptomology was assessed using the BDI-II. All participants completed the self-report inventory according to standard procedures.
Neuropsychological assessment
Subjects were administered a neuropsychological battery consisting of measures sensitive to neurocognitive impairment associated with HIV (Antinori et al. 2007). Assessments were completed the same day as the MRI. Standard testing procedures were utilized to assess three cognitive domains including: verbal learning and memory, executive functioning, and psychomotor/processing speed. Raw scores on each measure were recorded but were not normed as norms include correction for potential differences in gender. The following measures were used to evaluate neuropsychological functioning:
-
Verbal Learning: total number of correct responses from three learning trials of the Hopkins Verbal Learning Test – Revised (HVLT-R; Benedict et al. 1998; Brandt and Benedict, 2001).
Verbal Memory: total number of correct responses from the Hopkins Verbal Learning Test – Revised delayed recall trial (HVLT-R delay; Benedict et al. 1998; Brandt and Benedict 2001).
Executive Function: completion time in seconds for the Trail Making Test, Part B (TMT-B; Reitan and Davison 1974); correct number of strings from the Letter-Number Sequencing Task (L-N Seq; Wechsler 1997); the total number of correct responses from the FAS Verbal Fluency task (Borkowski et al. 1967), and total correct on Verb Fluency (Piatt et al. 1999).
Psychomotor Speed/Processing Speed: correct number of responses on the Digit-Symbol Coding task (Wechsler 1997); time to completion on the Trail Making Test, Part A (TMT-A; Reitan and Davison 1974) and time to completion on the Grooved Pegboard Test non-dominant (ND) hand (Klove 1963).
Neuroimaging
All images were acquired using a 3T Siemens Tim Trio whole body MR scanner (Siemens AG, Erlangen Germany) with a 12-channel transmit/receive head coil. Structural images were obtained using a T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence with the following parameters: time of repetition (TR) = 2,400 ms, echo time (TE) = 3.16 ms, inversion time (TI) = 1,000 ms, flip angle = 8°, 162 slices, and voxel size = 1 × 1 × 1 mm3. All images were acquired in the sagittal plane. Motion was minimized by instructing participants to fixate on a plus sign centered on the screen.
Volumes within specific predefined regions of interest (ROIs) were quantified using the FreeSurfer software suite (v5.1) (Martinos Center, Harvard University, Boston, MA, USA; http://surfer.mmr.mgh.harvard.edu). Individual MP-RAGE scans were transformed into a template space, the skull was removed, and the brain segmented into white matter (WM), grey matter (GM), and ventricles. A surface deformation program was used to further segment brain regions into parcellated subcortical and cortical regions of interest (ROIs) (Fischl et al. 2002; Desikan et al. 2006; Fischl and Dale 2000). Images for all the subjects were then aligned onto a common atlas (MNI305;Fischl et al. 2002). Strategic ROIs analyzed for this specific analysis comprised of areas that have been previously shown to be affected by HIV including: hippocampus, caudate, putamen, nucleus accumbens, corpus callosum, amygdala, total WM, and total GM (Thompson et al. 2005; Stout et al. 1998; Ances et al. 2012; Heaps et al. 2012, Ortega et al. 2013). A trained rater (JMH) independently confirmed the segmentation of these ROIs making necessary manual edits when required. Each volume was normalized to total intracranial volume using a least square residual regression model (Ances et al. 2012).
Statistical analysis
All statistical analyses were conducted utilizing SPSS (Version 21). Independent sample t tests and chi-squared analyses were conducted to assess if the four groups were similar for age, education, gender, race, and depression scores (Table 1). The groups significantly differed with regard to age and race; therefore, these variables were included as covariates in subsequent analyses. Depression scores were significantly higher in the HIV+ female group compared to the other groups; however, the depression scores were not correlated with ROI volumes (r values ranging from= −0.009 – 0.08; all p values > 0.22). Depression scores were weakly correlated with only two of the neuropsychological measures, verb fluency (r=.15; p=0.02) and TMT-A (r=.15; p=0.02). Due to the lack of relationship between depression scores and outcome variables, we did not include depression as a covariate in the primary analysis to maximize power. However, to ensure full correction for depression scores we completed a secondary covariate analysis correcting for depression.
The HIV+ group was significantly older than the HIV− group; therefore age was used as a covariate in all subsequent analyses to compare HIV+ versus HIV− individuals. Separate factorial analyses of covariance (ANCOVAs with age as a covariate) were conducted to determine the impact of HIV status and gender on cognition and the interaction between HIV and gender on these outcomes. The 8 brain ROIs were entered as the primary outcome measures for the neuroimaging analysis. For the neuropsychological analysis, the three cognitive domains of verbal learning and memory, psychomotor/processing speed, and executive function were entered by domain in subsequent factorial ANCOVAs with age and race included as covariates. HIV serostatus and gender were entered as the independent variables. The raw scores on the measures for each cognitive domain were entered as dependent variables. Finally, univariate analyses were examined in order to determine individual dependent variable contributions to the main effects.
Pearson correlations were conducted to determine the relationships between clinical markers of disease burden (CD4 and plasma viral load (VL), brain volumetrics, and neuropsychological performance in the HIV+ group. Since both plasma CD4 (nadir and recent) and HIV VL have a non-normal distribution we square root transformed the CD4 variable and natural log transformed plasma VL to achieve normal distributions for the correlation analyses.
Results
Neuropsychological performance
Executive Function
A significant main effect for HIV status was identified on executive function measures (Wilks’ λ=0.905, F(4,205) =5.36, p<.001, η2=.095). Exploratory analyses revealed HIV status had a significant effect on verb fluency (F(1, 215) = 16.99, p<.0001, η2=.076) and L-N Seq (F(1,215) = 8.14, p =.005, η2=.038) with HIV+ individuals achieving fewer correct responses on both tests. HIV status did not have a significant effect on FAS (F(1, 215) = 0.030, p=.863, η2=.000) or TMT-B (F(1, 215) = 1.69, p=.196, η2=.008). There was no significant main effect of gender on executive function measures (Wilks’ λ=.980, F(4,205) =1.05, p=0.4, η2=.02; Table 2). In addition, the interaction between HIV and gender was not significant for the executive function domain (Wilks’ λ=0.981, F(4,205) =1.006, p=.41, η2=.02).
Table 2.
Effects of gender and HIV status on cognitive domain scores
|
Verbal Learning and Memory Domain
| |||
|---|---|---|---|
| Male | Female | ||
| HVLT-R Learning (total # correct) | HIV− | 22.63 (4.63) | 23.59 (4.43) |
| HIV+ | 21.35 (5.11) | 22.13 (4.59) | |
|
| |||
| HVLT-R Delayed Recall (total # correct) | HIV− | 8.03 (3.60) | 8.10 (2.49) |
| HIV+ | 7.05 (2.97) | 7.75 (2.84) | |
|
Psychomotor Speed Domain
| |||
|---|---|---|---|
| Male | Female | ||
| Grooved Peg-ND (sec) | HIV− | 81.54 (25.78) | 78.30 (17.68) |
| HIV+ | 91.35 (33.70) | 93.83 (45.82) | |
|
| |||
| Digit Symbol (sec) | HIV− | 75.76 (17.70) | 81.06 (17.80) |
| HIV+ | 66.30 (18.27) | 72.75 (19.32) | |
|
| |||
| TMT-A (sec) | HIV− | 27.14 (10.11) | 27.78 (10.00) |
| HIV+ | 35.75 (21.05) | 31.29 (11.17) | |
|
Executive Function Domain
| |||
|---|---|---|---|
| Male | Female | ||
| L-N Seq (total # correct) | HIV− | 10.05(2.89) | 10.26 (3.01) |
| HIV+ | 8.68(3.37) | 8.33 (2.55) | |
|
| |||
| TMT-B (sec) | HIV− | 75.19 (35.76) | 73.48 (45.82) |
| HIV+ | 93.68(48.51) | 79.46(35.50) | |
|
| |||
| Verb Fluency (total # correct) | HIV− | 16.69(8.07) | 17.21(7.49) |
| HIV+ | 12.77(6.00) | 11.92(4.88) | |
|
| |||
| FAS (total # correct) | HIV− | 39.29(13.43) | 39.72(9.70) |
| HIV+ | 34.71(11.22) | 43.54(66.84) | |
FAS= FAS Verbal Fluency task, TMT =Trail Making Test, L-N Seq = Letter-Number Sequencing Task, Grooved Peg-ND= Grooved Pegboard Test non-dominant, HVLT-R Learning =Hopkins Verbal Learning Test – Revised, HVLT-R Delayed Recall =Hopkins Verbal Learning Test – Revised delayed recall trial.
Mean scores and standard deviations presented for all tasks
Psychomotor/Processing Speed
There was a main effect for HIV status (Wilks’ λ= .959, F(3,200) =2.84, p=.04, η2=.04). Exploratory analyses revealed that the HIV+ group performed significantly worse than the HIV− group on the Digit Symbol Test (F(1, 209) =7.24 p=.008 η2=.034), Grooved Peg ND (F(1, 209) =4.03, p=.046, η2=.019), and TMT-A (F(1, 209) =4.95, p=.027, η2=0.024 (Table 2)). There was no significant main effect of gender on psychomotor/processing speed (Wilks’ λ= 0.969, F(3,200) =2.13, p=.10, η2=.03). Additionally, there was no significant interaction between HIV and gender in the psychomotor/processing domain (Wilks’ λ= 0.984, F(3,200) =1.09, p=.36, η2=.02).
Learning and Memory
There was no effect of HIV (Wilks’ λ=0.992, F(2,215) =.826, p=0.44, η2=.008) or gender (Wilks’ λ=0.992, F(2,215) =0.895, p=.41, η2=.008) on learning and memory. In addition, there was no significant interaction between HIV and gender in the learning and memory domain (Wilks’ λ= 0.99, F(2,213) =1.05, p=.35, η2=.01).
We ran supplementary analyses of the neuropsychological measures in the HIV+ group to determine whether if significant differences existed between males and females on any of the neuropsychological measures. Using ANCOVA models with age as a covariate, there were no significant differences between the HIV+ males and females in any of the neuropsychological domains. In general, HIV+ females performed better than males on the measures of learning and memory and psychomotor speed. Results on executive function tasks were mixed with males performing better on some while females achieved better scores on others (Table 2).
Brain Imaging
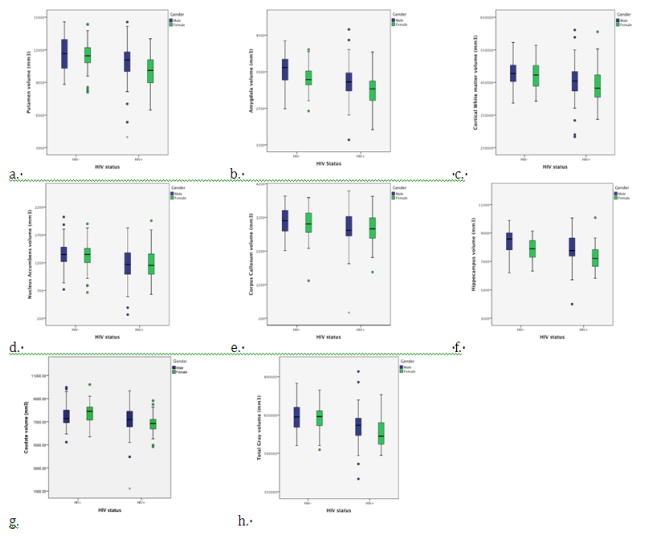
A significant main effect of HIV status on brain volumetrics was observed for the following ROIs: Total GM (F(1, 228)= 19.47 p<0.0001), Total WM (F(1, 228)= 9.94 p=0.002), Caudate (F(1, 228)= 9.93 p=0.002), Hippocampus (F(1, 228)= 14.53 p<0.0001), Amygdala (F(1, 228)= 16.67 p<0.0001), Nucleus Accumbens (F(1, 228)= 8.64 p=0.004), Corpus Callosum (F(1, 228)= 5.04 p=0.036), and Putamen (F(1, 228)= 13.06 p<0.0001) (Table 3). There was also a significant main effect of gender for the following ROIs: Total GM F(1, 228)= 7.15 p=0.008), Hippocampus F(1, 228)= 21.41 p<0.0001), Amygdala F(1, 228)= 14.84 p<0.0001), and Putamen F(1, 228)= 9.97 p<0.002). The interaction between gender and HIV was not significant for any of the selected brain volumes. A secondary analysis that included depression scores as a covariate did not affect the significant main effects for the neuroimaging measures.
Table 3.
Effects of HIV on select brain regions
| HIV− n=91 |
HIV+ n=137 |
Significance | |
|---|---|---|---|
| Total Gray Matter | 637289.98 (52872.06) | 597779.31 (61492.77) | p<0.001 |
| Thalamus | 14008.46 (1576.94) | 13132.14 (1786.94) | p=0.004 |
| Caudate | 7619.20 (1027.76) | 7074.89 (1052.13) | p=0.002 |
| Putamen | 11515.84 (1455.32) | 10592.80 (1660.51) | p<0.001 |
| Hippocampus | 8161.53 (837.15) | 7710.52 (1008.74) | p<0.001 |
| Nucleus Accumbens | 1346.04 (268.03) | 1176.11 (287.69) | p=0.004 |
| Corpus Callosum | 3042.77 (440.40) | 2868.10 (511.51) | p=0.036 |
| Amygdala | 3417.63 (405.26) | 3166.67 (454.73) | p<0.001 |
| Cortical White Matter | 473387.97 (41573.38) | 450185.74 (53797.89) | p=0.001 |
Values represent the mean (cm3) and standard deviation for each group. For all regions except the corpus callosum an HIV effect was observed after FDR.
For the HIV+ group correlation analyses examined the relationship between brain integrity measures (neuropsychological performance and volumetrics) and laboratory values (current plasma CD4 T-cell count, nadir plasma CD4 T-cell count, and plasma HIV RNA viral load). There were no significant associations between the cognitive measures and laboratory values after controlling for multiple comparisons using a false discovery rate (FDR; min p value required 0.006, lowest p value=0.03). In general, laboratory values were not correlated with ROI volumes. Only a correlation between higher nadir CD4 and larger thalamus volume (r=0.27, p=0.004) was preserved after correction for multiple comparisons.
Discussion
The present study investigated whether differences exist between males and females on measures of cognition and brain volumetrics among HIV+ individuals in the modern HAART era. Overall, we observed an effect of HIV, but not gender on brain integrity measures. Our study did not reveal a significant interaction between HIV and gender for cognition or volumetric measures. Specifically, no significant differences between males and females were evident on measures of executive function, psychomotor/processing speed, verbal learning and memory, or brain volumes across a number of regions sensitive to the effects of HIV (Grant et al.1999; Woods et al. 2009). Overall, our findings coincide with past research demonstrating no significant gender differences in cognitive status associated with HIV among patients examined in the pre-HAART era (Ge et al. 2003, Ances et al. 2006, Pfefferbaum et al. 2006, 2007). The present study extends this literature by examining a cohort of patients on stable HAART.
In the pre-HAART era some studies reported an increased risk for HIV-associated dementia (Chiesi et al. 1996a; 1996b) and/or encephalitis (Morlat et al. 1992) among HIV+ females at the time of AIDS diagnosis. Further, Robertson et al. (1996) observed greater neurocognitive dysfunction and abnormal visual evoked potentials among HIV+ females compared to males. In the HAART era, HIV+ females exhibited significantly poorer performance on tasks of motor function and learning when compared to HIV− females, whereas, no differences were revealed between HIV+ and HIV− males (Martin et al. 2011). Although, analyses between males and females were not conducted, different neurocognitive profiles may exist for males and females. Additional work conducted in Zambia reported that HIV+ females exhibited greater cognitive impairment compared to HIV+ males (Hestad et al. 2012), an effect possibly driven by gender differences in access to health care and other resources as well as comorbid conditions experienced more frequently by females. Finally, a study at five major centers in the US (CHARTER; CNS HIV Anti-Retroviral Therapy Effects Research) investigating disease progression demonstrated that asymptomatic HIV+ females had a three-fold increase in risk for developing more severe forms of cognitive impairment when compared to asymptomatic HIV+ males (Grant et al. 2014).
In contrast to the literature cited above, other studies have reported no significant difference in the frequency or severity of cognitive dysfunction secondary to HIV according to gender. Bouwman et al. (1998) reported that intravenous drug use was a stronger predictor of cognitive impairment than gender among an HIV+ sample. Robertson et al. (2004) identified no effects of gender on cognitive outcomes at baseline or after a 3-year longitudinal follow-up. It is important to note that a large percentage of individuals in the latter study were not on HAART and therefore the results may not be generalizable to a cohort of patients on stable HAART regimens. The issue of treatment status is important, as pre-HAART outcomes may not generalize to the post-HAART era. Although our study and others have failed to show a significant difference between males and females this is not to say that the groups perform equally, or have equivalent changes with regard to brain integrity. To determine equivalence would require statistical methods that were not performed in this study.
Although previous literature has suggested it is possible that females are more vulnerable to the effects of HIV, the results of the current study do not support these findings. Maki and colleagues recently reported variables related to cognitive reserve (e.g. education, reading level, and age) were more likely to contribute to cognitive impairment than HIV status in a large cohort of HIV+ and HIV− females (Maki et al. 2015). Our strict HAART requirement and inclusion of males (both HIV− and HIV+) further extends these results implying cognitive gender differences do not exist in the HAART era.
Alternatively, a significant serostatus effect was observed on cognitive measures with HIV+ individuals performing significantly worse on two measures of executive functioning (i.e. Verb Fluency and L-N Seq). Previous studies have demonstrated that neurocognitive impairment is present in HIV+ patients despite successful viral suppression and immune system improvements (Woods et al. 2009; Heaton et al. 2010; Antinori et al. 2007). Evidence of residual impairments in executive function is highly problematic given the importance of these cognitive skills for effective implementation of activities of daily living. Executive function predicts adherence to HIV medications, driving abilities, financial management and related high-level instrumental activities of daily living (Rog et al. 2014; Thames et al. 2013; Cattie et al. 2012; Boyle et al. 2002, 2003; Tomaszewski et al. 2009; Scott et al. 2011). The chronic nature of executive deficits and the importance of these skills to successfully engage in critical aspects of daily living underscores the need for interventions to improve executive skills among HIV+ individuals already on treatment.
In terms of brain volumetrics, the HIV+ group had significantly lower brain volumes in all ROIs when compared to HIV− controls. Evidence of residual brain volumetric abnormalities among seropositive individuals on effective HAART is consistent with previous work (Thompson et al. 2005; Ances et al. 2006; Becker et al. 2011; Archibald et al. 2004), and emphasizes the chronic nature of brain dysfunction associated with HIV. Results of the present study provide further evidence that these residual brain abnormalities appear consistent among both males and females.
Similarly, there were significant differences in brain volumetrics between males and females independent of HIV status. Overall, males displayed larger total GM volume, amygdala, subcortical GM volume, putamen, and hippocampal regions when compared to females. Although neuroimaging studies have established similar sex-specific dimorphisms in healthy individuals (Lüders et al. 2002; Raz et al. 2001; Good et al. 2001), few studies have been completed to date that were designed to examine brain volume differences between HIV+ males and females. Previously, our group identified no differences between seropositive males and females in terms of caudate volume (Ances et al. 2006). Similarly, work by Pfferbaum et al. (2006, 2007) and Ge et al. (2003) revealed no gender-specific brain volume differences among HIV+ individuals. The current study extends this literature by investigating additional regions of interest including both cortical and subcortical brain ROIs.
In summary, results from the present study provide additional evidence of neuropsychological and neuroimaging abnormalities in HIV+ individuals on stable HAART, however, there were no significant differences between males and females. The study highlights the importance of clinical outcomes for both males and females infected with HIV as cognitive difficulties were present despite stable HAART with a majority having viral suppression. Consistent with other studies, results from the present investigation highlight the persistence of neuropsychological impairments in the HAART era.
Figure 1.
Boxplots of the volumes (mm3) for HIV infected (HIV+) and HIV uninfected (HIV−) groups. Lines within each box represent the mean values for volumes of each group and bars represent standard deviation within each of the following regions: a. Putamen, b: Amygdala, c. White Matter, d. Nucleus Accumbens, e. Corpus Callosum, f. Hippocampus, g. Caudate, h. Total Gray Matter.¶
The effect of HIV on the Corpus Callosum does not survive false discovery rate (FDR) correction. However, for all other regions the effect of HIV but not gender was significant after FDR correction.¶
Acknowledgments
This work was supported by the following grants: the National Institute of Mental Health (R21MH099979) (BA) (R01MH085604) (RP), the National Institute of Aging (R01 NS052420) (RP), and the National Institute of Nursing Research (R01NR012657, R01NR014449, R01NR012907) (BA). Research was conducted and supported by the Washington University Institute of Clinical and Translational Sciences (UL1 TR000448 from the National Center for Advancing Translational Sciences).
Footnotes
The content of this study is solely the responsibility of the authors and does not necessarily represent the official view of NIH.
Conflict of Interest
The funding sources were not involved in the study and Ashley M. Behrman-Lay, Robert H. Paul, Jodi Heaps-Woodruff, Christina Usher, Laurie M. Baker, and Beau M. Ances declare that they have no conflict of interest.
References
- 1.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66(6):862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Clinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem Human Immunodeficiency Virus encephalitis and dendritic loss. Arch Neurol. 2004;61(3):369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Beck AT, Steer RA, Beck . Beck Depression Inventory. 2. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 6.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM. Multicenter AIDS Cohort Study (2011). Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 5(2):77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: normative data and analysis of interform and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 8.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 9.Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50(6):1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- 10.Boyle PA, Cohen RA, Paul R, Moser D, Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int J Geriatr Psychiatry. 2002;17(2):164–169. doi: 10.1002/gps.539. [DOI] [PubMed] [Google Scholar]
- 11.Boyle PA, Paul R, Moser D, Zawacki T, Gordon N, Cohen R. Cognitive and neurological predictors of functional impairment in vascular dementia. Am J Geriatr Psychiatry. 2003;11(1):103–106. [PubMed] [Google Scholar]
- 12.Brandt J, Benedict RHB. Professional manual. Psychological Assessment Resources, Inc; Lutz: 2001. Hopkins Verbal Learning Test—Revised. [Google Scholar]
- 13.Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, van Sighem A, Miro JM, Podzamczer D, Olson A, Arribas JR, Moreno S, Meyer L, del Romero J, Dabis F, Bucher HC, Wandeler G, Vourli G, Skoutelis A, Lanoy E, Gasnault J, Costagliola D, Hernán MA HIV-CAUSAL Collaboration . Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83(2):134–141. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cattie JE, Doyle K, Weber E, Grant I, Woods SP HIV Neurobehavioral Research Program (HNRP) Group . Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol. 2012;34(9):906–918. doi: 10.1080/13803395.2012.692772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention. HIV/AIDS surveillance report. 2011;23:1–84. [Google Scholar]
- 16.Chiesi A, Seeber AC, Dally LG, Floridia M, Rezza G, Vella S. AIDS dementia complex in the Italian National AIDS Registry: temporal trends (1987–93) and differential incidence according to mode of transmission of HIV-1 infection. J Neurol Sci. 1996a;144(1–2):107–113. doi: 10.1016/s0022-510x(96)00192-x. [DOI] [PubMed] [Google Scholar]
- 17.Chiesi A, Vella S, Dally LG, Pendersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996b;11(1):39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, Westbrook ML, Mulligan RC, Jerskey BA, White TL, Navia B, Tashima KT. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc. 2012;18(4):657–658. doi: 10.1017/S1355617712000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maquire RP, Hyman BT, Albert MS, Killany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischl B, David H, Salat EB, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 22.Ge Y, Kolson DL, Babb JS, Mannon LJ, Grossman RI. Whole brain imaging of HIV-infected patients: quantitative analysis of magnetization transfer ratio histogram and fractional brain volume. AJNR Am J Neuroradiol. 2003;24(1):82–87. [PMC free article] [PubMed] [Google Scholar]
- 23.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 24.Goodkin K, Wilkie FL, Concha M, Asthana D, Shapshak P, Douyon R, Fujimura RK, LoPiccolo C. Subtle neuropsychological impairment and minor cognitive-motor disorder in HIV-1 infection. Neuroradiological, neurophysiological, neuroimmunological, and virological correlates. Neuroimaging Clin N Am. 1997;7(3):561–579. [PubMed] [Google Scholar]
- 25.Grant I, Franklin DR, Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK CHARTER Group . Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant I, Marcotte TD, Heaton RK HNRC Group . Neurocognitive complications of HIV disease. Psychol Sci. 1999;10(3):191–195. [Google Scholar]
- 27.Heaps JM, Joska J, Hoare J, Ortega M, Agrawal A, Seedat S, Ances BM, Stein DJ, Paul R. Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol. 2012;18(3):151–156. doi: 10.1007/s13365-012-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER STUDY. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reichs C, Grant I HNRC Group . The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 30.Hestad K, Aukrist P, Tønseth S, Reitan SK. Depression has a strong relationship with alterations in the immune, endocrine and neural system. Curr Psychiatry Rev. 2009;5:287–297. [Google Scholar]
- 31.Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR, Jr, Imasiku ML, Kalima K, Heaton RK. Sex differences in neuropsychological performance as an effect of immunodeficiency virus infection: A pilot study in Zambia, Africa. J Nerv Ment Dis. 2012;200(4):336–342. doi: 10.1097/NMD.0b013e31824cc225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, Chandler J, Grant I. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Arch Neurol. 1993;50(3):250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- 33.Kauf TL, Roskell N, Shearer A, Gazzard B, Mauskopf J, Davis EA, Nimsch C. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health. 2008;11(7):1144–1153. doi: 10.1111/j.1524-4733.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 34.Klove H. Grooved pegboard. Lafayette Instruments; Indiana: 1963. [Google Scholar]
- 35.Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- 36.Lüders E, Steinmetz H, Jäncke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13(17):2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]
- 37.Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K. Cognitive function in women with HIV: Findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol. 2011;33(1):112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason KI, Campbell A, Hawkins P, Madhere S, Johnson K, Takushi-Chinen R. Neuropsychological functioning in HIV-positive African-American women with a history of drug use. J Natl Med Assoc. 1998;90(11):665–674. [PMC free article] [PubMed] [Google Scholar]
- 40.Morlat P, Parneix P, Douard D, Lacoste D, Dupon M, Chėne G, Pellegrin JL, Ragnaud JM, Dabis F. Women and HIV infection: a cohort study of 483 HIV-infected women in Bordeaux, France, 1985–1991. The Group d’Epidémiologi Clinique du SIDA en Aquitaine. AIDS. 1992;6(10):1187–1193. [PubMed] [Google Scholar]
- 41.Ortega M, Heaps JM, Joska J, Vaida F, Seedat S, Stein DJ, Paul R, Ances BM. HIV clades B and C are associated with reduced brain volumetrics. J Neuroviol. 2013;19(5):479–487. doi: 10.1007/s13365-013-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26(3):353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 43.Pereira M, Canavarro MC. Gender and age differences in quality of life and the impact of psychopathological symptoms among HIV-infected patients. AIDS Behav. 2011;15(8):1857–1869. doi: 10.1007/s10461-011-9928-8. [DOI] [PubMed] [Google Scholar]
- 44.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130(Pt 1):48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 45.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. NeuroImage. 2006;33(1):239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 46.Piatt AL, Fiels JA, Paolo AM, Troster AI. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologica. 1999;37(13):1499–1503. doi: 10.1016/s0028-3932(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 47.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22(6):1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 48.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 49.Reitan RM, Davison LA. Clinical neuropsychology: current status and applications. Hemisphere; New York: 1974. [Google Scholar]
- 50.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson K, Wilkins J, Messenheimer J, Robertson W, Hall C. Gender differences in HIV neurological progression: a preliminary study. J NeuroAIDS. 1996;1:166. [Google Scholar]
- 52.Robertson KR, Kapoor C, Robertson WT, Fiscus S, Ford S, Hall CD. No gender difference in the progression of nervous system disease in HIV infection. J Acquir Immune Defic Syndr. 2004;36(3):817–822. doi: 10.1097/00126334-200407010-00008. [DOI] [PubMed] [Google Scholar]
- 53.Rog LA, Park LQ, Harvey DJ, Huang C-J, Mackin S, Farias ST. The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. Clinical Neuropsychol. 2014;28(2):215–236. doi: 10.1080/13854046.2013.876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Grant I, Marcotte TD. San Diego HIV Neurobehavioral Research Center (HNRC) Group (2011) A neuropsychological investigation of multitasking in HIV infection: Implications for everyday functioning. Neuropsychology. 25(4):511–519. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spies G, Fennema-Notestine C, Archibald SL, Cherner M, Seedat S. Neurocognitive deficits in HIV-infected women and victims of childhood trauma. AIDS Care. 2012;24(9):1126–1135. doi: 10.1080/09540121.2012.687813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurovirol. 1998;55(2):161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- 57.Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, Laidlaw DH, Taylor LE, Flanigan T, Navia B, Cohen R, Tashima K. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav. 2010;4(1):68–79. doi: 10.1007/s11682-009-9086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH. Functional disability in medication management and driving among individuals with HIV: A 1-year follow up study. J Clin Exp Neuropsychol. 2013;35(1):49–58. doi: 10.1080/13803395.2012.747596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Alzenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging od the brain in human immunodeficiency virus-positive patients. AJNR Am J Neuroradiol. 2005;26(9):2275–2281. [PMC free article] [PubMed] [Google Scholar]
- 61.Tomaszewski FS, Cahn-Weiner DA, Harvey DJ, Reed BR, Mungas D, Kramer JH, Chui H. Longitudinal changes in memory and executive functioning are associated with longitudinal changes in instrumental activities of daily living in older adults. Clin Neuropsychol. 2009;23(3):446–461. doi: 10.1080/13854040802360558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.UNAIDS. [Accessed April 16, 2014];Global Report UNAIDS report on the global AIDS epidemic 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 63.Vellozzi C, Brooks JT, Bush TJ, Conley LJ, Henry K, Carpenter CC, Overton ET, Hammer J, Wood K, Holmberg SD SUN Study Investigators . The study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN Study) Am J Epidemiol. 2009;69(5):642–652. doi: 10.1093/aje/kwn361. [DOI] [PubMed] [Google Scholar]
- 64.Wechsler D. WAIS-III: Weschsler Adult Intelligence Scale. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 65.Willig JH, Abroms S, Westfall AO, Routman J, Adusumilli S, Varshney M, Allison J, Chatham A, Raper JL, Kaslow RA, Saag MS, Mugavero MJ. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22(15):1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wojna V, Skolasky RL, Hechavarría R, Mayo R, Seines O, McArthur JC, Meiēndez LM, Maldonado E, Zorrilla CD, García H, Kraiselburd E, Nath A. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12(5):356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- 67.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]