Abstract
Precise temporal control of neuro-differentiation and postdifferentiation events is necessary for the creation of appropriate wiring diagram in the brain. To make advances in the treatment of neurodevelopmental and neurodegenerative disorders, and traumatic brain injury, it is important to understand these mechanisms. Caenorhabditis elegans has emerged as a revolutionary tool for the study of neural circuits due to its genetic homology to vertebrates and ease of genetic manipulation. microRNA (miRNA), a ubiquitous class of small non-coding RNA, that inhibits the expression of target genes, has emerged as an important timing control molecule through research conducted on C. elegans. This review will focus on the temporal control of neurodifferentiation and post-differentiation events exerted by the two conserved miRNAs, lin-4 and let-7. We summarize recent findings on the role of lin-4 as a timing regulator controlling transition of sequential events in neuronal pathfinding and synaptic remodeling, and the role of let-7 as a timing regulator that limits the regeneration potential of post-differentiated AVM neurons as they age.
Introduction
Caenorhabditis elegans is a transparent nematode and the first organism to have its whole genome successfully sequenced (Corsi et al., 2015). The 100Mb genome encodes approximately 20,000 proteins and controls the formation of precisely 302 neurons in the adult hermaphrodite (White et al., 1986). Forward and reverse genetic screens have taken advantage of the sequenced genome in C. elegans in order to identify critical genes, which are approximately 40% orthologous to humans (Corsi et al., 2015). Combined with our knowledge of the entirely sequenced genome, the animal’s transparency, and invariant cellular lineage, C. elegans serves as an ideal model for the development of novel genetic tools. At 20 °C, C. elegans experiences a 16 hour embryogenesis, followed by four larval stages (L1-L4). The majority of C. elegans are hermaphrodites that are capable of self-fertilization, which allows easy maintenance of desired strains. However, if heterozygous or novel combinations of mutant strains are desired, temperature stresses are able to induce male orientation in hermaphrodites, which can be used for creating genetic crosses that lead to the desired progeny. C. elegans has the unique ability to survive through extraordinary stresses such as starvation, by molting into the alternative “dauer” larval pathway in the L2 stage and can also withstand long-term freezing. This unparalleled durability allows researchers to store libraries of different mutant genotypes without the constant strain upkeep or the painstaking rederivation procedures (Corsi et al., 2015).
Precise control of the spatial and temporal parameters in the wiring and rewiring of neural networks is critical to the function of neural circuits. However many fundamental questions about the architecture of even the simplest neural networks remain unanswered. Given the high degree of gene homology between C. elegans and vertebrates, it is likely that the discoveries in this animal model will be critical to understanding the conserved processes in the assembly and reorganization of neural circuits in higher organisms. In fact, the first microRNA (miRNA) lin-4, which was identified in C. elegans (Lee et al., 1993), underscores the value of this animal model. miRNA is a short non-coding RNA molecule that is now known to be one of the fundamental regulators of neural pathways. It is a post-transcriptional regulator of gene expression via gene silencing (Chiu et al., 2014), a reversible process in which the miRNA binds to the complementary 3’ UTR region of the target transcript to repress translation. A multistep process controls miRNA biogenesis. miRNA is transcribed by RNA polymerase II, cleaved by Drosha to create pre-miRNA, exported from the nucleus to cytoplasm by Exportin-5, and further cleaved by Dicer in the cytoplasm to create the final, mature miRNA of 20–25 nucleotides (Vella & Slack, 2005). The mature miRNA is assembled into a silencing complex, called miRISC (miRNA-induced silencing complex), which requires the activity of Argonaute proteins ALG-1 or ALG-2 to search for target sequences. These Argonaute proteins also play an important role in miRNA processing and maturation (Duchaine et al., 2006; Grishok et al., 2001).
The interactions between miRNAs and their transcript targets do not follow a 1:1 relationship, thus one miRNA can regulate multiple transcripts and one transcript can be downregulated by multiple miRNAs (Chiu et al., 2014). One such example is the seven lin-4 miRNA binding sites on the 3’UTR of lin-14 transcript (Lee et al., 1993; Wightman et al., 1993). There is also mounting evidence that the mechanism of action of miRNAs extend beyond the traditional silencing via binding to the 3’UTR regions of complementary mRNA targets. miRNA have been shown to create aberrant splicing, transcript deadenylation and degradation, and even found to be active in the nucleus (Londin et al 2014; Stroynowska- Czerwinska et al 2014). Due to the ease of miRNA transcription and the variability of interactions possible, miRNAs have a pleiotropic role in the nervous system. For example, a null mutation in lin-4 in C. elegans inappropriately delays the occurrence of early developmental events in later stages (Lee et al., 1993) as well as prolongs axon pathfinding (Zou et al., 2012). Not surprisingly, overexpression of lin-14, the target of lin-4, results in a similar phenotype as the lin-4 mutants. The concept of miRNA-mediated inhibition and subsequent changes in protein expression profile is central to exploring the role of miRNA in the nervous system. The remainder of this review will focus on the recent advancement in our understanding of temporal control exerted by the lin-4 and let-7 miRNAs on neuronal pathfinding, synaptic reorganization, and neuronal regeneration in C. elegans.
lin-4 miRNA Temporally Controls Axonal Guidance
lin-4 miRNA is homologous to vertebrate miR-125a and miR-125b miRNAs (Zou et al., 2012). In C. elegans it is highly enriched in the nervous system (Zou et al., 2012) and has multiple targets including but not limited to hbl-1 (a homolog of Drosophila hunchback gene), lin-41, and lin-14 (Abrahante et al., 2003; Lin et al., 2003; Slack et al., 2000). lin-14 is a heterochronic gene well known for its ability to control the timing of tissue remodeling during the transition from L1 to L2 stage in hypodermal, vulval, muscle, and neuronal cells (Ambros & Horvitz, 1984; Hallam & Jin, 1998). Since lin-4 and lin-14 interactions are known to be important for timing mitotic cell development in C. elegans, it was proposed that this miRNA regulatory circuit may continue to play a role in timing the differentiation events of postmitotic cells, such as neurons.
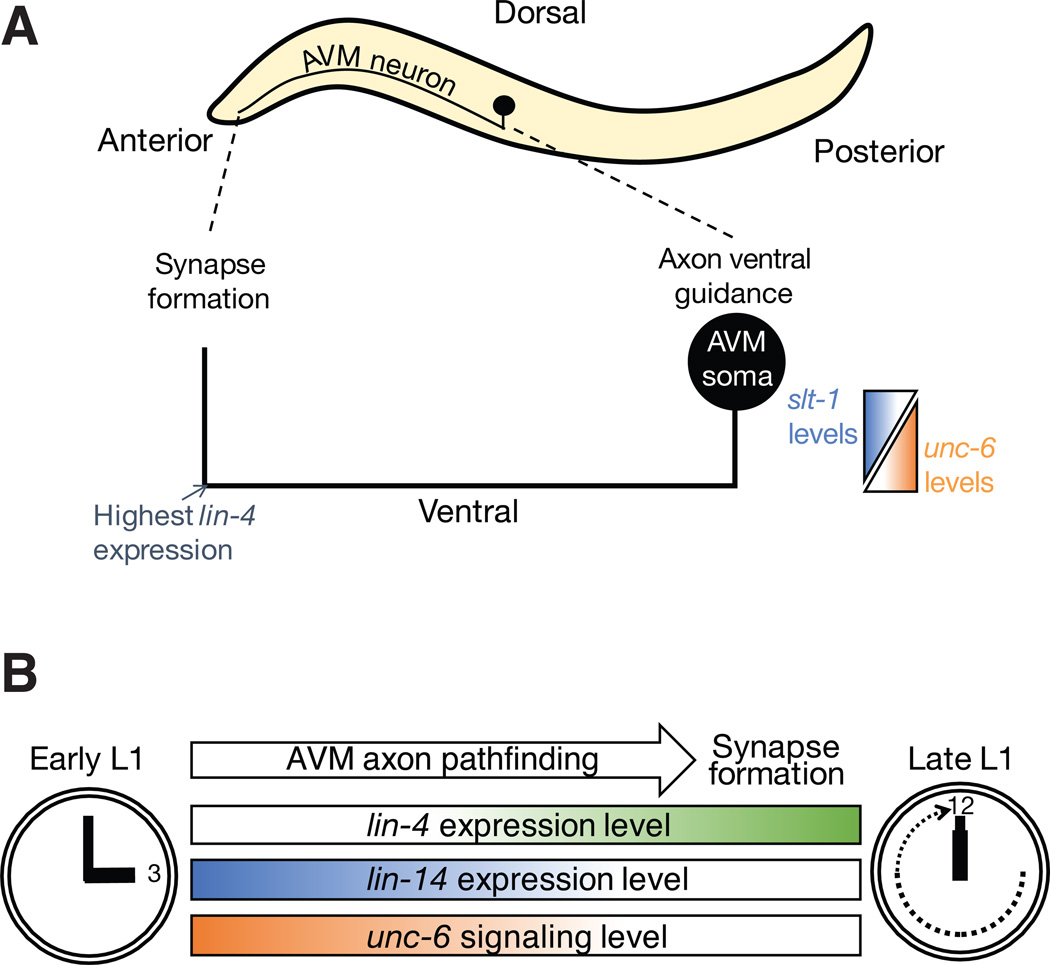
It has been demonstrated that the guidance process of the AVM axonal growth cones to their correct location in the ventral midline during L1 stage depends on both UNC-6 (Netrin) attraction and SLT-1 repulsion cues (Chang et al., 2004; Fig. 1A). The interactions of UNC-40 receptor with its cofactor MADD-2 is crucial in UNC-6 (Netrin)-mediated guidance signaling (Hao et al., 2010; Alexander et al., 2010) whereas SAX-3 and EVA-1 receptor interactions are important in SLT-1-mediated guidance signaling (Fujisawa et al., 2007). UNC-6 (Netrin) is secreted by the ventral nerve cord motor neurons and serves as a guidance cue. It exerts an attractive effect on the axon via the UNC-40 receptor, which is the homolog of vertebrates’ netrin receptors Deleted in Colorectal Cancer (DCC). Proper axonal guidance of the AVM neuron to the ventral midline also requires a repulsive cue that exerts an effect on the axon to move away from the dorsal midline. This repulsive cue from dorsal body wall muscles is provided by SLT-1 (member of the slit family of guidance cues) and is registered by the SAX-3 and EVA-1 co-receptors on the axonal growth cone. Once the AVM axon reaches the ventral midline, it migrates anteriorly toward the neuropil region where axon pathfinding ceases and synapse formation begins. (Fig. 1A).
Fig. 1. Temporal regulation of neuronal connectivity by the lin-4-lin-14 pathway.
(A) The AVM axon initially projects ventrally towards the ventral nerve cord, guided by the repulsive SLT-1 and the attractive UNC-6 guidance cues. The highest level of lin-4 expression occurs when the axon reaches its most anterior position. At this point, the axon no longer responds to UNC-6 attractive guidance cues in the ventral nerve cord and projects dorsally to form synapses. (B) Temporal control of transition from axon pathfinding to synapse formation by lin-4 miRNA functioning through the unc-6 signaling.
Genetic analyses were employed to examine the role of lin-4-lin-14 circuit in AVM response to guidance cues. As both UNC-6 and SLT-1 are necessary for the AVM axon ventral guidance (Fig. 1A), the slt-1 loss of function mutant, slt-1(ok255), provided a sensitized genetic background that specifically revealed the role of lin-4 in UNC-6-mediated AVM axon ventral attraction. While unc-6; lin-4 double mutants displayed the same phenotype as unc-6 single mutants, slt-1; lin-4 double mutants showed a milder phenotype compared to the slt-1 single mutants due to enhanced axonal attraction to UNC-6 (Zou et al., 2012). This observation suggests that lin-4 acts within the UNC-6 (netrin)-mediated pathway as lin-4 loss of function only presents with a phenotype when the SLT-1 pathway is absent but the UNC-6 pathway is intact. In slt-1; lin-4 double mutants where LIN-14 levels are increased, the response to the attractive UNC-6 cue is enhanced such that the defect caused by the absence of dorsal repulsion from SLT-1 is compensated.
Having determined the relationship between lin-4, LIN-14, and UNC-6, additional interactions between lin-4, EVA-1 and SAX-3 (C. elegans homolog of vertebrate Robo) were examined in order to further elucidate how lin-4 regulates AVM axon pathfinding. SAX-3 and EVA-1 are co-receptors that bind SLT-1. All three components are necessary for the repulsive effect of SLT-1 on AVM neurons. It is important to note that SAX-3 has an additional role in inhibiting UNC-40 receptor binding to UNC-6, independent of its role in SLT-1-mediated repulsion of the AVM axon. Genetic analysis of eva-1; lin-4 and sax-3; lin-4 double mutants further revealed that the lin-4 loss of function mutation was able to suppress the eva-1 mutant phenotype but not the sax-3 mutant phenotype (Zou et al., 2012). This result suggests that lin-4 regulation of UNC-6-mediated AVM axon guidance depends on SAX-3 receptors.
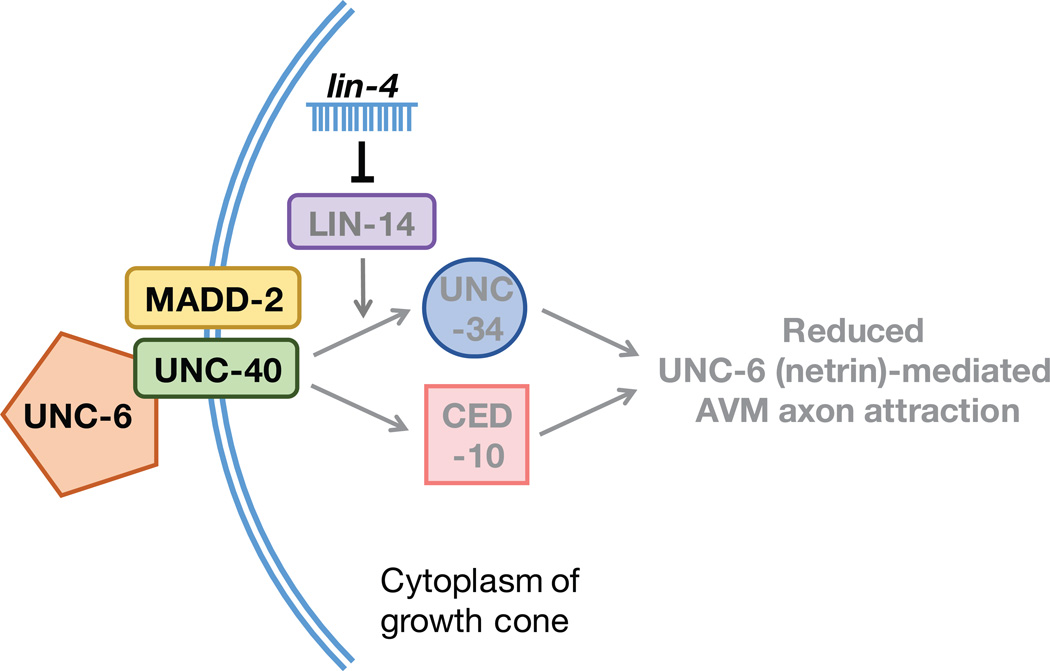
Netrin-mediated axon pathfinding is temporally regulated by the lin-4-lin-14 pathway, but how this timing pathway regulates UNC-40 receptor signaling remains unclear. There are two known downstream pathways that mediate UNC-40 signaling in AVM neurons (Lundquist et al., 2001; Gitai et al., 2003). One involves UNC-34, a homolog of Enabled in Drosophila, which is a member of the vasodilator-stimulated phosphoprotein (VASP) family of actin cytoskeleton regulators. The other relies on CED-10, a Rac guanosine triphosphatase that is conserved across all animal systems. Triple mutant analyses demonstrated that the temporal effect of the lin-4-lin-14 pathway on axon pathfinding is mediated by the receptor UNC-40 and its cofactor MADD-2 through a combination of the downstream UNC-34- and CED-10-dependent pathways (Fig. 2).
Fig. 2. Model of negative control of UNC-6-mediated AVM axon attraction by lin-4 miRNA.
The lin-4-lin-14 circuit inhibits both CED-10 and UNC-34 downstream pathways that mediate the UNC-40 receptor signaling.
By using genetic analyses, it was shown that lin-4 miRNA participates in limiting UNC-6-mediated axon attraction by repressing LIN-14 expression. Reduced expression of LIN-14 downregulates UNC-40/MADD-2 signaling, which results in decreasing the growth cone response to the UNC-6 guidance cue (Fig. 1B). Increased lin-4 expression signifies maturation as the lin-4-mediated reduction in guidance cue responsiveness is necessary for the AVM axon to halt its migration and to begin synapse formation. Interestingly, in the timing of development of the C. elegans hermaphrodite specific neurons (HSNs), lin-4 acts to suppress the expression of lin-14 and lin-28 to promote HSN axon initiation in a guidance cue independent manner (Olsson-Carter and Slack, 2010). Although, lin-4 is ubiquitously expressed in the C. elegans nervous system, there is much on the function and role of lin-4 to be uncovered.
The lin-4-lin-14 Pathway Regulates the Timing of Synaptic Reorganization
It was previously shown that the LIN-14 signalling temporally controls synaptic remodelling of dorsal D-type (DD) motor neurons, rewiring connectivity and changing the innervation of muscles from ventral to dorsal direction in C. elegans (Hallam and Jin, 1998), More recently, LIN-14 was found to cooperate with UNC-30 to prolong the expression of oig-1, a single immunoglobulin domain protein, during the embryonic and L1 stages. The reduction of LIN-14 expression in L2 and later stages contributes to the temporal down-regulation of oig-1 in the DD motor neurons, resulting in synaptic rewiring. The extended expression of oig-1 was seen when lin-14 expression was prolonged by the removal of lin-4, which causes a delay in synaptic rewiring (Howell et al., 2015). Thus the lin-4-lin-14 regulatory pathway appears to regulate the timing of this synaptic respecification event. This study suggests that lin-4-mediated down-regulation of lin-14 is necessary in the temporal control of neuronal connectivity beyond the AVM neuron.
It is also interesting to note that UNC-30 acts with the UNC-55 nuclear hormone receptor, to specify the VD synaptic wiring pattern in a spatially controlled manner (Howell et al., 2015). Taken together, in this spatiotemporal control system, the UNC-55 nuclear hormone receptor expressing in VD but not DD neurons provides a spatial code whereas the LIN-14 transcription factor expressing only in the L1 stage provides a temporal code. The UNC-30 transcription factor likely further restricts the UNC-55 and LIN-14 functions within the nervous system. Whether a similar intersectional transcriptional strategy is used in timing neuronal connectivity elsewhere remains to be seen.
Temporal Control of Neuronal Regeneration by let-7 miRNA
let-7 miRNA has been detected in a variety of species, including humans (Pasquinelli et al., 2000), who have a highly conserved paralog expressed in the brain (Pena et al., 2009). The two best known targets of let-7 in C. elegans are LIN-28 and LIN-41. Recently, the interaction between let-7 and LIN-28 has drawn much attention, due to the LIN-28’s role in stem cells and tumors (Rehfeld et al., 2015). lin-41, encoding a heterochronic, tripartite motif protein, is the direct target of let-7 in neurons (Zou et al., 2013).
A number of C. elegans neurons, including the AVM neuron, show increased levels of let-7 as they age. In AVM neurons, let-7 plays a significant role in the decline of the regeneration potential as neurons age (Zou et al., 2013; Chiu & Chang, 2013; Chiu et al., 2011). The regeneration potential of AVM axons steeply declines in the late L3 stage of development, as evidenced by the stunted regrowth of severed AVM axons that were cut using a femptosecond laser. To demonstrate that this limited regeneration is miRNA dependent, the regeneration ability of dcr-1 and alg-1 mutants was investigated. Both dcr-1 and alg-1 are required for miRNA processing, but their miRNA targets are different (Zou et al., 2013). Following femtosecond laser ablation of AVM axons in the L3 stage of development, dcr-1 mutants showed the same limited axon regeneration capacity as WT axons. However, alg-1 mutants presented with increased axonal outgrowth from the point of ablation, which was 2.5 times the length of the axon regrown in WT, demonstrating that Argonaute proteins are required in restricting the regeneration potential of the AVM axon (Zou et al., 2013).
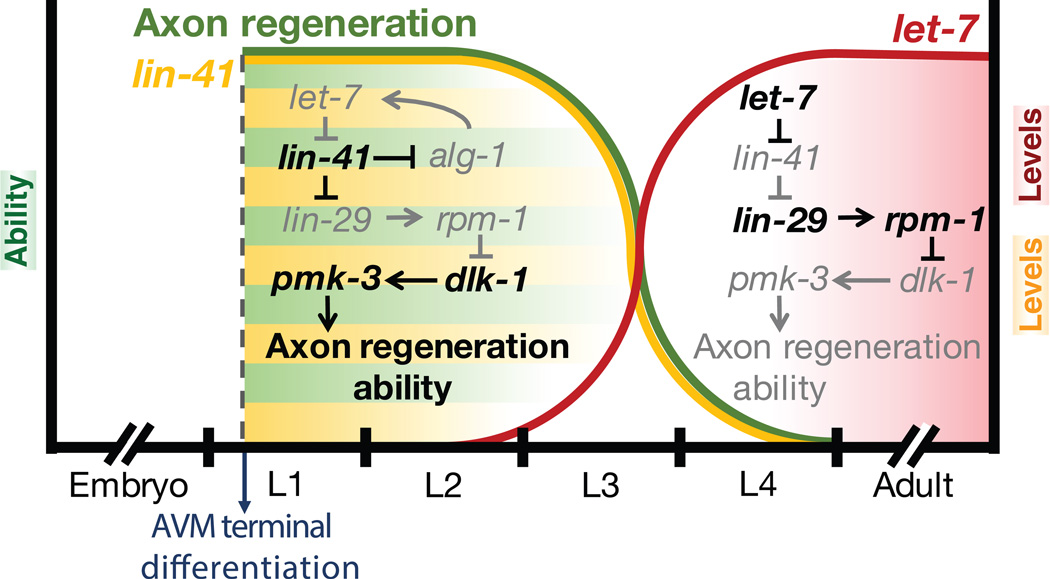
Stem-loop reverse transcription polymerase chain reaction (RT-PCR) identified miRNA candidates that could contribute to regeneration decline in later stages. An increased level of let-7 miRNA was detected beginning in the L3 stage of C. elegans development and continued to approximately day 4 of the adult stage. A target of let-7 miRNA, LIN-41, begins to show a significant decrease in the L3 stage, demonstrating an inverse relationship between let-7 and LIN-41 expression. let-7 miRNA inhibition of LIN-41 was confirmed through genetic analyses. let-7 loss of function mutants displayed enhanced AVM axon regeneration potential while lin-41 mutations suppressed the let-7 mutant phenotype. Evidence further showed that let-7-lin-41 act via the lin-29 transcription factor to control AVM axon regeneration (Fig. 3). It has been known that Mitogen Activated Protein Kinase (MAPK) cascade, in which RPM-1 is a negative regulator of DLK-1 (MAPK kinase kinase) and PMK-3 (p38 kinase), controls axonal regeneration in C. elegans (Hammarlund et al., 2009; Yan et al., 2009). Through genetic analysis, it appears that the timing of the MAPK pathway inactivation is controlled by the let-7-lin-41-lin-29 circuit (Zou et al., 2013; Fig. 3).
Fig. 3. Model of temporal regulation of axonal regeneration by let-7 miRNA functioning through the MAPK pathway.
The let-7-lin-41 circuit inhibits the function of the MAPK pathway leading to limited axonal regeneration in older neurons.
The importance of let-7 as a regulatory timing microRNA for neuronal regeneration was underscored by the effect of the forced early expression of this miRNA. Forced early expression of let-7 at the first larval stage, caused precocious decline in axonal regeneration ability (Zou et al., 2013). The timing of let-7 expression in WT animals is reciprocally controlled by its direct target, LIN-41 (Fig. 3). In lin-41 loss of function mutants, let-7 is prematurely expressed in the L1 stage. This reciprocal inhibition of let-7 by LIN-41 proteins seems to be dependent on the Argonaute proteins, as evidenced by the analysis of lin-41; alg-1 double mutant phenotypes. lin-41; alg-1 double mutants showed no premature expression of let-7 and displayed a WT-like increase in let-7 only from the L3 stage onwards. Co-immunoprecipitation of LIN-41 and ALG-1 provides further support that these two proteins interact directly. A comparison between let-7 expression in lin-41 mutants and lin-41; alg-1 double mutants revealed an important caveat to LIN-41’s control of let-7 expression. The let-7 expression remains increased in the double mutant during the later stage of development indicates that LIN-41 is able to exert its inhibitory effect on let-7 expression via ALG-1 only during the early stage of development. Another ALG-1-independent mechanism must come into play for let-7 biogenesis in the later stage.
The reduction of plasticity at the level of the AVM neuronal regeneration has thus been demonstrated to depend on the let-7-lin-41 interaction, but much is left undefined about the other key players in this plasticity pathway. The precise role of let-7 in other pathways that control neuronal regeneration potential, such as the MAPK pathway, also remains unclear. Even the temporal control of let-7 expression remains elusive as it is not known what triggers the increased let-7 expression and the inversely correlated decline of LIN-41 at the later stages in WT neurons. Furthermore, limited neuronal regeneration in aged neurons is not entirely dependent on let-7, as the limited regeneration potential continues into aged neurons even after let-7 levels are reduced following the fourth day of the adult stage (Zou et al., 2013). Thus, in order to fully understand the mechanisms that limit neuronal regeneration in adulthood, it is essential to find other contributors that decrease regeneration capacity in aged neurons following the decline of let-7.
Future directions
C. elegans provides the field of developmental and regenerative neuroscience with an elegant model that is unparalleled in its ability to be genetically modified, quickly propagated, and easily visualized. The simplicity of the animal should not be underestimated, as its 40% homologous genome has already provided us with insight into fundamental biological pathways, such as the mechanisms of miRNA modulation. Although we have gained significant insight into the control exerted by the lin-4 and let-7 miRNAs on the timing of differentiation and postdifferentiation events in postmitotic neurons, a full understanding of the regulatory circuitry still largely remains elusive. It is hopeful that continuing the efforts to reveal the conserved timing mechanisms at play in the wiring and rewiring of neural circuits will lead to progress to our eventual goal of applying this knowledge to targeted therapy for neurodevelopmental and neurodegenerative disorders, and traumatic brain injury.
Acknowledgments
This work was supported by grants from the NSF (IOS-1455758), the March of Dimes Foundation, the Whitehall Foundation Research Award, and the NIH (5R01GM111320) to C.C..
References
- Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev Cell. 2003;4:625–637. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Alexander M, Selman G, Seetharaman A, Chan KK, D’Souza SA, Byrne AB, Roy PJ. MADD-2, a homolog of the Opitz syndrome protein MID1, regulates guidance to the midline through UNC-40 in Caenorhabditis elegans. Dev. Cell. 2010;18:961–972. doi: 10.1016/j.devcel.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitaz HR. Heterochronic Mutants of the Nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Chang C, Yu TW, Bargmann CI, Tessier-Lavigne M. Inhibition of Netrin-mediated axon attraction by a receptor protein tyrosine phosphatase. Science. 2004;305:103–106. doi: 10.1126/science.1096983. [DOI] [PubMed] [Google Scholar]
- Chang S, Johnston RJ, Jr, Frokjaer-Jensen C, Lockery S, Hobert O. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature. 2004;430:785–789. doi: 10.1038/nature02752. [DOI] [PubMed] [Google Scholar]
- Chiu H, Alqadah A, Chang C. The role of microRNAs in regulating neuronal connectivity. Front Cell Neurosci. 2014;7:1–6. doi: 10.3389/fncel.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Alqadah A, Chuang C-F, Chang C. C. elegans as a genetic model to identify novel cellular and molecular mechanisms underlying nervous system regeneration. Cell Adhesion & Migration. 2011;5:387–394. doi: 10.4161/cam.5.5.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Chang C. Rejuvenating nerve cells in adults. Aging. 2013;5:485–486. doi: 10.18632/aging.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. A Transparent window into biology: A primer on Caenorhabditis elegans. In: WormBook, editor. The C elegans Research Community. WormBook; 2015. http:www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm A, Jin Y. The Dlk-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, Yates JR, III, Mello CC. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Wrana JL, Culotti JG. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science. 2007;317:1934–1938. doi: 10.1126/science.1144874. [DOI] [PubMed] [Google Scholar]
- Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The Netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through Enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon Regeneration Requires a Conserved MAP Kinase Pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JC, Adler CE, Mebane L, Gertler FB, Bargmann CI, Tessier-Lavigne M. The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in Netrin-mediated axon attraction and branching. Dev. Cell. 2010;18:950–960. doi: 10.1016/j.devcel.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K, White JG, Hobert O. Spatiotemporal control of a novel synaptic organizer molecule. Nature. 2015;523:83–87. doi: 10.1038/nature14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C elegans hunchback homolog, hbl-, controls temporal patterning and is a probable microRNA target. Dev. Cell. 2003;4:639–650. doi: 10.1016/s1534-5807(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Londin ER, Adijanto J, Philp N, Novelli A, Vitale E, Perria C, Serra G, Alesi V, Surrey S, Fortina P. Donor splice-site mutation in CUL4B is likely cause of X-linked intellectual disability. American Journal of Medical Genetics Part A. 2014;164:2294–2299. doi: 10.1002/ajmg.a.36629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Reddien PW, Hartwieg E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 2001;128:4475–4488. doi: 10.1242/dev.128.22.4475. [DOI] [PubMed] [Google Scholar]
- Olsson-Carter K, Slack FJ. A Developmental Timing Switch Promotes Axon Outgrowth Independent of Known Guidance Receptors. PLoS Genetics. 2010;6:e1001054. doi: 10.1371/journal.pgen.1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale M, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- Pena JTG, Sohn-Lee C, Rouhanifard SH, Ludwig J, Hafner M, Mihailovic A, Lim C, Holoch D, Berninger P, Zavolan M, Tuschl T. miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nat Methods. 2009;6:139–141. doi: 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld F, Rohde AM, Nguyen DTT, Wulczyn FG. Lin28 and let-7: ancient milestones on the road from pluripotency to neurogenesis. Cell Tissue Res. 2015;359:145–160. doi: 10.1007/s00441-014-1872-2. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;4:659–669. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- Stroynowska-Czerwinska A, Fiszer A, Krzyzosiak WJ. The panorama of miRNA-mediated mechanisms in mammalian cells. Cell Mol Life Sci. 2014;71:2253–2270. doi: 10.1007/s00018-013-1551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella MC, Slack FJ. C. elegans microRNAs. In: WormBook, editor. The C. elegans Research Community. WormBook; 2005. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Zou Y, Chiu H, Domenger D, Chuang C-F, Chang C. The lin-4 microRNA targets the LIN-14 transcription factor to inhibit netrin-mediated axon attraction. Sci. Signal. 2012;5:ra43. doi: 10.1126/scisignal.2002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Chiu H, Zinovyeva A, Ambros V, Chuang C-F, Chang C. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science. 2013;340:372–376. doi: 10.1126/science.1231321. [DOI] [PMC free article] [PubMed] [Google Scholar]