Abstract
Background
Airway epithelial cells (AEC) are increasingly recognized as a major signaling center in the pathogenesis of allergic asthma. A previous study demonstrated that epithelial growth factor receptor (EGFR) signaling in AEC regulated key features of allergic airway disease. However, it is unclear what mediators are regulated by EGFR signaling in AEC, although the production of the pro-inflammatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) is EGFR-dependent in keratinocytes.
Objectives
To determine if EGFR signaling regulates GM-CSF production by human AEC downstream of the clinically relevant mediators house dust mite (HDM) and interleukin (IL)-17A and in a mouse model of established allergic asthma.
Methods
EGFR inhibitors were used to determine whether EGFR signaling regulates GM-CSF production by cultured human AEC in response to HDM and IL-17A. The roles of EGFR ligands, p38 mitogen activated protein kinase (MAPK), and tumor necrosis factor alpha (TNFα) converting enzyme (TACE) were also assessed. To determine if EGFR regulates GM-CSF as well as key asthma characteristics in vivo, mice were chronically exposed to HDM to establish allergic airway disease and then treated with the EGFR inhibitor Erlotinib.
Results
EGFR inhibition reduced HDM and IL-17A induced GM-CSF production in a dose-dependent manner in cultured human AEC. GM-CSF production also required amphiregulin, p38 MAPK signaling, and protease/TACE activity. In mice with established allergic airway disease, EGFR inhibition reduced levels of GM-CSF and TNFα, as well as airway hyperreactivity, cellular inflammation, smooth muscle thickening, and goblet cell metaplasia without changes in IgE and Th1, Th2, and Th17 cytokines.
Conclusions and Clinical Relevance
Results link HDM, IL-17A, amphiregulin, EGFR and GM-CSF in a mechanistic pathway in AEC, and demonstrate that EGFR regulates GM-CSF production and the severity of established disease in a clinically relevant asthma model. These results identify the EGFR→GM-CSF axis as a target for therapeutic development.
Keywords: EGFR, IL-17A, house dust mite, chronic asthma
Introduction
Allergic asthma is a complex, immune-driven chronic lung disease characterized by airflow limitation from bronchospasm due to increased airway hyperreactivity (AHR), bronchial wall thickening due to chronic inflammation, airway smooth muscle (ASM) thickening, and goblet cell metaplasia and mucus hypersecretion (1). While current therapies are effective in mild to moderate asthma, symptom control remains challenging and is a major cause of morbidity and mortality in severe refractory asthma (2). Airway epithelial cells (AEC) represent potential therapeutic targets as they are the site of initial contact with environmental stimuli such as allergens and are thought to play an important role in asthma pathogenesis through initiation and/or chronic maintenance of disease (3-5). However, despite their pathogenic importance, signaling mechanisms governing AEC responses to external stimuli that drive the critical clinical features of asthma are poorly understood.
Epidermal growth factor (EGF) receptor (EGFR) was initially identified as a potential contributor to asthma pathogenesis by genome wide association studies and polymorphism linkage analysis that associated EGFR with AHR in asthma patients (6, 7). EGFR signaling is activated by a variety of factors known to initiate or exacerbate asthma symptoms including allergens, viruses, and pollutants suggesting its potential importance as a common pathway mechanistically linking phenotypically distinct asthma populations (8-11). Increased EGFR expression was detected in the airway epithelium, submucosal glands, and ASM of asthma patients and correlated with disease severity (12-15). EGFR is similarly expressed in mice, and active (phosphorylated) EGFR expression is increased in the airway epithelium with chronic exposure to the clinically-relevant allergen house dust mite (HDM) (16). EGFR expression also correlates with goblet cell metaplasia (17, 18), and drives many of characteristic features of allergic asthma in animal models (19-21). Our previous study determined that EGFR signaling in AEC regulated characteristics of allergic asthma in a HDM-induced model (16). However, the molecular mediators and cytokines induced by HDM and regulated by EGFR signaling in AEC are unclear.
A complex array of mediators derived from multiple cell types contributes to asthma pathogenesis, however, it is not clear how they are linked and regulated. For example, expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-17A are increased and correlate to disease severity in asthma patients (22, 23). GM-CSF also contributes to disease in experimental asthma models by promoting allergic responses via the priming and activating of macrophages and dendritic cells (24-26). EGFR signaling mediates GM-CSF expression and secretion by keratinocytes (27), but if and how this may occur in AEC is unknown. IL-17A and HDM induce GM-CSF production in human bronchial epithelial cells (28, 29), but whether this is EGFR dependent is unclear. IL-17A and HDM also activate p38 mitogen-activated protein kinase (MAPK) (30, 31), which can directly activate the tumor necrosis factor alpha (TNFα) converting enzyme (TACE) to cleave and release EGFR ligands (32, 33). To date, published reports have not linked these important asthma mediators into a specific allergen-stimulated pathway.
The present study focused on AEC, since data from asthma patients showed that GMCSF and EGFR were increased in AEC and IL-17A was increased in inflammatory cells in close proximity to AEC (13, 22, 23). In vitro studies using human AEC, including primary cultures, were used to test the hypothesis that EGFR signaling regulates GM-CSF production downstream of important asthma mediators. An in vivo model of established allergic disease was also used to examine this question in vivo, since clinical findings demonstrated increased GM-CSF, EGFR, and IL-17A levels in bronchial biopsies of patients with established disease. Results identified a pathway linking HDM, IL-17A, p38, TACE, and EGFR to allergen-induced GM-CSF production in AEC. Additionally, inhibition of EGFR reduced GM-CSF levels as well as key features of asthma. Collectively, these data identify the EGFR→GM-CSF axis in AEC as a potential target for asthma therapy.
Methods and Materials
Cell Culture
Human bronchial epithelial cells (HBEC; HBEC3-KT CRL-4051, ATCC) were cultured as a monolayer in keratinocyte serum free media (KSFM; 17005-042, Life Technologies). HBEC were passaged onto 12-well plates with 100,000 cells per well, then allowed to recover for 24 hours to reach approximately 80% confluency, and then media was switched to supplement free media for 18 hours as the cell were approximately 99% confluent prior to stimulation with transforming growth factor alpha (TGFα; 72 nM, 100-16A, Peprotech), epidermal growth factor (EGF; 72 nM, AF-100-15, Peprotech), heparin-binding EGF-like growth factor (HB-EGF; 72 nM, 100-47, Peprotech), amphiregulin (AREG; 72 nM, 100-55B, Peprotech), HDM (10, 25, 100 μg dry weight, D. pteronyssinus XPB82D3A25, Greer Laboratories), IL-13 (100 ng, 130-093-953, Miltenyi Biotec), and IL-17A (1-100 ng, 130-093-959, Miltenyi Biotec) with or without the EGFR inhibitors Erlotinib (0.1-0.5 μM, OSI Pharmaceuticals) or AG1478 (0.1-0.5 μm, 658548, EMD Millipore), Actinomycin D (1 μg/mL, A9415, Sigma), the protease inhibitor GM6001 (20 μM, 364206, EMD Millipore), TACE inhibitor TAPI-1 (20 μM, 579053, EMD Millipore), the p38 inhibitor SB202190 (2-5 μM, S7067, Sigma), the MEK1/2 inhibitor ARRY142886 (1 μM, 1183194, Otava), or a neutralizing antibody to amphiregulin (0.3-1 μg/mL, AF262, R&D Systems). To determine any effects of the inhibitors on cell viability HBEC were exposed to the highest dose of each inhibitor for 24 hours in supplement free media. Representative pictures were taken to determine any changes in morphology, and then cells were collected and counted to determine any effects on cell number; data is presented in supplemental material (S Fig. 1).
Primary HBEC were provided via a collaboration with Dr. Scott Randell obtained from donor lungs and cryopreserved as previously described (34). Primary cells were thawed and cultured as a monolayer in bronchial epithelial growth media (BEGM; CC-3170, Lonza) on Purecol (5005-B, Advanced Biomatrix) coated tissue-culture dishes. Primary cells were passaged to P2 on 12-well plates with 100,000 cells per well, then allowed to recover for 48 hours to reach approximately 80% confluency, and then media was switched to supplement free media for 18 hours as the cell were approximately 99% confluent prior to stimulation with HDM (100 μg) and IL-17A (25 ng) with or without the EGFR inhibitor Erlotinib (0.1-0.5 μM). Media was removed and stored at −80°C for measurement of GM-CSF by ELISA (432001, Biolegend) following manufacturer's instructions.
Animals, Allergen Exposure, and Erlotinib Treatment
The Institutional Animal Care and Use Committee (IACUC) at Cincinnati Children's Hospital Medical Center approved all protocols used for the animals in this study. For the chronic exposure protocol, adult (6-8wk) FVB/N female mice were treated with house dust mite (HDM; 50 μg protein, D. pteronyssinus XPB82D3A25, Greer Laboratories) or 0.9% saline intranasally (i.n.) three times a week for six weeks. A group of mice were given intraperitoneal (i.p.) injections of Erlotinib (100 mg/kg, OSI Pharmaceuticals), an EGFR inhibitor, the day of and day after HDM exposure beginning the fourth week of HDM exposure and continued until the end of the study. 24 hours after the last HDM exposure, lung mechanics were assessed and tissues were harvested for analysis.
Lung Mechanics, Histology, Immunostaining, and Airway Smooth Muscle Analysis
Mice were connected to a flexiVent system (FX1 Model, Scireq), and mechanical ventilation was initiated at 150 breaths/minute with a tidal volume of 10 mL/kg and a positive end-expiratory pressure of 3 cm H2O. Repeated measurements of Snapshot 150 and Quickprime 3 were taken after two deep inflation maneuvers followed by nebulization for 10 seconds of 1X PBS (baseline) and then increasing doses of methacholine (acetyl-b-methylcholine chloride A2251, Sigma) at 12.5, 25 and 50 mg/mL.
The left lung was inflation-fixed at 25cm pressure with 4% paraformaldehyde (PFA), processed and embedded in paraffin, and then 6μm sections were cut and collected onto poly-L-Lysine coated slides. Immunostaining of chloride channel calcium activated family member (CLCA3; primary antibody 1:12,500, ab46512, Abcam) and α-smooth muscle actin (α-SMA; primary antibody 1:20,000, A2547, Sigma; secondary antibody 1:200; 1070-08, Southern Biotech) were performed on lung sections, developed with diaminobenzidine, and counter-stained with 10% hematoxylin. Images were acquired using a microscope and camera (Axioplan 2, Carl Zeiss).
Morphometric analysis was performed on lung sections after immunostaining for α-SMA and images of cross-sectioned airways were acquired, as described previously (16). The area of smooth muscle staining was determined by pixel thresholding using Metamorph software (Molecular Devices), and the square root of airway smooth muscle area was corrected to the internal perimeter of the measured airway. The values of all cross-sectioned airways were averaged to generate a value for each animal. Measurements were performed by an observer blinded to the identity of the slides.
Western Blot Analysis
Western blots were performed on lung homogenates. Membranes were incubated with primary antibodies for chloride channel calcium activated family member 3 (CLCA3; 1:5000; ab46512, Abcam) and pan-Actin (C4 Actin; 1:40,000; 7 Hills Bioreagents), and secondary antibodies for goat-anti-rabbit or goat-anti-mouse (1:10,000; 401393 and 401215, Calbiochem). Luminata Forte (WBLUF0500, Millipore) was used to develop blots and a CCD camera LAS 4000 (Fujifilm) was used to acquire digital images. Densitometry measurements were performed using Multi Gauge 3.0 software (Fujifilm).
Allergic Sensitization, Cellular Inflammation, and Cytokines
Blood was collected and serum extracted by centrifugation and HDM-specific IgG and IgE were measured by ELISA. Plates were coated with 0.01% HDM overnight and then blocked with 1% BSA for 1 hour. Samples were incubated for 1 hour (IgG 1:5000, IgE 1:2), then biotin-anti-mouseIgE (1:250; 553419, BD Biosciences) was applied for 1 hour, and then streptavidin-HRP (1:100; DY998, R&D Systems) was added for 30 minutes. The plate was developed by adding tetramethylbenzidine substrate (555214, BD Biosciences), neutralized with 2N H2SO4, and then absorbance was measured at 450nm using a spectrophotometer (Synergy2, BioTek).
Bronchoalveolar lavage fluid (BALF) was collected and inflammatory influx was determined by counting the total number of cells. A portion of the BALF cell suspension was differentially stained with Kwik Diff kit (9990700, Thermo Scientific), and the percentages of macrophages, lymphocytes, neutrophils, and eosinophils were calculated. IL-4, −5, −17A, Eotaxin, IFNγ, TNFα, and GM-CSF were measured in BALF using multiplex cytokine/chemokine panel I and Luminex xMAP technology (PXMCYTO-70K, Millipore) following the manufacturer's instructions. IL-13 mRNA levels were measured by qPCR from mRNA isolated from lung tissue using TaqMan primers for IL-13 (Mm00434204_m1) corrected to β-actin (Mm00607939_s1) and utilizing a StepOne Plus real-time PCR system (Applied Biosystems).
Statistical Analysis
Statistical analyses were performed using Prism 5 software (Graphpad). For all graphs one-way ANOVA with Tukey and Bonferroni multiple comparisons were used for statistical comparison, and the mean of each group is plotted with error bars representing the standard error of the mean (±SEM).
Results
Regulation of GM-CSF Downstream of HDM in Human AEC
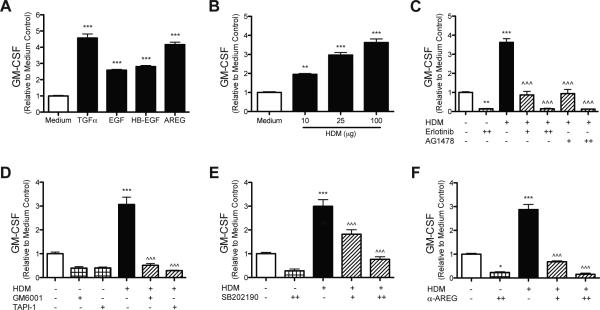
To determine if EGFR signaling regulates GM-CSF production by AEC we utilized cultured human bronchial epithelial cells (HBEC) and stimulated EGFR signaling with several EGFR ligands. After 24 hours, GM-CSF levels were increased in response to TGFα (239.6 pg/mL ± 14.6), EGF (154.7 pg/mL ± 4.8), HB-EGF (171.6 pg/mL ± 3.6), and amphiregulin (231.1 pg/mL ± 11.2) compared to medium only (58.6 pg/mL ± 3.9) (Fig. 1a). To determine if GM-CSF production is induced by exposure to HDM, a clinically relevant allergen, HBEC were stimulated with several concentrations of HDM. GM-CSF levels increased and were positively correlated with the concentration of HDM (112.9 pg/mL ± 6.0 at 100μg) compared to medium only (34.6 pg/mL ± 2.5) (Fig. 1b). To determine if HDM induced increases in GM-CSF were dependent on EGFR signaling, HBEC were stimulated with HDM with or without the EGFR inhibitors Erlotinib or AG1478. EGFR inhibition with either inhibitor blocked GM-CSF levels in a dose-dependent manner (Fig. 1c). Since HDM exposure can induce p38 signaling, which in turn can induce TACE activity, HBEC were exposed to HDM with or without the broad-spectrum metalloproteinase inhibitor GM6001, a TACE inhibitor TAPI-1, as well as the p38 inhibitor SB202190. HDM-induced increases in GM-CSF were blocked by GM6001, TAPI-1, and SB202190 (Fig. 1d,e). Collectively, these data indicate that HDM-induced GM-CSF production by AEC is dependent on p38, protease/TACE activity, and EGFR signaling. Since both EGFR ligands and HDM stimulation increased GM-CSF production and it is known that HDM exposure can lead to EGFR ligand release via shedding, we sought to determine whether EGFR ligands (amphiregulin or TGFα) were inducing GM-CSF production after HDM stimulation. HBEC were stimulated with HDM with or without neutralizing antibodies to TGFα or amphiregulin, since TGFα and amphiregulin were the two most potent EGFR ligands inducing GM-CSF production (Fig. 1a). Neutralizing TGFα after HDM exposure did not reduce GM-CSF levels (data not shown). However, neutralizing amphiregulin blocked the increase in GM-CSF production after HDM stimulation in a dose-dependent manner (Fig. 1f). These data indicate that amphiregulin is the major EGFR ligand released by epithelial cells in response to HDM exposure that induces GM-CSF production.
Figure 1. EGFR signaling, protease/TACE activity, and p38 MAPK signaling are required for GMCSF production by HBEC stimulated with HDM.
HBEC were stimulated with (A) equimolar concentrations (72 nM) of TGFα, EGF, HB-EGF, and AREG; (B) HDM (10, 25, or 100 μg dry weight); (C) HDM (100 μg) with or without EGFR inhibitors Erlotinib (0.1 μM [+] or 0.25 μM [++]) or AG1478 (0.25 μM [+] or 0.5 μM [++]); (D) HDM (100 μg) with or without the protease inhibitor GM6001 (20μM) or the TACE inhibitor TAPI-1 (20 μM); (E) HDM (100 μg) with or without the p38 inhibitor SB202190 (2 μM [+] or 5 μM [++]); (F) HDM (100 μg) with or without neutralizing antibodies to AREG (0.3 μg/mL [+] or 1 μg/mL [++]). GMCSF was measured in the media by ELISA 24 hours after stimulation (A-F), and statistical significance was determined by comparing samples to the medium only group (* p<0.05, ** p<0.01, *** p<0.001) or compared to the HDM stimulated group (^^^ p<0.001); n=2 (two independent experiments, each performed in triplicate) for each group in (A-F).
Regulation of GM-CSF Downstream of IL-17A in Human AEC
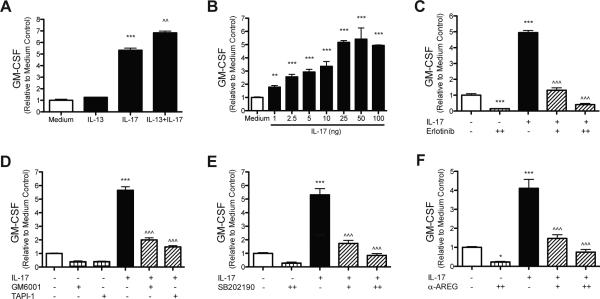
To determine whether EGFR signaling regulates GM-CSF production downstream of important cytokine mediators in allergic asthma, HBEC were stimulated with IL-13 and IL-17A. GM-CSF levels were not significantly increased by IL-13 (225.6 pg/mL ± 2.3) compared to medium only (179.7 pg/mL ± 17.9), but IL-17A induced a 5-fold increase in GM-CSF production (957.7 pg/mL ± 32.9), and there was some synergy with the combination of IL-13 and IL-17A (1,229.0 pg/mL ± 26.3) (Fig. 2a). To determine the dose dependent relationship between IL-17A and GM-CSF, HBEC were stimulated with increasing doses of IL-17A, from 1 to 100ng. 1ng of IL-17A was sufficient to increase GM-CSF production by AEC (Fig. 2b). To determine the timing of IL-17A induced GM-CSF production, HBEC were harvested at different times beginning at 30 minutes and continuing until 24 hours after IL-17A stimulation. GM-CSF levels increased between 2 and 4 hours after IL-17A exposure, suggesting transcriptional regulation (S Fig. 1). To test this, HBEC were stimulated with IL-17A with or without Actinomycin D, an inhibitor of transcription. Actinomycin D blocked the IL-17A induced increases in GM-CSF production, indicating that the increase in GM-CSF production is dependent on de novo transcription following IL-17A stimulation (S Fig. 2). Since HDM utilized EGFR signaling to induce GM-CSF production, we sought to determine if IL-17A-dependent increases in GM-CSF were also mediated via EGFR signaling. HBEC were exposed to IL-17A (25ng) with or without the EGFR inhibitor Erlotinib. Increases in GM-CSF induced by IL-17A were blocked with Erlotinib in a dose-dependent manner (Fig. 2c). Since IL-17A signaling can induce p38 signaling, which in turn can induce TACE activity, HBEC were exposed to IL-17A with or without the broad-spectrum metalloproteinase inhibitor GM6001, a TACE inhibitor TAPI-1, as well as the p38 inhibitor SB202190. Both GM6001 and TAPI-1 attenuated IL-17A induced increases in GM-CSF production (Fig. 2e). GM-CSF production by IL-17A stimulation was blocked by p38 inhibition (Fig. 2f). In summary, these data indicate that GM-CSF production by AEC following IL-17A stimulation is dependent on p38, protease/TACE activity, and EGFR signaling and also suggest that IL-17A utilizes a similar pathway to induce GM-CSF production. We sought to determine whether EGFR ligands amphiregulin or TGFα were also inducing GM-CSF production after IL-17A stimulation. HBEC were stimulated with IL-17A with or without neutralizing antibodies to amphiregulin or TGFα. Neutralizing TGFα after HDM exposure did not reduce GM-CSF levels (data not shown), however, neutralizing amphiregulin blocked IL-17A stimulated increases in GM-CSF production in a dose-dependent manner (Fig. 2f). These data indicate that amphiregulin is also the main EGFR ligand released by HBEC in response to IL-17A to induce GM-CSF production. These data also suggest that both HDM and IL-17A seem to be utilizing a similar pathway (HDM/IL-17A → MAPKp38 → TACE → amphiregulin→EGFR→GM-CSF) in human AEC.
Figure 2. IL-17A induced GMCSF production by HBEC requires EGFR signaling, and protease/TACE activity, and p38 MAPK signaling.
HBEC were stimulated with (A) IL-13 (100 ng), IL-17A (100 ng), or a combination of both IL-13 and -17A; (B) increasing amounts of IL-17A (1-100 ng); (C) IL-17A (25 ng) with or with the EGFR inhibitor Erlotinib (0.1 μM [+] or 0.25 μM [++]); (D) IL-17A (25 ng) with or without the protease inhibitor GM6001 (20 μM) or the TACE inhibitor TAPI-1 (20 μM); (E) IL-17A (25 ng) with or without the p38 inhibitor SB202190 (2 μM [+] or 5 μM [++]); (F) IL-17A (25ng) with or without neutralizing antibodies to AREG (0.3 μg/mL [+] or 1 μg/mL [++]). GMCSF was measured in the media by ELISA 24 hours after stimulation (A-F), and statistical significance was determined by comparing samples to the medium only group (* p<0.05, ** p<0.01, *** p<0.001) or compared to the IL-17A stimulated group (^^^ p<0.001); n=2 (two independent experiments, each performed in triplicate) for each group in (A-F).
p38 Signaling Upstream of EGFR in GM-CSF production in Human AEC
Since p38 signaling is known to be downstream of HDM and IL-17A stimulation and also possibly EGFR activation, we sought to determine if p38 signaling is downstream of EGFR dependent GM-CSF production. EGFR activation was stimulated directly in HBEC with amphiregulin and p38 signaling was inhibited with SB202190. Inhibiting p38 signaling after direct stimulation of EGFR did not reduce GM-CSF production, although inhibition of MEK1/2 with ARRY142886 did block the increases in GM-CSF (S Fig. 3). These data, together with the data from the HDM and IL-17A experiments (Fig 1e, 2e), indicate that p38 signaling is downstream of HDM and IL-17A but upstream of EGFR.
Regulation of GM-CSF Production in Primary Human AEC
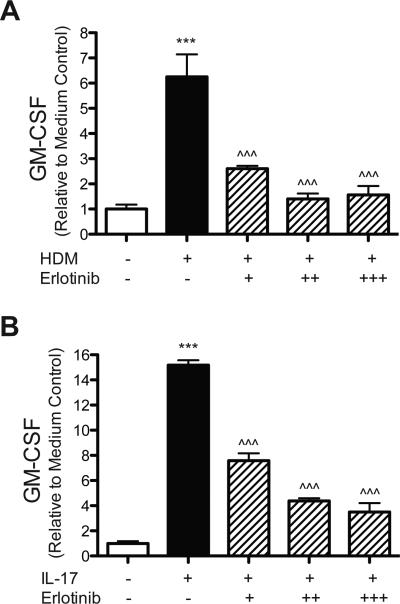
To confirm that EGFR signaling regulates GM-CSF production in primary human AEC, primary cells were stimulated with HDM or IL-17A with or without the EGFR inhibitor Erlotinib. Both HDM and IL-17A stimulation increased GM-CSF production (532.7 pg/mL ± 76.5 and 1,294.0 pg/mL ± 33.0 respectively) compared to medium only (85.3 pg/mL ± 15.1), and these increases were blocked in a dose-dependent manner with Erlotinib (Fig. 3a,b). These data confirm the findings from HBEC that EGFR signaling regulates GM-CSF production in AEC in response to HDM and IL-17A.
Figure 3. HDM and IL-17A induced GMCSF production by primary human airway epithelial cells requires EGFR signaling.
Primary human airway epithelial cells were stimulated with (A) HDM (100 μg) or (B) IL-17A (25 ng) with or with the EGFR inhibitor Erlotinib (0.1 μM [+], 0.25 μM [++], or 0.5 μM [+++]). GMCSF was measured in the media by ELISA 24 hours after stimulation, and statistical significance was determined by comparing samples to the medium only group (*** p<0.001) or compared to the HDM stimulated group (^^^ p<0.001); single experiment performed in triplicate for each group in (A,B).
EGFR Regulation of Cytokine Production in vivo
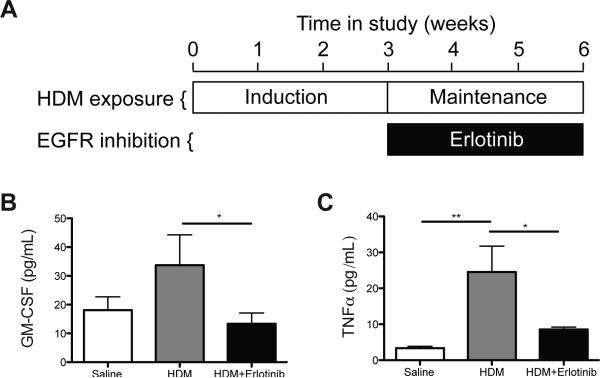
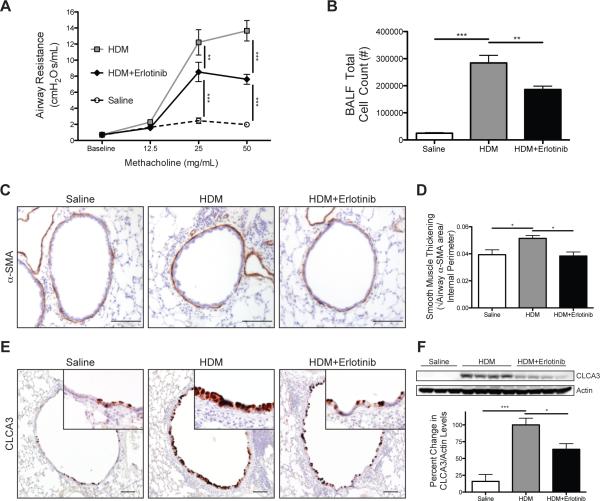
Since our in vitro data showed that EGFR signaling regulates GM-CSF production, we utilized a mouse model of established allergic asthma to determine whether EGFR signaling regulated GM-CSF production in vivo. Mice were chronically exposed to HDM to establish disease and then subsequently treated with the EGFR inhibitor Erlotinib (Fig. 4a). The impact of EGFR inhibition on cytokine production was determined by measuring cytokine levels in the lungs of exposed mice. GM-CSF and TNFα levels were reduced with EGFR inhibition (Fig. 4b,c). However, while IL-4, −5, −13, −17A, and eotaxin (CCL11) were increased and IFNγ was decreased with HDM exposure, Erlotinib treatment did not alter levels of these cytokines (S Fig. 4a-f). These data demonstrate that in a model of established allergic asthma EGFR inhibition does not alter the levels of Th1, Th2, or Th17 cytokines, but does reduce GM-CSF and TNFα levels.
Figure 4. EGFR inhibition reduced GM-CSF and TNFα levels in mice with established allergic asthma.
(A) Study protocol; adult wild-type FVB/N mice were exposed intranasally (i.n.) to 50 μg (protein) HDM or saline 3 times a week for 6 weeks. A group of mice were also treated with HDM for 6 weeks and received intraperitoneal (i.p.) injections of Erlotinib (100mg/kg) 6 times a week for the last 3 weeks of HDM exposure. The cytokines GM-CSF (B) and TNFα (C) were measured in bronchoalveolar lavage fluid by multiplex ELISA. * p<0.05, ** p<0.01; n=6 for each group in (B,C), which represents the total number of individual mice used in vivo.
EGFR Regulation of Key Asthma Characteristics in Established Disease
Since EGFR signaling was demonstrated to regulate GM-CSF production in vivo and both EGFR and GM-CSF have been previously shown to play important roles in the pathogenesis of asthma in experimental models, we assessed the impact of EGFR inhibition on key features of allergic asthma in a chronic model of allergic airway disease. Airway resistance in response to methacholine was reduced in mice that received Erlotinib (Fig. 5a). Pulmonary cellular inflammation was increased in mice exposed to HDM, but reduced with Erlotinib treatment (Fig. 5b). Differential staining revealed that the decrease in inflammatory cell influx with EGFR inhibition was primarily due to reductions in eosinophil numbers (S Fig. 5a). To assess whether EGFR inhibition altered allergic sensitization, HDM-specific IgG and IgE were measured in the serum of exposed mice. HDM-specific IgG and IgE levels were increased in mice exposed to HDM chronically and unaltered with Erlotinib treatment (S Fig. 5b,c). Taken together, these data indicate that EGFR signaling plays a role in mediating AHR and inflammatory cell influx into the lung during ongoing allergic airway disease but not allergic sensitization.
Figure 5. EGFR inhibition attenuated key asthma characteristics in mice with established allergic airway disease.
(A) Airway resistance was measured using a flexiVent system after nebulizing PBS (baseline) and then increasing doses of methacholine into the lungs after the therapeutic intervention of Erlotinib that began the week 4 of exposures. (B) Inflammatory cell influx was determined by counting the number of cells present in the bronchoalveolar lavage fluid (BALF). (C) Immunostaining for the alpha-smooth muscle actin (α-SMA) on lung sections (positive staining shown in brown); scale bar 100 μm. (D) Quantitation of airway smooth muscle thickening determined by morphometric analysis of α-SMA stained area corrected to internal perimeter of the airway. (E) Immunostaining for the goblet cell protein chloride channel calcium activated family member 3 (CLCA3) on lung sections (positive staining shown in brown); scale bar 100 μm. (F) Representative Western blots and densitometry analysis for CLCA3 and Actin in lung homogenates; both antibodies were applied to the same blot and each lane represents individual mice. * p<0.05, ** p<0.01, ***p<0.001; n=12-20 for each group in (A,B), n=5 for each group in (D), n=6-15 for each group in (F), which represents the total number of individual mice used in vivo.
To determine the effects of EGFR inhibition on ASM thickening, another key feature of chronic asthma, the amount of smooth muscle thickening was determined by measuring alpha smooth muscle actin (α-SMA) positive stained area around the airways (Fig. 5c). ASM thickening was modestly increased in mice chronically exposed to HDM, and this increase was blocked with Erlotinib treatments (Fig. 5d). These data suggest that EGFR signaling contributes to ASM remodeling in ongoing allergic airway disease.
To determine the effect of inhibiting EGFR on goblet cells, immunostaining was performed on lung sections for the goblet cell marker chloride channel calcium activated family member 3 (CLCA3). Occasional CLCA3 positive cells were seen in the large conducting airways of saline control mice (Fig. 5e). Mice exposed chronically to HDM had increased CLCA3 staining in the large conducting airways. CLCA3 staining was decreased in HDM exposed mice that received Erlotinib. To quantitate these changes, western blot analysis was performed on lung homogenates for CLCA3 and densitometry quantifications were assessed (Fig. 5f). CLCA3 protein levels in the lungs of mice chronically exposed to HDM were increased compared to saline controls. This increase was attenuated with Erlotinib treatment. These data indicate a role for EGFR signaling in the regulation of goblet cell metaplasia in ongoing allergic airway disease.
Discussion
In this report, multiple EGFR ligands induced GM-CSF production by human AEC in vitro. HDM or IL-17A stimulated AEC increase GM-CSF production and this required active EGFR signaling as well as protease/TACE activity and p38 MAPK signaling. HDM or IL-17A also induced primary human AEC to increase EGFR-dependent GM-CSF production. In vivo EGFR inhibition reduced GM-CSF and TNFα levels as well as AHR, cellular inflammation, ASM thickening, and goblet cell metaplasia in the airways of mice with established allergic airway disease without altering HDM-specific IgG and IgE, Th1 (IFNγ), Th2 (IL-4, IL-5, IL-13, eotaxin), and Th17 (IL-17A) cytokines. Results demonstrate that EGFR signaling regulates GMCSF production both in vitro and in vivo and pharmacologic inhibition of EGFR signaling reduces the severity of established allergic airway disease in a clinically relevant mouse model. These data identify a novel allergen-activated signaling mechanism in AEC, which suggest the EGFR→GM-CSF axis might represent molecular targets for future therapeutic development.
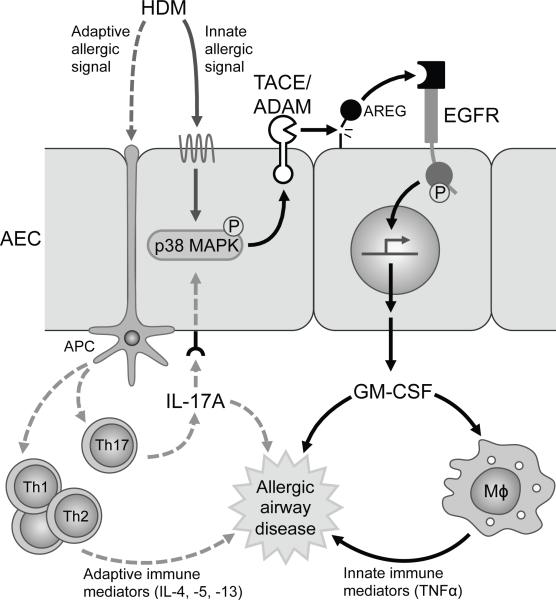
Our results identify a novel mechanism of EGFR signaling in AEC and suggest this pathway may be an important driver of extrinsic asthma (Fig. 6). In this pathway, HDM and IL-17A utilize EGFR signaling to stimulate AEC to produce and locally secrete GM-CSF, an autoimmune/inflammatory mediator with potent regulatory effects on tissue-resident macrophages as well as neutrophils and eosinophils. This mechanism (HDM/IL-17A → MAPKp38→protease/TACE→amphiregulin→EGFR→GM-CSF) is supported by and links findings from a number of reports on the individual components of this pathway (27-31). However, this study is the first to mechanistically place IL-17A, amphiregulin, EGFR, and GMCSF in a pathway in AEC, show that both HDM and IL-17A stimulate AEC directly by activating this EGFR→GM-CSF signaling axis, and suggest that HDM and IL-17A contribute to asthma pathogenesis via amphiregulin and EGFR mediated GM-CSF production from AEC.
Figure 6. Schematic of proposed pathway of innate allergic signaling mechanism in airway epithelial cells.
HDM and IL-17A activate p38 MAPK signaling in airway epithelial cells (AEC) to induce TNFα converting enzyme (TACE) or another member of the a disintegrin and metalloproteinases (ADAM) family to cleave EGFR ligands such as amphiregulin (AREG), on adjacent cells or on its own cell surface, that activate EGFR signaling, which drives the production of GM-CSF. GM-CSF contributes to allergic airway disease via the activation and priming of macrophages (Mϕ) to produce innate immune mediators and possibly through direct effects on the airway. Antigen presenting cells (APC) can process and present HDM antigens to activated T cells (Th1, Th2, Th17) that then release adaptive immune mediators and cytokines that contribute to the pathogenesis of allergic airway disease.
The observation that EGFR inhibition reduced allergen-induced airway disease without affecting numerous mediators of adaptive immunity in vivo and also blocked HDM and IL-17A dependent activation of the EGFR→GM-CSF axis in cultured and primary human AEC in vitro has important biological implications. First, this mechanism is an ‘innate allergic’ signaling pathway that acts locally to determine airway host responses and asthma severity, which may function independent of and in parallel to or down-stream from known adaptive immune pathways. Second, the observation that IL-17A levels were unaffected by Erlotinib in vivo and caused an exposure-dependent activation of the EGFR → GM-CSF axis in isolated AECs suggests that IL-17A may serve to integrate innate and adaptive host responses locally. Third, this pathway may drive airway disease by priming the host defense functions of tissue-resident macrophages or other myeloid cells and by promoting survival. Prior studies have shown that GM-CSF stimulates the proliferation of macrophages and hyperplasia of type II AEC (35). Fourth, it is possible that GM-CSF may modify disease severity by regulating the functional capacity of local, tissue-resident macrophages. This is supported by the following observations: 1) both TNFα and GM-CSF were increased in HDM-exposed mice; 2) EGFR inhibition in HDM-exposed mice reduced the levels of both TNFα and GM-CSF (albeit, not to pre-exposure baseline levels); 3) cultured HDM-exposed AEC secreted GM-CSF but not TNFα, indicating the increased TNFα in HDM-exposed mice was most likely from cells other than AEC; 4) GM-CSF, by regulating alveolar macrophage maturation, determines their capacity for TNFα secretion as well as other cytokines (36); 5) TNFα expression is increased in airway tissues in allergic asthma (37, 38), and we have observed increased TNFα immunostaining in macrophages from lungs of mice chronically exposed to HDM (unpublished results). These observations may shed light on the interpretation of results of clinical trials evaluating TNFα inhibition as therapy of asthma, which have reported mixed efficacy results (39-43), as GM-CSF regulated pathways may vary among patients comprising asthma subgroups on the basis of difference in environmental or genetic modifiers (44).
Inhibiting EGFR in our model of established allergic airway disease reduced ASM thickening, AHR, and eosinophil number. Our in vitro studies show that EGFR regulates GM-CSF production by AEC. Others have reported that GM-CSF can recruit α-smooth muscle actin positive myofibroblasts (45), which could potentially contribute to ASM thickening and AHR. However, whether GM-CSF directly stimulates ASM cell proliferation/hyperplasia and AHR is unclear. Since GM-CSF is a known survival signal for granulocytes including eosinophils (46), reducing GM-CSF by inhibiting EGFR could account for the decrease in eosinophils in our model. The observation that Erlotinib markedly reduced the severity of established allergic airway disease in mice identifies EGFR as a potential molecular target for development of asthma therapy. EGFR may play an important role in other lung diseases and has been identified as a target for therapeutic development in COPD and pulmonary fibrosis (47). Erlotinib is a small molecule inhibitor currently approved as a non-DNA modifying cancer therapy with clinical trials documenting it is safe and well-tolerated with only mild/moderate adverse effects (48). EGFR inhibition did not completely reduce features of allergic asthma in our model of established disease. This is not surprising given that allergens activate a complex network of inter-dependent innate and adaptive immune host responses with local and systemic components. However, the observation that multiple distinct asthma triggers activate EGFR signaling not only suggests EGFR may function as an ‘environmental sensor’ but that it may function as a common pathogenic driver in otherwise mechanistically and phenotypically distinct forms of asthma patients (8-11).
The observation that HDM and IL-17A induced GM-CSF production is downstream of amphiregulin and EGFR in AEC and dependent on p38 MAPK as well as protease/TACE activity identifies the EGFR→GM-CSF axis as a pathway with multiple molecular targets for potential future therapeutic development. This conclusion is supported by a human clinical trial (KaloBios KB003) that is underway to evaluate anti-GM-CSF therapy in severe asthma. Further, GM-CSF signaling inhibition using an anti-GM-CSF receptor α monoclonal antibody was therapeutically effective and safe in patents with rheumatoid arthritis, despite the potential for anti-GM-CSF therapy to impede lung surfactant clearance (49). While GM-CSF represents a potentially novel therapeutic target in severe refractory asthma, balancing the potential deleterious side effects while improving respiratory function is potentially a challenge.
Our results indicate that amphiregulin is the main EGFR ligand responsible for the activation of EGFR signaling and induction of GM-CSF production by AEC after stimulation with HDM and IL-17A. Amphiregulin is a mitogenic growth factor that is known to play a role in epithelial repair after acute lung injury (50). Amphiregulin produced by innate lymphoid cells and mast cells, also contributes to epithelial repair after viral infection and regulates mucus production (51, 52). Amphiregulin has also been linked to asthma, with higher levels of amphiregulin found in sputum samples from asthmatic children (53, 54). These data, together with our in vitro data, suggest that amphiregulin, which can activate the EGFR→GM-CSF axis, is another potential target for therapeutic development.
In conclusion our data mechanistically link HDM, IL-17A, amphiregulin, EGFR, and GM-CSF in a pathway in AEC and demonstrate the importance of EGFR signaling in regulating GM-CSF production by AEC. These data suggest the EGFR→GMCSF axis in AEC may represent an important innate signaling pathway in allergic asthma and a potential mediator of established disease in patients.
Supplementary Material
Acknowledgment
The authors thank Patricia Pastura and Anusha Sridharan for their excellent technical assistance. This work was supported by funding from NIH grants HL097135 (TDLC) and HL085453 (BCT).
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129:48–59. doi: 10.1016/j.jaci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–19. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 5.Heijink IH, Nawijn MC, Hackett T-L. Airway epithelial barrier function regulates the pathogenesis of allergic asthma. Clin Exp Allergy. 2014;44:620–30. doi: 10.1111/cea.12296. [DOI] [PubMed] [Google Scholar]
- 6.Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, et al. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383(6597):247–50. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Saito J, Ishida T, Munakata M. Polymorphism of egfr Intron1 is associated with susceptibility and severity of asthma. J Asthma. 2006;43(9):711–5. doi: 10.1080/02770900600925247. [DOI] [PubMed] [Google Scholar]
- 8.Monick MM, Cameron K, Staber J, Powers LS, Yarovinsky TO, Koland JG, et al. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem. 2005;280:2147–58. doi: 10.1074/jbc.M408745200. [DOI] [PubMed] [Google Scholar]
- 9.Koff JL, Shao MXG, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. American Journal of Physiology Lung Cellular and Molecular Physiology. 2008;294:L1068–75. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 10.Eierhoff T, Hrincius ER, Rescher U, Ludwig S, Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao D, Tal TL, Graves LM, Gilmour I, Linak W, Reed W, et al. Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. American Journal of Physiology Lung Cellular and Molecular Physiology. 2007;292:L422. doi: 10.1152/ajplung.00204.2006. [DOI] [PubMed] [Google Scholar]
- 12.Amishima M, Munakata M, Nasuhara Y, Sato a, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–12. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 13.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB. 2000;14:1362–74. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 14.Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate ST, et al. Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects. J Allergy Clin Immunol. 2002;109(1):75–81. doi: 10.1067/mai.2002.120274. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka Y, Hayashi K, Komurasaki T, Morimoto S, Ogihara T, Sobue K. EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem Biophys Res Commun. 2001;281(2):373–7. doi: 10.1006/bbrc.2001.4385. [DOI] [PubMed] [Google Scholar]
- 16.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. American Journal of Physiology Lung Cellular and Molecular Physiology. 2011:414–21. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadel JA. Role of epidermal growth factor receptor activation in regulating mucin synthesis. Respir Res. 2001;2:85–9. doi: 10.1186/rr43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeyama K, Fahy JV, Nadel Ja. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001;163:511–6. doi: 10.1164/ajrccm.163.2.2001038. [DOI] [PubMed] [Google Scholar]
- 19.Hur GY, Lee SY, Lee SH, Kim SJ, Lee KJ, Jung JY, et al. Potential use of an anticancer drug gefinitib, an EGFR inhibitor, on allergic airway inflammation. Exp Mol Med. 2007;39:367–75. doi: 10.1038/emm.2007.41. [DOI] [PubMed] [Google Scholar]
- 20.Tamaoka M, Hassan M, McGovern T, Ramos-Barbón D, Jo T, Yoshizawa Y, et al. The epidermal growth factor receptor mediates allergic airway remodelling in the rat. Eur Respir J. 2008;32:1213–23. doi: 10.1183/09031936.00166907. [DOI] [PubMed] [Google Scholar]
- 21.Vargaftig BB, Singer M. Leukotrienes mediate part of Ova-induced lung effects in mice via EGFR. American Journal of Physiology Lung Cellular and Molecular Physiology. 2003;285:L808–18. doi: 10.1152/ajplung.00377.2002. [DOI] [PubMed] [Google Scholar]
- 22.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Saha S, Doe C, Mistry V, Siddiqui S, Parker D, Sleeman M, et al. Granulocyte-macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax. 2009;64:671–6. doi: 10.1136/thx.2008.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita N, Tashimo H, Ishida H, Kaneko F, Nakano J, Kato H, et al. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF). Cell Immunol. 2002;219:92–7. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 25.Cates EC, Fattouh R, Wattie J, Mark D, Goncharova S, Coyle AJ, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–92. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–31. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 27.Mascia F, Cataisson C, Lee T-C, Threadgill D, Mariani V, Amerio P, et al. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. J Invest Dermatol. 2010;130:682–93. doi: 10.1038/jid.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King C, Brennan S, Thompson PJ, Stewart Ga. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–51. [PubMed] [Google Scholar]
- 29.Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, Kaneko T, et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-a. Eur Respir J. 2003;21:387–93. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 30.Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS. IL-17A Induces Eotaxin-1/CC Chemokine Ligand 11 Expression in Human Airway Smooth Muscle Cells: Role of MAPK (Erk1/2, JNK, and p38) Pathways. J Immunol. 2006;177:4064–71. doi: 10.4049/jimmunol.177.6.4064. [DOI] [PubMed] [Google Scholar]
- 31.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, et al. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–23. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 32.Sahin U, Weskamp G, Kelly K, Zhou H-M, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–79. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-Differentiated Human Airway Epithelial Cell Cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 35.Huffman Reed JA, Rice WR, Zsengelle ZK, Wert SE, Dranoff G, Whitsett JA. GM-CSF enhances lung growth and causes alveolar type II epithelial cell hyperplasia in transgenic mice. Am J Physiol. 1997;273:L715–25. doi: 10.1152/ajplung.1997.273.4.L715. [DOI] [PubMed] [Google Scholar]
- 36.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett Ja, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–67. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 37.Ying S, Robinson DS, Varney V, Meng Q, Tsicopoulos A, Moqbel R, et al. TNF alpha mRNA expression in allergic inflammation. Clin Exp Allergy. 1991;21:745–50. doi: 10.1111/j.1365-2222.1991.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 38.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, et al. Interleukin-4, -5, and -6 and Tumor Necrosis Factor-alpha in Normal and Asthmatic Airways : Evidence for the Human Mast Cell as a Source of These Cytokines. Am J Respir Cell Mol Biol. 1994;10:471–80. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 39.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. doi: 10.1016/j.jaci.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 41.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174:753–62. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén S-E, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179:549–58. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- 43.Morjaria JB, Chauhan aJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63:584–91. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
- 44.Ober C, Thompson EE. Rethinking genetic models of asthma: the role of environmental modifiers. Curr Opin Immunol. 2005;17:670–8. doi: 10.1016/j.coi.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Vyalov S, Desmoulière A, Gabbiani G. GM-CSF-induced granulation tissue formation: relationships between macrophage and myofibroblast accumulation. Virchows Archiv B Cell Pathology. 1993;63:231–9. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- 46.Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, et al. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. Eur Respir J. 1998;12(4):872–8. doi: 10.1183/09031936.98.12040872. [DOI] [PubMed] [Google Scholar]
- 47.Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. Eur Respir J. 2014:1–10. doi: 10.1183/09031936.00146413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbst R. Erlotinib (Tarceva): An update on the clinical trial program. Semin Oncol. 2003;30:34–46. [PubMed] [Google Scholar]
- 49.Nair JR, Edwards SW, Moots RJ. Mavrilimumab, a human monoclonal GM-CSF receptor-α antibody for the management of rheumatoid arthritis: a novel approach to therapy. Expert Opin Biol Ther. 2012;12:1661–8. doi: 10.1517/14712598.2012.732062. [DOI] [PubMed] [Google Scholar]
- 50.Fukumoto J, Harada C, Kawaguchi T, Suetsugu S, Maeyama T, Inoshima I, et al. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(2):L131–8. doi: 10.1152/ajplung.90576.2008. [DOI] [PubMed] [Google Scholar]
- 51.Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. FcepsilonRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol. 2005;115(2):272–9. doi: 10.1016/j.jaci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering Ta, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enomoto Y, Orihara K, Takamasu T, Matsuda A, Gon Y, Saito H, et al. Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol. 2009;124:913–20.e7. doi: 10.1016/j.jaci.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 54.Kyung WK, Hye MJ, Yeo HP, Bong SC, Myung HS, Kim KE. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest. 2009;136:805–10. doi: 10.1378/chest.08-2972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.