Abstract
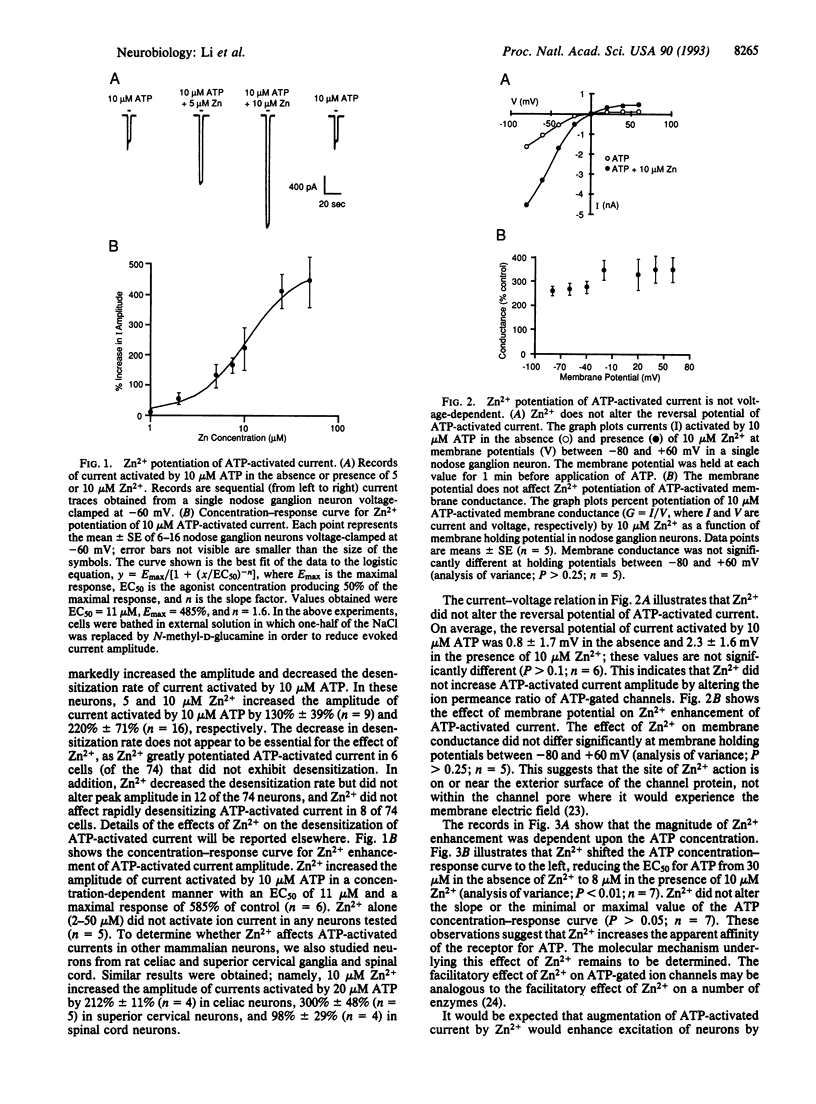
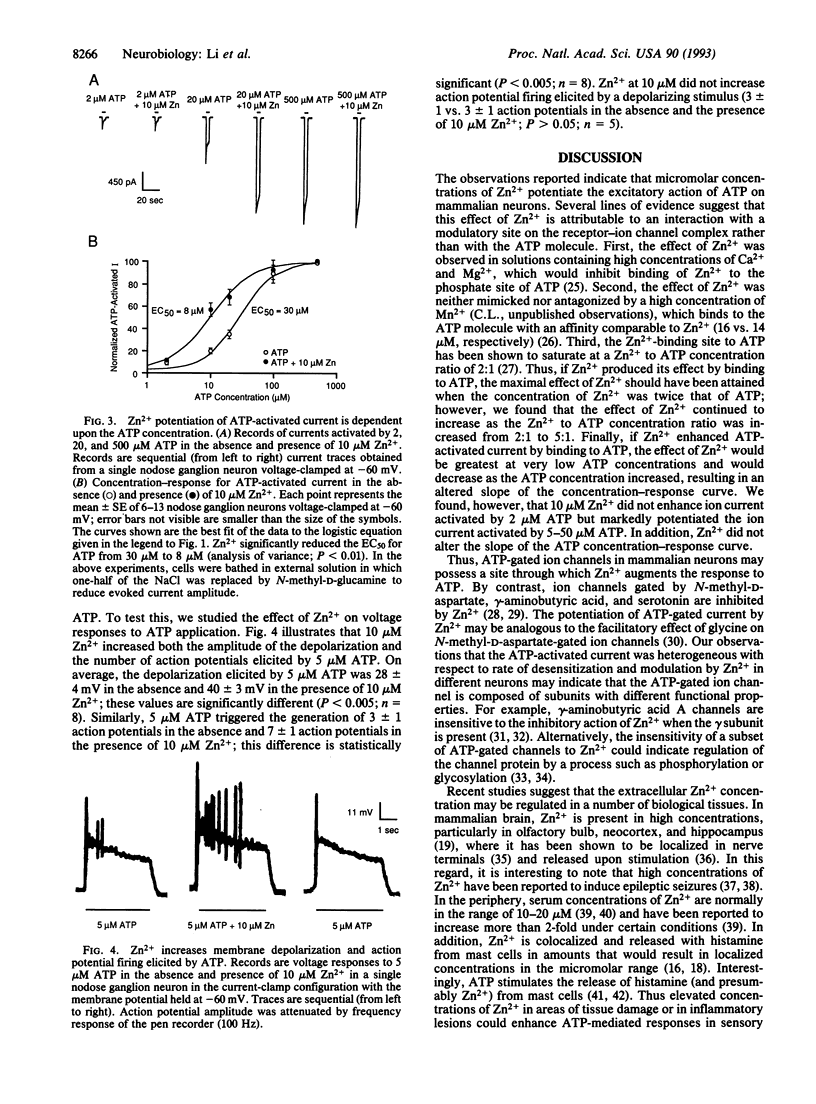
Despite the increasing recognition that ATP is an important extracellular excitatory mediator in the nervous system, the regulation of ATP receptors is poorly understood. Because the extracellular Zn2+ concentration is regulated in a variety of biological tissues, we studied modulation of the ATP-gated cation channel by Zn2+ in mammalian neurons using the whole-cell patch-clamp technique. In approximately 73% of cells tested, the amplitude of ATP-activated membrane ion current increased up to 5-fold in the presence of micromolar concentrations of Zn2+. The characteristics of this action suggest that Zn2+ increases the apparent affinity of the receptor for ATP. In addition, Zn2+ increased membrane depolarization and action potential firing elicited by ATP. These observations suggest that Zn2+ may play a physiological role in regulating the excitatory action of ATP on mammalian neurons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angyal A. M., Archer G. T. The zinc content of rat mast cells. Aust J Exp Biol Med Sci. 1968 Feb;46(1):119–121. doi: 10.1038/icb.1968.10. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Born G. V., Kratzer M. A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol. 1984 Sep;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Kasakov L., Wong H. K. Direct evidence for ATP release from non-adrenergic, non-cholinergic ("purinergic") nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978 May 15;49(2):145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Chung S. H., Gabrielsson B., Norris D. K. Transition metal ions in epilepsy: an overview. Adv Exp Med Biol. 1986;203:545–555. doi: 10.1007/978-1-4684-7971-3_42. [DOI] [PubMed] [Google Scholar]
- Dahlquist R., Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Pierre T. S., Minnich J. L., Barbeau A. Determination of Na + , K + , Mg 2+ , Cu 2+ , Zn 2+ , and Mn 2+ in rat brain regions. Can J Biochem. 1973 Jan;51(1):87–92. doi: 10.1139/o73-010. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Flynn A., Pories W. J., Strain W. H., Hill O. A., Jr Mineral element correlation with adenohypophyseal-adrenal cortex function and stress. Science. 1971 Sep 10;173(4001):1035–1036. doi: 10.1126/science.173.4001.1035. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Glassman T. A., Cooper C., Kuntz G. P., Swift T. J. Nuclear magnetic resonance evidence for a Zn2-ATP complex. FEBS Lett. 1974 Feb 1;39(1):73–74. doi: 10.1016/0014-5793(74)80019-0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G. T. Heavy metals in rat mast cell granules. Lab Invest. 1967 Dec;17(6):588–598. [PubMed] [Google Scholar]
- HOLTON F. A., HOLTON P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol. 1954 Oct 28;126(1):124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Jaffar Z. H., Pearce F. L. Histamine secretion from mast cells stimulated with ATP. Agents Actions. 1990 Apr;30(1-2):64–66. doi: 10.1007/BF01968999. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- KERP L. [Importance of zinc for histamine storage in mast cells]. Int Arch Allergy Appl Immunol. 1963;22:112–123. [PubMed] [Google Scholar]
- Keele C. A. Chemical causes of pain and itch. Annu Rev Med. 1970;21:67–74. doi: 10.1146/annurev.me.21.020170.000435. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y., Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992 Sep 17;359(6392):241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Bevan S., Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull. 1991 Jul;47(3):534–548. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- Rimai L., Heyde M. E. An investigation by Raman spectroscopy of the base-proton dissociation of ATP in aqueous solution and the interactions of ATP with Zn++ and Mn++. Biochem Biophys Res Commun. 1970 Oct 23;41(2):313–320. doi: 10.1016/0006-291x(70)90505-x. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Moss S. J., Xie X., Huganir R. L. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991 Aug;103(4):1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Moss S. J., Blackstone C. D., Huganir R. L. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992 May;6(8):2514–2523. [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C., Eusebi F., Miledi R. Glycosylation is required for maintenance of functional voltage-activated channels in growing neocortical neurons of the rat. Proc R Soc Lond B Biol Sci. 1990 Mar 22;239(1295):119–127. doi: 10.1098/rspb.1990.0011. [DOI] [PubMed] [Google Scholar]


