Abstract
Individuals infected with HIV are living longer due to effective treatment with combination antiretroviral therapy (cART). Despite these advances, HIV-associated neurocognitive disorders (HAND) remain prevalent. In this study, we analyzed resting state functional connectivity (rs-fc) data from HIV-infected and matched HIV-uninfected adults aged 60 years and older to determine associations between HIV status, neuropsychological performance, and clinical variables. HIV-infected participants with detectable plasma HIV RNA exhibited decreased rs-fc within the salience (SAL) network compared to HIV-infected participants with suppressed plasma HIV RNA. We did not identify differences in rs-fc within HIV-infected individuals by HAND status. Our analysis identifies focal deficits in the SAL network that may be mitigated with suppression of plasma virus. However, these findings suggest that rs-fc may not be sensitive as a marker of HAND among individuals with suppressed plasma viral loads.
Keywords: HIV, cognition, functional MRI, network connectivity
INTRODUCTION
Older individuals make up an increasing portion of the HIV-infected population. Currently, about half of all HIV-infected individuals in the United States are 50 years of age and older. This population represents a group of individuals with unique aspects of disease course and complications (Brooks et al. 2012). The expansion of this older demographic is, in large part, due to combination antiretroviral therapy (cART) that has increased life expectancy. There are few data on individuals over age 60 years; yet, elucidating the effects of HIV on brain function in this unique population is particularly important due to the increased risk of neurodegenerative disorders at older ages. Moreover, evidence suggests that HIV and its treatments may exacerbate normal age-related health concerns, and this oldest group has both extended duration of HIV and longer exposure to antiretroviral treatments (High et al. 2012). It remains unclear whether HIV has an additive or even accelerative effect on the aging process (Pathai et al. 2014).
HIV infection can be accompanied by cognitive deterioration, known as HIV-associated neurocognitive disorders (HAND) in 30 - 50% of individuals (Heaton et al. 2011). The frequency of cognitive dysfunction and its impact on everyday function increases with age (Vance et al. 2013). The virus enters the brain soon after infection, causing dysfunction as measured by CSF cytokines and magnetic resonance spectroscopy (MRS) (Valcour et al. 2012). While cART has decreased the incidence of the severe form of HAND (e.g. HIV-associated dementia (HAD)), less severe manifestations of cognitive dysfunction persist (Heaton et al. 2011).
Both aging and HIV infection have a negative impact on overall neuropsychological (NP) and everyday functional performance (Glisky 2007; Woods et al. 2009). In particular, HIV-uninfected older compared to younger individuals exhibit decreased performance on measures of memory, executive function, processing speed, and motor function (Hedden et al. 2012). Similarly, older HIV-infected individuals tend to exhibit greater deficits in multiple cognitive domains, particularly executive functioning (Sacktor et al. 2007). Older HIV-infected individuals also have greater declines in daily functioning measures (e.g. instrumental and basic activities of daily living) (Morgan et al. 2012).
Current methods that use NP testing to establish HAND may miss subtle changes in brain function seen in older HIV-infected individuals. Observed changes in NP performance may reflect a combination of factors including HIV effects, side effects of cART, or low-level inflammation within the brain due to persistent viral reservoirs. Neuroimaging using techniques such as blood-oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) has become a useful tool in the assessment of the neurophysiological effects of HIV and aging (Ortega et al. 2015; Thomas et al. 2015). Spontaneous fluctuations in the BOLD signal unmask intrinsic brain network maps. Resting state functional connectivity (rs-fc) can be obtained without the participant performing a particular task and without use of contrast agents (Zhang and Raichle 2010).
Recent imaging research identified changes in rs-fc due to age and various neurodegenerative diseases. Age is associated with a reduction of functional correlations within certain resting state networks (RSNs) (e.g. default mode network (DMN)) (Andrews-Hanna et al. 2007; Yao et al. 2013; Geerligs et al. 2014). Selective brain vulnerability correlates with age-associated neurodegenerative disorders (Seeley et al. 2009). A resting state signature for HIV may exist. In past work, HIV infection was associated with abnormalities in distinct network cortical hubs while aging was associated with widespread decreases in rs-fc (Thomas et al. 2015).
The assessment of HIV virologic and immunological status (e.g. plasma CD4 count, plasma HIV RNA), as they impact clinical outcomes is well established. However, the relationship between such measures and brain function among individuals on cART remains less clear (Kaul 2009). As a non-invasive means to evaluate brain function, rs-fc could assist in more tailored medical management, especially among older HIV-infected patients where co-morbidities and polypharmacy are frequent (Nightingale et al. 2014). In the present study, we combined NP testing and rs-fc to assess brain function within an older cohort of HIV-infected and HIV-uninfected adults (≥60 years old) to investigate relationships between measures of brain connectivity (rs-fc), cognition (NP testing), and clinical measures (including plasma HIV RNA).
METHODS
Selection of participants
HIV-infected participants (n=52) were enrolled in the UCSF HIV Over 60 Cohort, a study conducted at the University of California San Francisco (UCSF) to understand cognition in elder (≥60 years) HIV-infected individuals. All were recruited by community advertisements and physician referrals with no selection criteria related to cognitive symptoms. For the present study, we selected images from the first scanning session from all subjects who had completed neuroimaging using a Siemens 3T MRI. One participant was excluded due to preexisting white matter changes thought to be associated with chronic inflammatory demyelinating polyneuropathy (CIDP). Most HIV-infected participants (94%) had been on stable cART for at least 12 months prior to neuroimaging.
Healthy HIV-uninfected volunteers (n=29) had enrolled in studies conducted by the UCSF Memory and Aging Center. Individuals from these studies of normal controls were included in the present study if they were between 60 and 70 years old, free of other neurological disorders, and had normal neuropsychological testing performance with no symptoms, as determined by a consensus conference including a behavioral neurologist. We initially selected only male control participants between 60 and 70 years of age and then supplemented this group with 1:1 random sampling of women to match the HIV-infected group by gender. Healthy HIV-uninfected participants underwent the same neuroimaging protocols on the same MRI scanner as HIV-infected participants. All participants signed informed consent forms approved by the UCSF Committee on Human Research.
Cognitive and clinical characterization
All participants completed a 90-minute cognitive battery, as previously described (Chiao et al. 2013). The NP battery assessed the following domains: Memory (delayed and immediate recalls of the California Verbal Learning Test II and Story Recall tasks, delayed recall of the Benson Figure Copy task); Executive (Modified Trails test, Trails B test, Stroop interference task, phonemic fluency (D words), and digits backwards); Attention (California Verbal Learning Test, Digits forward); Visuospatial (Visual Object and Space Perception Battery, Rey Complex figure copy trial); and Psychomotor function (Trails A, Digit Symbol Modalities Test, and Stroop color naming). Individual tests were standardized to an age- and education-adjusted z-score using published normative data. A composite domain score was calculated as the arithmetic mean of z-scores from individual tests constituting each cognitive domain. A global neuropsychological score (NPZ-global) was calculated by averaging individual z-scores across all tests. All participants underwent a comprehensive neurological examination, first symptoms questionnaire and, when possible, proxy informant interview to assess functional capabilities (47 of 52 HIV-infected participants).
HIV-infected participants additionally completed the Grooved Pegboard and Finger Tapping tests for dominant and non-dominant hands to conform with 2007 “Frascati” HAND diagnostic criteria (Antinori et al. 2007). HIV-infected participants with HAND were classified as having asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), or HIV-associated dementia (HAD) in a consensus conference that included at least one neuropsychologist and one behaviorally trained neurologist using clinical acumen and guided by Frascati criteria.
Clinical variables
We used a structured physician interview that included questions about self-reported duration of infection, nadir CD4 cell count, antiretroviral history, opportunistic infections, and other important medical history variables. We measured plasma HIV RNA and CD4 cell count if not available from clinical records within 3 months of the study visit. HIV-infected subjects with no recent laboratory data available were excluded from analyses examining effects of viral load (2 of 52 HIV-infected individuals).
Image acquisition, pre-processing and quality assurance
Participants were scanned using a 3T Siemens Tim Trio with a 12-channel head coil. Structural magnetic resonance imaging scans were obtained with T1-weighted Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequences [matrix: 1.0 × 1.0 × 1.0 mm3, repetition time (TR) = 2300 ms, echo time (TE) = 2.98 ms, flip angle (FA) = 9°, inversion time = 900 ms]. rs-fc scans were collected using a gradient spin-echo sequence (voxel size = 2.5 × 2.5 × 3.0 mm3, TR = 2000 ms, TE = 27 ms, FA = 80°, field of view (FoV) = 230 mm) that was sensitive to blood-oxygen-level dependent (BOLD) contrast. A total of 36 3.0 mm-thick slices were acquired parallel to the anterior commissure/posterior commissure plane. A single rs-fc scan (240 volumes) was acquired for each participant.
The preprocessing of rs-fc data followed conventional methods, as previously described (Shulman et al. 2010; Brier et al. 2014). Preprocessing included correction for slice-dependent intensity differences (Hacker et al. 2013), rigid body correction for head movement within and across runs, and atlas transformation. A frame censoring technique was performed to minimize motion-related BOLD signal changes (Smyser et al. 2010; Power et al. 2012). Briefly, for each frame, we calculated a root mean square (RMS) of signal intensity change over the previous frame. Frames with large RMS signal intensity changes (> 0.7%) were removed. In order to facilitate a temporal filtering procedure, removed frames were replaced voxel by voxel with linearly interpolated values (Power et al. 2014). The replaced frames were discarded after temporal filtering.
Additional preprocessing steps were carried out in preparation for the correlation analysis. First, several sources of spurious variance were removed from the data by linear regression: (1) six parameters generated from rigid body correction of head motion; (2) the whole brain signal averaged from a mask region in atlas space; and (3) signal from ventricles and deep cerebral white matter. Second, the residual BOLD time series underwent a temporal low-pass filtering (< 0.1 Hz) and spatial smoothing with a 6-mm full width at half maximum Gaussian blur. Controversy remains concerning the inclusion of global signal regression (GSR) as it may differentially impact estimates of rs-fc across groups (Saad et al. 2013). To address this potential concern, we compared beta values from GSR for all primary comparisons: HIV-uninfected and HIV-infected individuals, among HIV-uninfected individuals vs. HIV-infected individuals with normal cognition vs. HIV-infected individuals with ANI or MND, and between individuals with detectable vs. undetectable plasma HIV RNA within the HIV-infected group. We found no group-wise differences in the beta values from GSR for any of these comparisons (all p ≥ 0.05).
We applied the previously described quality assurance criteria that excluded an individual's data set with RMS from BOLD signal variance (across all frames) > 2.5% or head motion exceeding 1.25 mm (Wang et al. 2012). In addition, any individual with more than 40% of frames removed was excluded from subsequent analyses (Brier et al. 2014). However, we found that all individuals met quality assurance measures and none were excluded. The number of removed frames was not different by any of the primary comparison groups described above (all p ≥ 0.09).
Participant-level analysis of rs-fc within RSNs
For each participant, a cross correlation matrix was constructed from the time-series derived from 36 regions of interest (ROIs) that included 5 RSNs (DMN, dorsal attention (DAN), control (CON), salience (SAL), and sensorimotor (SMN) networks). The cross-correlation matrices were averaged separately across HIV-infected and HIV-uninfected individuals. For each RSN, a composite score was calculated by averaging Fisher's z-transformed correlation coefficients across all ROI pairs included within a particular RSN to reduce the dimensionality of the statistical analysis (Brier et al. 2012).
Statistical analysis
Demographic variables and NP test scores were compared between HIV-infected and HIV-uninfected individuals using t tests. Treating composite scores of each RSN as a dependent variable, the effects of HIV (HIV-infected vs. HIV-uninfected) and HAND (HIV-uninfected vs. HIV-infected with no cognitive impairment vs. HIV-infected with ANI or MND) were analyzed separately using multivariate analyses of variance. Similarly, the effect of plasma HIV RNA detectability on RSN composite scores was examined within HIV-infected participants using multivariate analyses of variance. We used multivariate linear regression to test the effects of current and nadir CD4 cell counts on RSN composite scores. The relationships between RSN composite scores and NP performance were investigated separately within HIV-infected and HIV-uninfected individuals using Pearson correlations with a false discovery rate of p < 0.05 after correction for multiple comparisons.
RESULTS
Participant demographics
The final sample included 52 HIV-infected and 29 HIV-uninfected participants (Table 1). By design, the HIV-infected and HIV-uninfected groups did not differ by gender, but the HIV-uninfected cohort was slightly older and reported a greater number of years of education. All HIV-infected participants that met HAND criteria (n = 27) were classified as having ANI or MND, with no cases of HAD. Significant differences were observed between HIV-infected and HIV-uninfected groups for each of the NPZ-domain scores and for the NPZ-global score.
Table 1.
Demographic, medical, laboratory and neuropsychological testing comparisons between HIV-infected and HIV-uninfected individuals
| HIV-uninfected | HIV-infected | |
|---|---|---|
| N | 29 | 52 |
| Age (years) (SD)* | 65 (2) | 64 (3) |
| Sex (% Male) | 90 | 94 |
| Education (years) (SD)* | 18 (2) | 16 (2) |
| Duration of HIV infection (years) (SD) | NA | 20 (7) |
| Log plasma viral load (IQR) | NA | 2.2 (1.88 - 2.16) |
| Detectable viral load | 15 (28.8%) | |
| Plasma CD4 cell count (cells/mm3) (IQR) | NA | 504 (356 - 650) |
| Nadir CD4 cell count (cells/ mm3) (IQR) | NA | 194 (77 - 297) |
| Diagnosis (n, %) | ||
| Normal Cognition | 29 (100%) | 25 (48%) |
| Asymptomatic Neurocognitive Impairment | 13 (25%) | |
| Mild Neurocognitive Disorder | 14 (27%) | |
| HIV-Associated Dementia | 0 | |
| Neuropsychological Z (NPZ)-scores (SD) | ||
| Memory* | 0.6 (0.7) | −0.4 (0.8) |
| Executive function* | 0.3 (0.6) | −0.5 (0.8) |
| Psycho-motor* | 0.3 (0.8) | −0.3 (0.8) |
| Visuospatial* | 0.1 (0.6) | −0.5 (0.8) |
| Attention* | 0.6 (1.1) | −0.5 (0.9) |
| Global* | 0.3 (0.4) | −0.5 (0.6) |
p < 0.05; NA = not available; IQR = interquartile range
Effects of HIV on RSNs
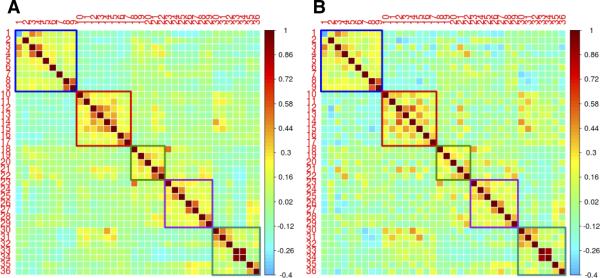
We examined five RSN composite scores and noted no significant differences between HIV-infected and HIV-uninfected groups (Figure 1). The within-network (intra-network) correlations are shown in blocks along the diagonal, while between-network (inter-network) correlations are shown in off-diagonal blocks. The computed matrices revealed only subtle differences in inter- and intra-network correlations between the two groups. Both matrices showed positive correlations within networks and negative correlations between networks.
Fig. 1. Region of interest (ROI) pair correlation matrices for HIV-infected (A) and HIV-uninfected (B) individuals.
ROIs are grouped by resting state network (RSN). Intra-network correlations are shown on diagonal blocks while inter-network correlations are shown in off-diagonal blocks. Colors indicate network membership: blue = DMN; red = DAN; green = CON; purple = SAL; teal = SMN. Units are z-transformed correlation coefficients.
DMN= default mode network; DAN= dorsal attention network; CON= control network; SAL= salience network; SMN= sensory-motor network
Correlations between HAND and RSN composite scores
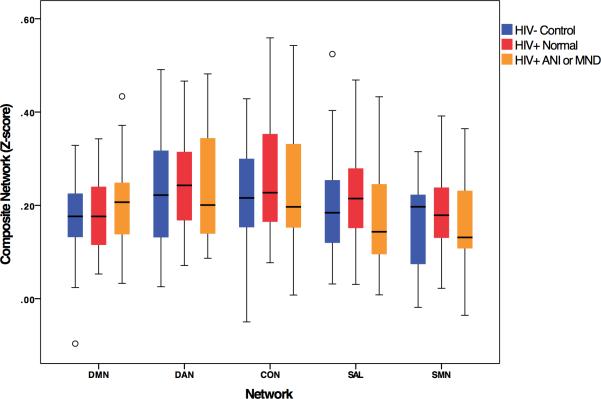
The ANI and MND groups were combined into a HAND group and compared to HIV-infected participants without HAND. No relationship was observed by HAND status on RSN composite scores (Figure 2). We also studied the relationship between NPZ-domain scores and RSN composite scores. Among HIV-uninfected controls, no correlations between NP test scores and rs-fc were found for any RSN. For the HIV-infected participants, a correlation between psychomotor function and SAL composite score (r = 0.293, uncorrected p = 0.035) was observed. However, this finding was not significant after correction for multiple comparisons.
Fig. 2. Association between cognitive diagnosis and resting state networks (RSNs).
A multivariate analysis of variance was conducted on RSN scores with factor of neurocognitive impairment status: HIV-uninfected control, HIV-infected with normal cognition, and HIV-infected with either Asymptomatic Neurocognitive Impairment (ANI) or Mild Neurocognitive Disorder (MND). No effect of HAND was found across networks.
Relationship between RSN composite scores and clinical variables
Within the HIV-infected group, 50/52 cases had plasma HIV RNA measured, among which 35 were undetectable (≤75 copies/mL) and 15 were detectable and had a mean (SD) log10 HIV RNA of 2.04 (0.83). We found a reduction in SAL composite scores for the HIV-infected group with detectable compared to undetectable plasma HIV RNA (p = 0.011, Figure 3). A subsequent Pearson correlation revealed no significant correlation between the SAL composite score and plasma HIV RNA value (r = −0.253, p = 0.363) among HIV-infected individuals with detectable HIV RNA; however, most detectable cases had very low levels. Similar analyses to investigate the effects of current and nadir CD4 cell count on rs-fc did not identify significant associations. A comparison of rs-fc data from HIV-infected individuals with detectable HIV RNA to HIV-uninfected controls yielded no significant differences for any RSN.
Fig. 3. Associations between plasma HIV RNA and RSNs in HIV-infected individuals [detectable (> 75 copies/mL) vs. undetectable plasma HIV RNA].
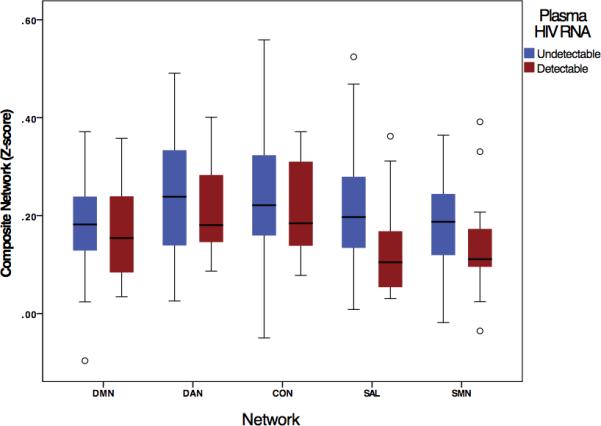
Analyses revealed a significant decrease in the composite score for the SAL network for HIV-infected individuals with detectable plasma HIV viral load (p = 0.011).
DISCUSSION
This work investigated rs-fc and NP performance in HIV-infected and uninfected individuals over 60 years old. HIV-infected individuals with detectable plasma HIV RNA had decreased rs-fc within the SAL compared to those with undetectable plasma HIV RNA. However, no relationship was found between domain-specific NP performance (memory, executive, attention, visuospatial, psychomotor) and rs-fc in the 5 RSNs (DMN, DAN, CON, SAL, SMN). We also found no effects of HAND diagnosis on rs-fc.
The relationship between rs-fc in the SAL network and detectability of plasma HIV RNA suggests that the virus may selectively affect distinct RSNs and raises the question of whether the virus or inflammatory pathways may alter rs-fc in the SAL, a network that provides emotional stimuli to neural systems (Seeley et al. 2009). Dysfunction in this network may explain the deficits in emotional-conflict processing seen in HIV-infected individuals (Schulte et al. 2011).
Previous research has focused on HIV RNA as a biomarker for cognitive dysfunction. However, with increased efficacy of cART, many HIV-infected individuals with HAND are virologically suppressed; thus, the relationship between plasma HIV RNA and cognition has become less important in the current cART era (Clifford and Ances 2013; Nightingale et al. 2014). Cerebrospinal fluid (CSF) rather than plasma HIV RNA may be more predictive of cognitive impairment; however, detectable CSF HIV RNA by standard assays and in the absence of detectable plasma HIV RNA (“CNS escape”) is rare (Eden et al. 2010). Future research comparing CSF HIV viral load with rs-fc may shed light on this issue, particularly in studies that have the capability of measuring single copy levels of CSF HIV RNA.
Of special interest is the lack of difference in rs-fc by HIV status. All HIV-infected individuals were receiving cART. The group's median duration of HIV infection exceeded two decades; thus, survivorship tendencies could exist. This survivorship bias could contribute to a negative finding. It was only when we evaluated HIV-infected individuals with detectable viral loads that we observed differences within the HIV-infected group. This suggests that cART restores networks within HIV-infected individuals once suppression of HIV RNA is achieved, such that these individuals are similar to HIV-uninfected controls. Our findings are in agreement with a past publication noting no differences in neural integrity using diffusor tensor imaging (DTI) in HIV-infected individuals after they received cART (Wright et al. 2012). However, our current findings are in contrast with recent publications evaluating the same participants enrolled in the current study that observed broad DTI and DTI connectivity differences by HIV status (Jahanshad et al. 2012; Nir et al. 2013). Thus, it remains less clear which modality may be most sensitive to HIV effects in the era of cART. Longitudinal studies using multiple neuroimaging modalities and particularly among participants before and after cART would be helpful.
Our findings are unique in that we examined an elder cohort of participants with well-controlled HIV. Individuals in our study were often highly educated and primarily male, further limiting generalizability to more diverse populations. As is now common among patients with access to cART, HIV-infected individuals in our study had less severe cognitive impairment and no subject met criteria for HAD. It would be of interest to expand the present cohort to include HIV-infected individuals with a wider range of HAND severity. In the current study, even after applying rather stringent criteria to control for excess movement, relatively few individuals were removed with missing data interpolated, strengthening our approach.
In sum, we observed a loss in rs-fc within the SAL network of older HIV-infected individuals with a detectable compared to undetectable plasma HIV RNA. The combination of HIV infection and advanced age might influence activity within certain functionally-defined RSNs. The present work suggests that rs-fc may be able to detect changes in brain function as a result of uncontrolled HIV RNA, but may be a less robust measure among those with suppressed virus or to distinguish HAND from non-HAND in a treated population.
ACKNOWLEDGEMENTS
We thank our study participants. We also thank Hannah Jang, Robin Ketelle, Katherine Rankin, and Stephanie Chiao for their involvement in the HIV Over 60 Cohort. This work was funded by National Institute of Health grants K23-AG032872 (VV), R01-NR012907 (BMA), R01-NR012657 (BMA), and R01-NR014449 (BMA); The Larry L. Hillblom Foundation (VV), the UCSF/GIVI Center For AIDS Research, and the UCSF AIDS Research Institute. Additional support from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
CONFLICT OF INTEREST
The authors, Anika Guha BA, Liang Wang MD, Aaron Tanenbaum BS BA, Pardis Esmaeili-Firidouni BS, Lauren A. Wendelken MS, Edgar Busovaca BA, Katherine Clifford BA, Akash Desai BS, Beau M. Ances MD PhD, Victor Valcour MD PhD, declare that they have no conflict of interest. Dr. Valcour has served as a consultant for ViiV Healthcare.
REFERENCES
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV- associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci. 2012;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Wang L, Fagan AM, Benzinger T, Morris JC, Ances BM. Unrecognized preclinical Alzheimer disease confounds rs fcMRI studies of normal aging. Neurology. 2014;83(18):1613–1619. doi: 10.1212/WNL.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: the public health perspective. Am J Public Health. 2012;102(8):1516–1526. doi: 10.2105/AJPH.2012.300844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, Miller B, Valcour V. Deficits in Self-Awareness Impact the Diagnosis of Asymptomatic Neurocognitive Impairment in HIV. AIDS Res Hum Retroviruses. 2013;29(6):949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Price RW, Gisslen M. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Brain Aging: Models, Methods, and Mechanisms. D. R. Riddle; Boca Raton (FL): 2007. Changes in Cognitive Function in Human Aging. [PubMed] [Google Scholar]
- Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, Corbetta M. Resting state network estimation in individual subjects. Neuroimage. 2013;82:616–633. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Jr., Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 60 Suppl. 2012;1:S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Valcour VG, Nir TM, Kohannim O, Busovaca E, Nicolas K, Thompson P. Disrupted brain networks in the aging HIV+ population. Brain Connect. 2012 doi: 10.1089/brain.2012.0105-Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22(3):315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Ellis R, Grant I, Woods SP. Synergistic effects of HIV infection and older age on daily functioning. Journal of acquired immune deficiency syndromes. 2012;61(3):341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, Solomon T. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139–1151. doi: 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Brier MR, Ances BM. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. 2015;29(6):703–712. doi: 10.1097/QAD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69(7):833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, Cox RW. Correcting brain-wide correlation differences in resting-state FMRI. Brain Connect. 2013;3(4):339–352. doi: 10.1089/brain.2013.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Shikuma C, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13(3):203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A. Disruption of emotion and conflict processing in HIV infection with and without alcoholism comorbidity. J Int Neuropsychol Soc. 2011;17(3):537–550. doi: 10.1017/S1355617711000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Ortega M, Benzinger TL, Ances BM. Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiol Aging. 2015;36(1):401–412. doi: 10.1016/j.neurobiolaging.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–282. doi: 10.1093/infdis/jis326. (PMID:22551810; PMCID:23490695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Gakumo CA. The impact of neuropsychological performance on everyday functioning between older and younger adults with and without HIV. J Assoc Nurses AIDS Care. 2013;24(2):112–125. doi: 10.1016/j.jana.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Roe CM, Snyder AZ, Brier MR, Thomas JB, Xiong C, Benzinger TL, Morris JC, Ances BM. Alzheimer disease family history impacts resting state functional connectivity. Annals of neurology. 2012;72(4):571–577. doi: 10.1002/ana.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS. 2012;26(12):1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Lu WL, Xu B, Li CB, Lin CP, Waxman D, Feng JF. The increase of the functional entropy of the human brain with age. Sci Rep. 2013;3:2853. doi: 10.1038/srep02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]