Abstract
Background
Sublingual immunotherapy (SLIT) with peanut changes clinical and immune responses in most peanut-allergic individuals, but the response is highly variable.
Objective
We sought to examine the component-specific effects of peanut SLIT and determine whether peanut component testing could predict the outcome of a double-blind, placebo-controlled food challenge (DBPCFC) after 12 months of peanut SLIT.
Methods
We included 33 subjects who underwent peanut SLIT with a DBPCFC of 2500 mg of peanut protein performed after 12 months on therapy. Plasma samples from baseline and after 12 months of peanut SLIT were assayed using ImmunoCAP for IgE and IgG4 against whole peanut, Ara h 1, Ara h 2, Ara h 3, Ara h 8, and Ara h 9.
Results
Following 12 months of SLIT, 10 subjects (30%) passed the DBPCFC without symptoms and were considered desensitized. Subjects that failed the DBPCFC tolerated a median of 460 mg peanut protein (range: 10–1710 mg). The desensitized group had significantly lower baseline levels of IgE against peanut (median 40.8 vs 231 kUA/L, p = 0.0082), Ara h 2 (median 17 vs 113 kUA/L, p=0.0082), and Ara h 3 (median 0.3 vs 8.5 kUA/L, p = 0.0396). ROC curves indicated that baseline IgE against peanut and Ara h 2 were equally effective at discriminating between the two groups (AUC = 0.7957, p = 0.007752 for both).
Conclusion and Clinical Relevance
In this cohort of subjects undergoing SLIT for peanut allergy, lower baseline levels of IgE against Ara h 2, Ara h 3, and peanut were associated with successful desensitization.
INTRODUCTION
Peanut allergy is a public health concern affecting greater than 1% of the US population.1 Reactions to peanut can be life threatening,2 and peanut-allergic patients and their families experience diminished quality of life.3 There are no available treatments for peanut allergy, and the current standard of care involves strict avoidance of peanut and access to self-injectable epinephrine. Our group and others are actively conducting clinical trials to determine the safety and efficacy of immunotherapy for the treatment of peanut allergy.4, 5
One approach under investigation is sublingual immunotherapy (SLIT), which involves daily administration of micrograms of peanut protein extract under the tongue. Although safe, clinical responses to peanut SLIT are highly variable, ranging from complete response in a minority of subjects, to others that do no better than placebo.6,7 Previous studies have shown that SLIT modulates IgE and IgG4 specific to whole peanut,6 and that peanut-specific IgE and salivary IgA at the time of challenge may correlate with amount of protein ingested in a double-blind placebo-controlled food challenge (DBPCFC) after 12 months of therapy. 6, 8 These end-of-therapy measures, however, cannot aid in the selection of SLIT subjects. Given the substantial heterogeneity in treatment responses, it would be a major advance to develop predictors of outcome to optimize the selection of individuals most likely to benefit prior to engaging in immunotherapy.
With the recent introduction of ImmunoCAP tests specific for the peanut component antigens Ara h 1, 2, 3, 8, and 9, there has been increased interest in the measurement of component-specific immunoglobulins as a way to improve peanut allergy diagnosis. Several studies have shown that Ara h 2-specific IgE can be useful in diagnosing peanut allergy,9–15 and that patients monosensitized to Ara h 8 may be clinically tolerant.16,17 While Ara h 1, 2, and 3 may be the major allergens in the United States, others have shown that component sensitization can vary by region; Ara h 9 appears to predominate in peanut-allergic patients in Spain and the Mediterranean18,19 and Ara h 8 predominates in the Swedish population.19 With the current evidence, component-specific testing is likely only relevant to clinical decision-making in specific situations.20
In this study, we sought to use component-specific analyses to examine for the first time the effects of SLIT on antibody reactivity to individual peanut allergens, and to determine if specific binding patterns could serve as a biomarker for clinical outcomes following peanut SLIT. We measured peanut- and peanut component-specific IgE and IgG4 in subjects who underwent 12 months of peanut SLIT followed by a DBPCFC to assess desensitization. We hypothesized that subjects with lower baseline IgE against the major peanut allergens, Ara h 1, 2, and 3, would be more likely to achieve desensitization than those with highly elevated IgE against the major allergens.
METHODS
SLIT Subjects
Plasma samples obtained from blood collected in sodium-heparin-containing tubes from 33 subjects on peanut SLIT were available for use in this study. 18 out of 33 subjects were enrolled in a previously described randomized, placebo-controlled trial of SLIT for peanut allergy,6 11 of whom were in the original, blinded treatment arm, and 7 of whom crossed-over and underwent open-label peanut SLIT after 12 months of sham treatment. Food challenge outcomes for an additional 9 who underwent open-label treatment have been previously reported,21 and 6 subjects who underwent open-label treatment are presented here for the first time. This study was approved by the Institutional Review Boards of Duke University and the University of North Carolina.
Subjects (1–11 years old at time of enrollment) had an initial peanut CAP-FEIA greater than 7 kUA/L and a clinical history of reaction to peanut. Baseline food challenges were not performed. Over 6 months of biweekly visits, subjects were built up from an initial daily dose of 0.25 mcg of peanut protein to a maintenance dose of 2000 mcg per day. After 6 additional months of maintenance dosing, subjects underwent a DBPCFC to 2500 mg of peanut protein. Plasma samples from 0 months of peanut SLIT and following 12 months of peanut SLIT were kept frozen at −20°C until time of analysis.
ImmunoCAP
Peanut- and peanut component-specific IgE and IgG4 were measured via ImmunoCAP 100 (Thermo Fisher, Uppsala, Sweden) according to the manufacturer’s specifications.
Statistical Analysis
If a sample had a measured IgE or IgG4 below the lower limit of detection (0.01 kUA/L for IgE or 0.001 mgA/L for IgG4), based on previous work,22 a value of half of this lower limit (0.005 kUA/L for IgE or 0.0005 mgA/L for IgG4) was used in statistical analyses, IgE/IgG4 calculations, and figures. Wilcoxon signed-rank tests for longitudinal tests, Mann-Whitney U for between-group tests, ROC curves, and simple linear regression analyses were calculated with GraphPad Prism 5 (La Jolla, CA). A p-value < 0.05 was considered significant.
RESULTS
Food Challenge Outcomes
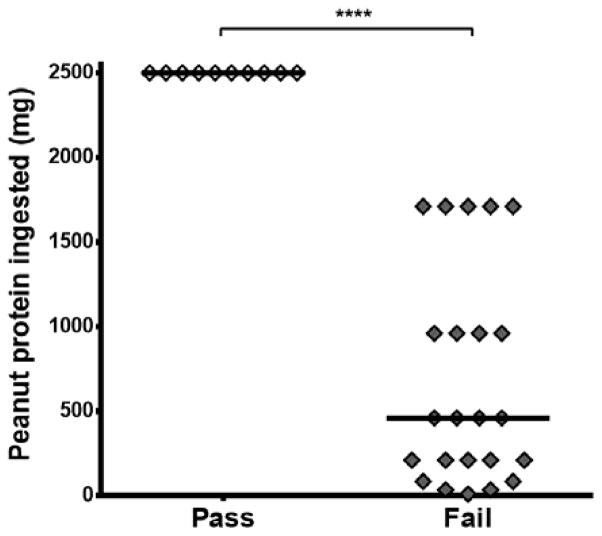
10/33 (30%) subjects completed the 2500 mg food challenge without symptoms and were considered fully desensitized. The remaining 23 subjects tolerated a median of 460 mg, with a range of 10–1710 mg (Figure 1).
Figure 1.
Peanut protein ingested during DBPCFC following 12 months of SLIT. 10 subjects passed the challenge by consuming 2500 mg without symptoms; 23 subjects failed the challenge. ****p<0.0001. Lines represent medians.
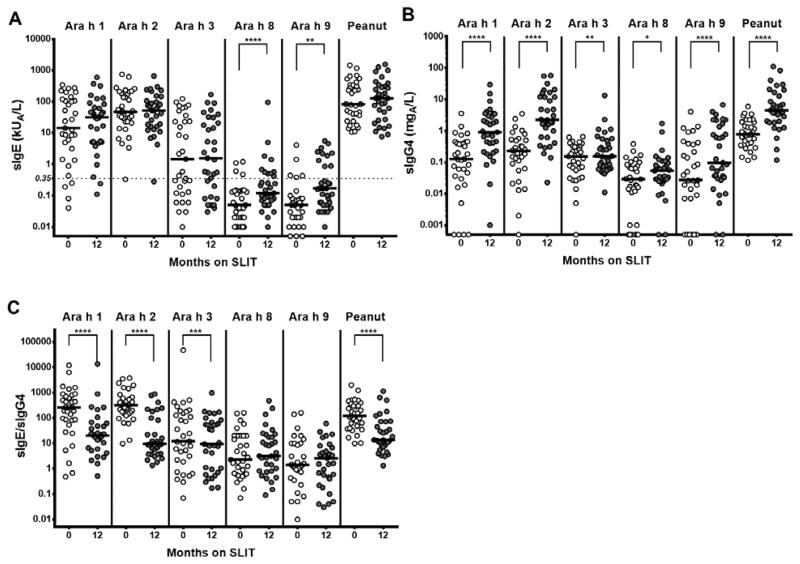
After 12 months of SLIT, peanut- and component-specific IgE remained stable, sIgG4 increased, and the sIgE/sIgG4 ratio decreased
Compared to baseline, Ara h 1-, Ara h 2-, Ara h 3-, and peanut-sIgE were not significantly different after 12 months of SLIT (Figure 2A). Ara h 8- and Ara h 9-specific IgE increased significantly (Medians, kUA/L: Ara h 8: 0.05 to 0.12, p <0.0001; Ara h 9: 0.05 to 0.17, p = 0.0018; see Online Supplemental Figure 1A for all data including IQR), but the median specific IgE stayed below 0.35 kUA/L. As shown in Figure 2B, specific IgG4 to peanut and all components significantly increased after 12 months of SLIT (Medians, mgA/L: Ara h 1: 0.127 to 0.905, p <0.0001; Ara h 2: 0.23 to 2.24, p <0.0001; Ara h 3: 0.154 to 0.156, p = 0.0038; Ara h 8: 0.029 to 0.054, p = 0.0184; Ara h 9: 0.027 to 0.096, p <0.0001; Peanut: 0.787 to 4.47, p <0.0001; see Online Supplemental Figure 1A for all data including IQR). The specific IgE/specific IgG4 ratio decreased from baseline for Ara h 1, Ara h 2, Ara h 3, and peanut (Figure 2C) (Medians: Ara h 1: 258 to 20.44, p <0.0001; Ara h 2: 322 to 9.5, p <0.0001; Ara h 3: 12 to 9.32, p = 0.0003; Peanut: 119.9 to 13.66, p <0.0001; see Online Supplemental Figure 1A for all data including IQR). Specific IgE/specific IgG4 was not significantly different for Ara h 8 and Ara h 9.
Figure 2.
Component- and peanut-specific immunoglobulin data over time on treatment for all subjects. A) Specific IgE; B) Specific IgG4; C) Specific IgE/specific IgG4. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Lines represent medians.
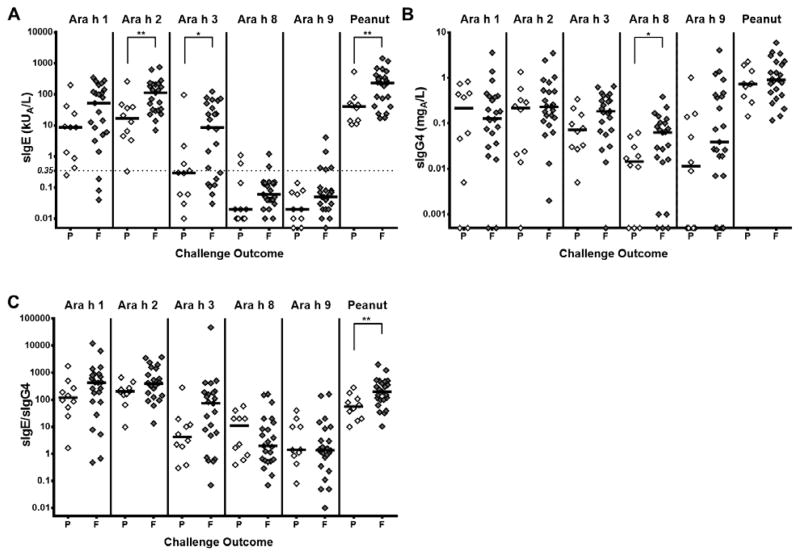
Baseline measurements were significantly different between passing and failing subjects
At baseline, Ara h 2-, Ara h 3-, and peanut-specific IgE were significantly higher in subjects who ultimately failed the 12 month food challenge (Medians, kUA/L: Ara h 2: median 17.0 vs 113, p = 0.0082; Ara h 3: 0.3 vs 8.5, p = 0.0396 Peanut: 40.8 vs 231, p = 0.0082; Figure 3A). As shown in Figure 3B, Ara h 8-specific IgG4 was significantly higher in the failing group (median 0.145 vs 0.063 mgA/L, p = 0.0349). Specific IgE/specific IgG4 ratios for the peanut antigens were not different between the groups that passed or failed the DBPCFC, but the peanut-specific IgE/peanut-specific IgG4 was significantly higher in those who failed the food challenge (median 55.87 vs 199.8 p = 0.0096, Figure 3C). See Online Supplemental Figure 1B for all data including IQR. IgE, IgG4, and IgE/IgG4 specific to peanut and the peanut antigens in samples from the time of the 12 month food challenge showed no significant differences between the two groups. (data not shown).
Figure 3.
Baseline component- and peanut-specific immunoglobulin data for subjects passing (P) and failing (F) the 12 month food challenge. A) Specific IgE; B) Specific IgG4; C) Specific IgE/Specific IgG4. *p<0.05; **p<0.01; ****p<0.0001. Lines represent
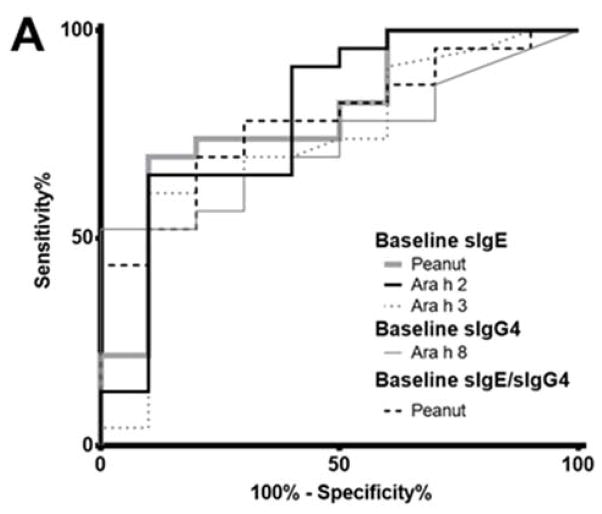
Discriminating between subjects that passed and failed the desensitization challenge
Only baseline values could differentiate between subjects passing and failing the desensitization challenge. The ROC analyses are shown in Figure 4. Peanut- and Ara h 2-sIgE were the best at discriminating between passing and failing groups (AUC = 0.7957, p = 0.007752), and optimal cut points for these discriminations were >81.30 kUA/L (65.22% sens., 90% spec.) for peanut-sIgE, >48.55 kUA/L (65.22% sens., 90% spec.) for Ara h 2-sIgE. Peanut-sIgE/IgG4 (AUC = 0.7826, p = 0.01092), Ara h 8-sIgG4 (AUC = 0.7326, p = 0.03615), and Ara h 3-sIgE (AUC = 0.7304, p = 0.03793) did not discriminate as well between the two groups. In addition, linear regression analyses considering amount ingested at challenge and IgE, IgG4, or IgE/IgG4 at baseline or at the time of challenge found no significant correlations (data not shown).
Figure 4.
Receiver operating characteristic (ROC) curves for factors significantly different for groups passing and failing the 12 month food challenge. A) Superimposed ROC curves; B) Optimal cut points, area under the curve (AUC) and p-value for each curve.
DISCUSSION
As reported here and in our previous publication,6 food challenge outcomes were variable after 12 months of peanut SLIT. These outcomes are in contrast to oral immunotherapy (OIT), in which participants ingest a maintenance dose 2000-fold larger than the dose used in SLIT, and a majority of subjects are able to pass a DBPCFC after 12 months of therapy.21 SLIT, however, may be easier to tolerate than OIT because of the smaller daily dose, sublingual administration, fewer allergic side effects, and liquid rather than flour vehicle.6 It would be useful, then, to find a parameter associated with successful desensitization so that appropriate subjects could be selected to receive SLIT. We used a component-resolved analysis to elucidate whether there are differences in peanut- and component-specific immunoglobulins between subjects passing and failing a DBPCFC following SLIT.
In our analysis, we measured IgE and IgG4 specific to Ara h 1, 2, 3, 8, 9 and peanut at baseline and after 12 months of SLIT. We found that specific IgG4 to all components and peanut significantly increased, and that the IgE/IgG4 ratio significantly decreased for Ara h 1, 2, 3, and whole peanut. We did not observe any significant changes in specific IgE to Ara h 1, 2, 3, and peanut, and specific IgE to Ara h 8 and 9 did not reach levels above 0.35 kUA/L, the value considered clinically significant. These results are consistent with previous findings regarding SLIT’s effect on peanut-specific IgE and specific IgG4,6 and show that the effect on IgE/IgG4 ratio was found only for Ara h 1, 2, 3, and whole peanut, but not Ara h 8 or 9. Our results with sublingual immunotherapy are also similar to those reported in a recent study of the humoral response to Ara h 1, 2, and 3 in Japanese children receiving peanut oral rush immunotherapy, in which the authors found an increase in IgG4 against Ara h 1, 2, 3 after 12 months of oral immunotherapy and no change in specific IgE against these components.23 The study, however, did not include certain elements of our study, such as food challenges to evaluate the effectiveness of therapy, and subjects sensitized to Ara h 8 or 9.
Our results may indicate that SLIT primarily modulates the humoral response, specifically the IgE/IgG4 ratio, to the three major allergens. They may, however, represent the sensitization profiles typically seen in U.S. patients with known systemic peanut allergy.19 The extent to which immunotherapy may alter allergen-specific immune responses does also depend on the amount of major allergen present in the treatment extract.
Comparing subjects who passed and failed the 12 month food challenge, we found that Ara h 2-, 3-, and peanut-specific IgE, Ara h 8-specific IgG4, and peanut-specific IgE/specific IgG4 were all higher at baseline in failing subjects. Using ROC curves to discriminate between the two groups, we found, based on the AUC, that peanut- and Ara h 2-specific IgE were equally effective at predicting the outcome of the food challenge, and that Ara h 8-specific IgG4 and Ara h 3-specific IgE were less effective. The optimal cut point for peanut-specific IgE was >81.30 kUA/L (69.57% sens., 90% spec.) and the cut point for Ara h 2-specific IgE was >48.55 kUA/L (65.22% sens., 90% spec.). Interestingly, these cut points are similar to those reported by Vickery et al. (peanut-specific IgE >85.5 kUA/L and Ara h 2-specific IgE >35.5 kUA/L) to discriminate between those who did and did not exhibit sustained unresponsiveness following long-term OIT. We do think it is important to note, however, that the present study is limited to the examination of outcomes after 12 months of continuous peanut SLIT treatment, and does not concern tolerance or sustained unresponsiveness in these subjects following cessation of treatment. Nonetheless, these studies may indicate that strong allergic responses are difficult to modify, regardless of treatment modality.24 From our data, we conclude that, in this pretreatment context, baseline component-resolved diagnosis does not add additional prognostic information above that offered by conventional peanut IgE measurements.
The higher Ara h 3-sIgE in failing subjects may reflect epitope spreading in those with a more severe phenotype,25 while the higher Ara h 8-sIgG4 may simply reflect the component’s cross-reactivity with the pollen pan-allergen, Bet v 1. It is also important to note that sIgE and sIgG4 values after 12 months of SLIT were not different in subjects passing or failing the challenge, indicating that using these time-of-challenge values would not be useful in predicting whether subjects had become desensitized. This is in contrast to peanut-specific salivary IgA, which has been correlated with challenge outcomes following SLIT.8 Our findings regarding the predictive value of time-of-challenge immunoglobulin measurements are similar to two studies that examined subjects on both peanut OIT and SLIT, which also found these measurements to be non-significant between passing and failing groups.21,26 These studies also found that peanut OIT induced greater changes in peanut-specific IgE and IgG4 when compared to peanut SLIT, 21,26 but did not include component-specific measurements.
Overall, we found that Ara h 2- and peanut-specific IgE at baseline were best at determining those subjects which would pass a food challenge after 12 months of peanut SLIT. Lower IgE levels at the beginning of treatment was associated with SLIT desensitization, suggesting that an intervention with SLIT in younger children with lower peanut-specific IgE would be a worthwhile treatment approach. These findings are generally consistent with other published work demonstrating the immunodominance of Ara h 2 in American peanut-allergic patients, and the inverse relationship between immunotherapy treatment outcomes and the strength of allergic priming. More study is necessary to identify robust immunotherapy-associated biomarkers.
Supplementary Material
Acknowledgments
We would like to thank Joe Jones and Thermo Fisher Scientific, Inc. for providing CAPs for this study
Funding: NIH-NCCAM R01-AT004435
Footnotes
Author Contributions:
Burk: study conception and design; data collection; data analysis and interpretation; manuscript drafting; critical revision; final manuscript approval
Kulis: study conception and design; data analysis and interpretation; manuscript drafting; critical revision; final manuscript approval
Leung: Data collection; critical revision; final manuscript approval
Kim: Data analysis and interpretation; critical revision; final manuscript approval
Burks: critical revision; final manuscript approval
Vickery: study conception and design; data analysis and interpretation; manuscript drafting; critical revision; final manuscript approval
CONFLICT OF INTEREST
(Each authors’ COI forms have been submitted separately)
References
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Sicherer SH. Quality of life in food allergy. Curr Opin Allergy Clin Immunol. 2011;11:236–42. doi: 10.1097/ACI.0b013e3283464cf0. [DOI] [PubMed] [Google Scholar]
- 4.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–73. doi: 10.1016/j.jaci.2010.12.1098. quiz 574–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran TP, Vickery BP, Burks AW. Oral and sublingual immunotherapy for food allergy: current progress and future directions. Curr Opin Immunol. 2013;25:781–7. doi: 10.1016/j.coi.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6. e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–27. e1–7. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1159–62. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–5. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Hong X, Caruso D, Kumar R, Liu R, Liu X, Wang G, et al. IgE, but not IgG4, antibodies to Ara h 2 distinguish peanut allergy from asymptomatic peanut sensitization. Allergy. 2012;67:1538–46. doi: 10.1111/all.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemans RJ, Liu X, Knulst AC, Knol MJ, Gmelig-Meyling F, Borst E, et al. IgE binding to peanut components by four different techniques: Ara h 2 is the most relevant in peanut allergic children and adults. Clin Exp Allergy. 2013;43:967–74. doi: 10.1111/cea.12136. [DOI] [PubMed] [Google Scholar]
- 12.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. e1–13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–63. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 14.Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011;154:216–26. doi: 10.1159/000321108. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JA, Glaumann S, Batelson S, Borres MP, Sampson HA, Nilsson C. The utility of peanut components in the diagnosis of IgE-mediated peanut allergy among distinct populations. J Allergy Clin Immunol Pract. 2013;1:75–82. doi: 10.1016/j.jaip.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Asarnoj A, Nilsson C, Lidholm J, Glaumann S, Östblom E, Hedlin G, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130:468–72. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Asarnoj A, Moverare R, Östblom E, Poorafshar M, Lilja G, Hedlin G, et al. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010;65:1189–95. doi: 10.1111/j.1398-9995.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 18.Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124:771–8. e5. doi: 10.1016/j.jaci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Vereda A, van Hage M, Ahlstedt S, Ibañez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127:603–7. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Sicherer SH, Wood RA. Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract. 2013;1:1–13. doi: 10.1016/j.jaip.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Chin SJ, Vickery BP, Kulis MD, Kim EH, Varshney P, Steele P, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132:476–8. e2. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Węgrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129:739–47. doi: 10.1016/j.jaci.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 23.Nozawa A, Okamoto Y, Movérare R, Borres MP, Kurihara K. Monitoring Ara h 1, 2 and 3-sIgE and sIgG4 antibodies in peanut allergic children receiving oral rush immunotherapy. Pediatr Allergy Immunol. 2014;25:323–8. doi: 10.1111/pai.12243. [DOI] [PubMed] [Google Scholar]
- 24.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743. e10. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, Wood RA. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. May;135:1275–1282. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.