Abstract
Objective
We recently demonstrated that low-density lipoprotein receptor related protein 1 (LRP1) is required for cardiovascular development in zebrafish. However, what role LRP1 plays in angiogenesis remains to be determined. To better understand the role of LRP1 in endothelial cell function, we investigated how LRP1 regulates mouse retinal angiogenesis.
Approach and results
Depletion of LRP1 in endothelial cells results in increased retinal neovascularization in a mouse model of oxygen-induced retinopathy. Specifically, retinas in mice lacking endothelial LRP1 have more branching points and angiogenic sprouts at the leading edge of the newly formed vasculature. Increased endothelial proliferation as detected by Ki67 staining was observed in LRP1 deleted retinal endothelium in response to hypoxia. Using an array of biochemical and cell biology approaches, we demonstrate that poly(ADP-ribose) polymerase-1 (PARP-1) directly interacts with LRP1 in human retinal microvascular endothelial cells (HRECs). This interaction between LRP1 and PARP-1 decreases under hypoxic condition. Moreover, LRP1 knockdown results in increased PARP-1 activity and subsequent phosphorylation of both retinoblastoma protein (Rb) and cyclin-dependent kinase 2 (CDK2), which function to promote cell cycle progression and angiogenesis.
Conclusions
Together, these data reveal a pivotal role for LRP1 in endothelial cell proliferation and retinal neovascularization induced by hypoxia. In addition, we demonstrate for the first time the interaction between LRP1 and PARP-1 and the LRP1-dependent regulation of PARP-1 signaling pathways. These data bring forth the possibility of novel therapeutic approaches for pathological angiogenesis.
Keywords: Low density lipoprotein receptor-related protein 1, Poly(ADP-Ribose) polymerase-1, hypoxia, endothelial cell proliferation, angiogenesis
INTRODUCTION
Angiogenesis is the process of new blood vessel growth from existing vascular networks. It occurs during embryonic development and throughout adulthood, and is initiated during wound healing and in pathological conditions such as retinopathy1. Pathological retinal angiogenesis generates physiologically deficient vessels and results in vision-threatening exudation and hemorrhage1. Several factors including vascular endothelial growth factor (VEGF), angiopoietins, Notch and Wnt have been shown as critical coordinators for retinal angiogenesis2–5. However, the exact molecular mechanisms of pathological retinal angiogenesis involved in retinopathy of prematurity and diabetic retinopathy remain elusive.
Low density lipoprotein receptor-related protein 1 (LRP1), a multifunctional member of the LDL receptor family, is involved in a variety of biological processes such as lipid metabolism, endocytosis and signal transduction6–8. Global deletion of the LRP1 gene in mice leads to embryonic lethality, demonstrating an essential role for LRP1 in development9. More recent work demonstrates that LRP1 deletion in embryo proper results in vascular developmental defects10. Tissue specific knockout mouse models show that LRP1 regulates cell proliferation and migration in smooth muscle cells, inflammation and efferocytosis in macrophages, suggesting that LRP1 plays important roles in atherosclerosis11–15. In endothelial cells, we previously demonstrated that LRP1 regulates vascular development through its interaction with BMP binding endothelial regulator (BMPER) and affecting BMP signaling16. However, whether LRP1 is involved in other signaling pathways in endothelial cells and regulates pathological angiogenesis remains unknown.
LRP1 is a heterodimer composed of an extracellular 515-kDa α chain (LRP1α) and an 85-kDa membrane-anchored cytoplasmic β chain (LRP1β), which remain non-covalently associated6–8. There are more than forty different ligands for LRP1, such as proteases, growth factors, extracellular matrix proteins and lipoproteins. The intracellular domain of LRP1β contains multiple serine, threonine and tyrosine residues that can be phosphorylated by PKA or Src8. This domain also associates with adaptor proteins via its two NPXY motifs to induce signals7. LRP1 mainly behaves as an endocytic receptor for its ligands. For example, we recently discovered that LRP1 modulates the endocytosis of BMP signaling complex in endothelial cells16. However, some other ligands, including tPA, α2-macroglobulin (α2M), apoE, matrix metalloproteinase 9, may activate Src/ERK and PI3K/Akt/PKCδ signaling pathways in neuronal cells17–21. Moreover, the LPS-induced intramembrane proteolysis of LRP1 enables the translocation of its intracellular domain into the nucleus and regulates transcriptional events of inflammatory genes22. Taken together, LRP1 may activate and integrate diverse downstream signaling pathways in response to different stimuli. Interestingly, LRP1 expression can be induced by hypoxia in smooth muscle cells and by fluvastatin and simvastatin in human brain microvascular endothelial cells23–25. It is plausible that LRP1 may also mediate endothelial cellular responses to diverse stimuli or stress through multiple signaling pathways besides of endocytosis. However, the LRP1-mediated function of these ligands and modulators in endothelial cells remain uncharacterized, warranting further investigation.
In this study, we investigated whether and how LRP1 deletion in endothelial cells regulates pathological angiogenesis by using a mouse model of oxygen-induced retinopathy (OIR). Our results demonstrate that LRP1 acts as a negative regulator of retinal angiogenesis under hypoxic condition. We also demonstrate that the regulatory role of LRP1 in endothelial cell proliferation and angiogenesis fulfills, at least in part, through the interaction of LRP1 with PARP-1. This interaction between LRP1 and PARP-1 broadens our understanding about the functional roles of LRP1 in endothelial cells and for the first time, reveals a novel regulatory role of LRP1 in the PARP-1/CDK2/Rb signaling pathway.
MATERIAL AND METHODS
Materials and Methods are available in the online-only supplement.
RESULTS
LRP1 Deletion in Retinal Endothelium Results in Increased Neovascularization in an Oxygen-Induced Retinopathy Mouse Model
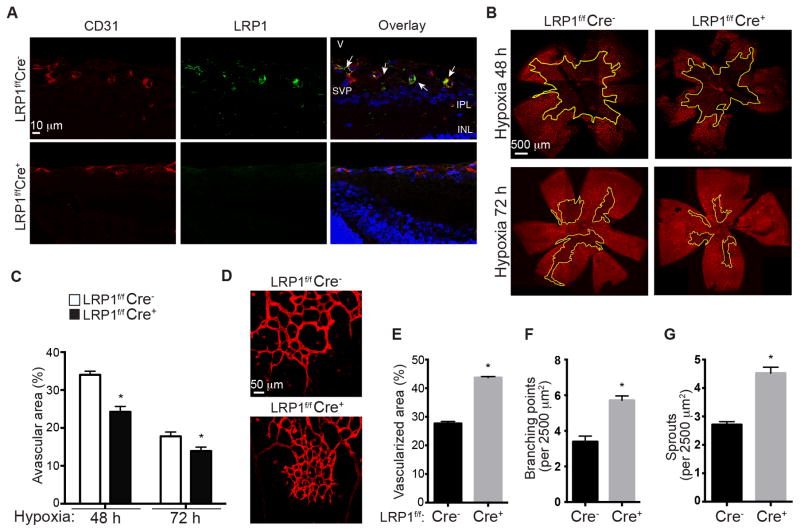
We recently discovered that LRP1 regulates vascular development in zebrafish16. To further determine the roles of LRP1 in pathological angiogenesis and its underlying mechanisms, we decided to utilize an oxygen-induced retinopathy (OIR) mouse model because retinal vasculature is a highly organized and easily approachable system to study. To elucidate the specific role of LRP1 in endothelial cells, we first crossed LRP1flox/flox (LRP1f/f) mice with Tie2Cre+ transgenic mice to generate LRP1f/f;Tie2Cre+/− mice. Tie2 promoter directs effective Cre expression, and hence LRP1 deletion, in endothelial cells (Figure 1A) as well as hematopoietic cells26. Since retinal vascularization begins at the optic nerve head and radiates outwards to cover the most superficial retinal layer during the first week of life (between postnatal days P0~P7) in mice27, we examined whether there is a difference during the development of this superficial retinal vasculature layer in LRP1f/f;Cre+ mice and LRP1f/f;Cre− littermate control mice. However, no obvious gross morphological difference was observed, indicating that LRP1 is not essential for retinal vasculature development (data not shown). We then investigated the role of LRP1 in pathological angiogenesis by using an OIR model28, 29. OIR model starts with postnatal day 7 (P7) mice being placed in a cage with constant 75% O2 for five days. This constant hyperoxia induces retinal capillary obliteration centrally28, 29. Mice were then placed back into room air to mimic relative hypoxia and permit retinal and intravitreous neovascularization. This model pathologically mimics the ischemia-induced angiogenesis observed in retinopathy of prematurity and proliferative diabetic retinopathy and is relevant in general to ischemic vascular diseases1. Using this model, we measured the detailed parameters of blood vessel formation following the onset of hypoxia. At 48 hours following the onset of hypoxia (P14), LRP1f/f;Cre+ mice displayed 29% more retinal vascularization compared to control littermates, and 22% more retinal vascularization at 72 hours (P15) (Figure 1B and 1C). This increase in intraretinal neovascularization observed in LRP1f/f;Cre+ mice was accompanied by a denser and more complex network of newly formed vessels (Figure 1D) as well as more vascularized area, greater number of branching points and more sprouts at the leading edge of the newly formed vessels in LRP1f/f;Cre+ mice, compared to their control littermates (Figure 1E–G). These observations demonstrate that LRP1 regulates angiogenic responses of retinal endothelial cells to the changes of oxygen tension.
Figure 1. Intraretinal neovascularization increases in retinal endothelium lacking LRP1 during hypoxia.
A, Loss of LRP1 expression in endothelial cells in LRP1f/f;Cre+ mouse retina. Images are sagittal views of mouse retinas at postnatal day P15. Tissue sections were stained with LRP1 (8G1 Ab, green) and CD31 (an endothelial cell specific marker, red) antibodies. The test for the specificity of this 8G1 antibody is shown in Figure SII. INL, inner nuclear layer; IPL, inner plexiform layer; SVP, superficial vascular plexus; V, vitreous. B–C, Loss of LRP1 in endothelial cells increased intraretinal neovascularization following onset of hypoxia for 48 hours at P14 and 72 hours at P15. (B) Confocal images of retinal flat mounts from LRP1f/f;Cre+/− oxygen-induced retinopathy (OIR) mice were stained with iso-lectin. Avascularized (yellow outline) areas are shown as a percentage of the total area of the retinal superficial vasculature layer (C). *, P<0.05 via two-way ANOVA analysis followed by Bonferroni multiple comparison test, n≥4. D–G, Analysis of angiogenic parameters for the retinal neovascularization. D, An increase in neovascularization is observed at the leading edge of LRP1f/f;Cre+ retinas, compared to LRP1f/f;Cre− littermates. Confocal images of retinal flat mounts stained with iso-lectin from P15 LRP1f/f;Cre+/− oxygen-induced retinopathy mice were used for analysis. Vascularized area (E), branching pints (F) and vessel sprout number (G) were quantified within leading edge of neovascularization area. *, P<0.05 via unpaired Student’s t-test, n=4 for LRP1f/f;Cre− and n=3 for LRP1f/f;Cre+ mice.
LRP1 Depleted Retinal Endothelium Displays Increased Proliferative Response Without Changes in the Interaction of Endothelial Cells with Astrocytes and Pericytes
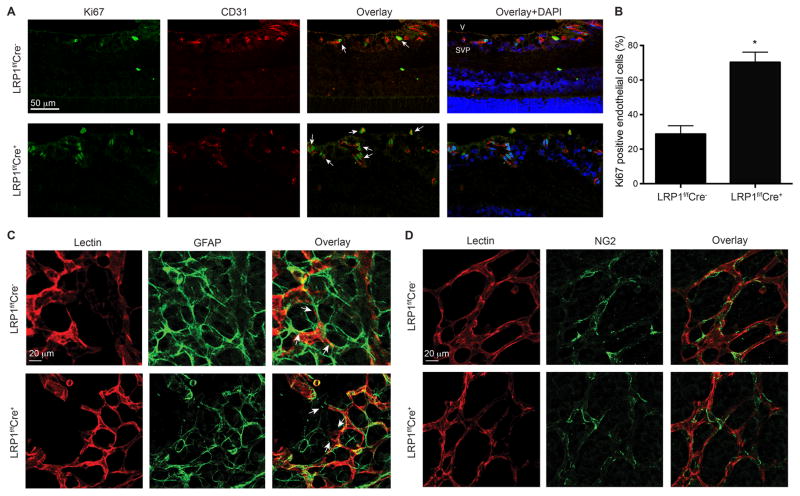
To determine which cellular processes during retinal neovascularization are affected by LRP1 depletion in endothelium, we examined endothelial cell proliferation, the interaction of endothelial cells with adjacent astrocytes and pericyte recruitment. First, we examined whether endothelial proliferation is affected during the increased angiogenic responses in LRP1f/f;Cre+ retinas. We stained retinal cells at 3 days following the onset of hypoxia (P15) with Ki67, a specific nuclear marker of cell proliferation. In retinas with LRP1 deletion in endothelial cells, we observed a significant increase in Ki67 positive endothelial cell number (Figure 2A and 2B), indicating that cell proliferation increased in LRP1 deleted vascular endothelium. Next, we investigated the interaction of astrocytes and the LRP1-deleted endothelial cells. Immunostaining of retinas following the onset of hypoxia (P14) with iso-lectin (to stain endothelial cells) and glial fibrillary acidic protein (GFAP, to stain astrocytes) revealed that the pattern of retinal astrocytic network in LRP1 depleted retinas was not obviously different from the control littermates. Moreover, we observed that endothelial cell filopodia at the tip of the growing vascular sprouts were similar between LRP1f/f;Cre+ and control retinas (Figure 2C). Specifically, both LRP1 depleted endothelial cells and control cells interacted with the astrocytic network similarly, suggesting that the communication between astrocytes and endothelial cells is not impaired by LRP1 depletion. Furthermore, we examined whether the recruitment of pericytes to the wall of the new vessels was abnormal in retinas with LRP1-depleted endothelium. Immunostaining of retinas with iso-lectin and NG2 (to stain pericytes) indicated that both LRP1f/f;Cre+ and control retinas displayed appropriate coverage of new vessels with pericytes, in a ratio of ~3:1 (endothelial cell: pericyte), suggesting that the process of pericyte recruitment is not disturbed in new vessels lacking LRP1 (Figure 2D). Based on our observation of these endothelial cellular processes, this infers that endothelial cell proliferation is regulated by LRP1, which may partly contribute to the increased angiogenic activity observed in LRP1f/f;Cre+ retinas.
Figure 2. LRP1 deletion in retinal endothelium displays increased proliferation without changes in the interaction of endothelial cells with astrocytes and pericytes.
A–B, Endothelial cell proliferation increases in LRP1f/f;Cre+ mice. (A) Retinal sagittal sections from P15 (72 hours of hypoxia) LRP1f/f;Cre+/− mice were stained with Ki67 (cell proliferation marker, green), CD31 (endothelial cell marker, red) and DAPI (blue). The percentage of Ki67 positive endothelial cells were counted and presented in B. Arrows, Ki67 positive endothelial cells. *, P<0.05 via unpaired Student’s t-test, n=6 for LRP1f/f;Cre− and n=5 for LRP1f/f;Cre+ mice. C–D, Normal astrocyte-endothelial filopodia interactions and pericyte recruitment are observed in both LRP1f/f;Cre+ and LRP1f/f;Cre− mouse retinas. Flat-mounted whole retinas from LRP1f/f;Cre+/− mice that were subjected to OIR for 48 hours were stained with iso-lectin (red), glial fibrillary acidic protein (GFAP; green in C) or NG2 (green in D). Arrows, endothelial filopodia.
LRP1 Knockdown in Human Retinal Microvascular Endothelial Cells (HRECs) Increases Angiogenesis, Endothelial Proliferation and Cell Cycle Progression in Response to Hypoxia
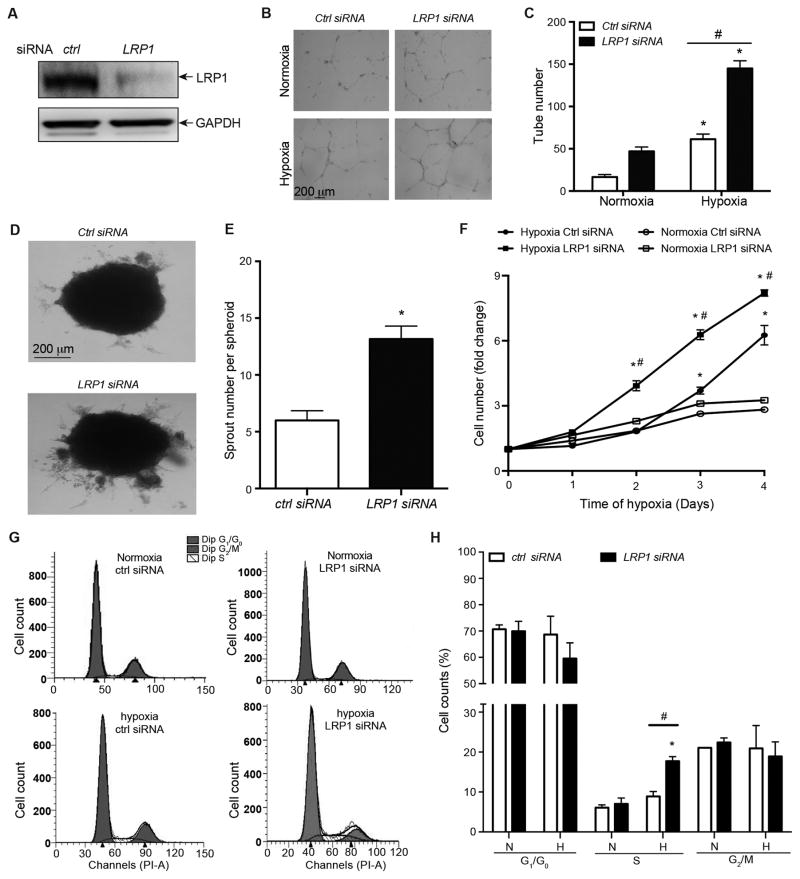
Our in vivo data (Figure 1 and 2) indicate that LRP1 depletion in retinal endothelium increases endothelial cell proliferation and retinal neovascularization. To identify the precise molecular and cellular events involved, we first tested whether LRP1 inhibits angiogenesis in cultured primary endothelial cells- Human Retinal Microvascular Endothelial Cells (HRECs). LRP1 is endogenously expressed in HRECs, and transfection of its specific siRNAs dramatically decreased its protein level (Figure 3A). In a Matrigel tubulogenesis assay, mild hypoxia (2% oxygen) predictably increased tube formation compared to normoxia. LRP1 knockdown in HRECs results in an additional ~2-fold increase in tube formation in mild hypoxia (Figure 3B and 3C). We also observed a similar increase in sprouting angiogenesis in response to hypoxia in LRP1 knockdown HRECs (Figure 3D and 3E). These in vitro data confirm our observations with LRP1f/f;Cre+ mouse retinas (Figure 1) and suggest that LRP1 is a negative regulator of angiogenesis. Since retinal angiogenesis and endothelial cell proliferation increases in LRP1f/f;Cre+ mice, we investigated the role of LRP1 in endothelial cell growth in HRECs. The growth curve of HRECs following LRP1 knockdown clearly shows that LRP1 knockdown in HRECs increases cell number during hypoxia, compared to control siRNA-transfected HRECs (Figure 3F). Lastly, we investigated whether LRP1 regulates endothelial proliferative response by affecting cell cycle progression. Significantly more HRECs lacking LRP1 progress into S phase from G1/G0 stage, compared to control cells in response to hypoxia (Figure 3G and 3H). These data establish that LRP1 is a negative regulator of retinal angiogenesis, at least in part through cell cycle arrest and the inhibitory effect on endothelial cell proliferation.
Figure 3. LRP1 knockdown in HRECs promotes angiogenesis, endothelial proliferation and cell cycle progression.
A, LRP1 protein level decreases in HRECs that were transfected with LRP1 siRNA, compared to control siRNA. Lysates of LRP1-knockdown or control HRECs treated were analyzed by Western blotting to detect LRP1 β chain at 85 kDa. B–C, LRP1 knockdown in HRECs increases tube formation. HRECs were incubated in normoxia (21% O2) or hypoxia (2% O2) condition for 24 hours on Matrigel coated plates. Phase contrast images were used for quantitative measurements of tube numbers per sample and shown in B. *, P<0.05 compared to the same HRECs at normoxia condition, #, P<0.05 compared to the control siRNA-treated HRECs at hypoxia condition, n=3. Analysis was two-way ANOVA followed by Bonferroni multiple comparison test. D–E, LRP1 knockdown in HRECs increases sprouting angiogenesis. Spheroid angiogenesis assays were performed with HRECs that were transfected with LRP1 or control siRNAs. Hypoxia (2% O2) was used to induce sprout formation. Images of HRECs spheroids demonstrate the formation of sprouts following 72 hours of hypoxia (2% O2) incubation. The number of sprouts per spheroid were counted and quantified in E. *, P<0.05 via unpaired Student’s t-test compared to control cells, n=6. F, LRP1 knockdown in HRECs increases hypoxia-induced cell growth. Cell numbers were counted daily in HRECs that were transfected with LRP1 or control siRNAs, and subsequently cultured in normoxia or hypoxia (2% O2) for 4 days. *, P<0.05, compared to same HRECs at day 0. #, P<0.05, compared to control siRNA-transfected HRECs that were incubated at hypoxia condition. n= 4. Analysis was two-way ANOVA followed by Bonferroni multiple comparison test. G–H, LRP1 knockdown increases cell cycle progression to S phase from G1/G0 stage. HRECs were transfected with LRP1 or control siRNA. Two days later, cells were incubated under normoxia (N, 21% O2) or hypoxia (H, 2% O2) for 24 hours. Cells were then stained with propidium iodide and analyzed by flow cytometry. The representative flow cytometry images are shown in G, and the percentages of cells at different cell cycle stages were quantified and present in H. *, P<0.05, compared to same cells under normoxia. #, P<0.05, compared to control siRNA-transfected HRECs under hypoxia. n=3. Analysis was two-way ANOVA followed Fisher’s LSD multiple comparison test.
LRP1 Interacts with Poly(ADP-Ribose) Polymerase-1 (PARP-1) in HRECs
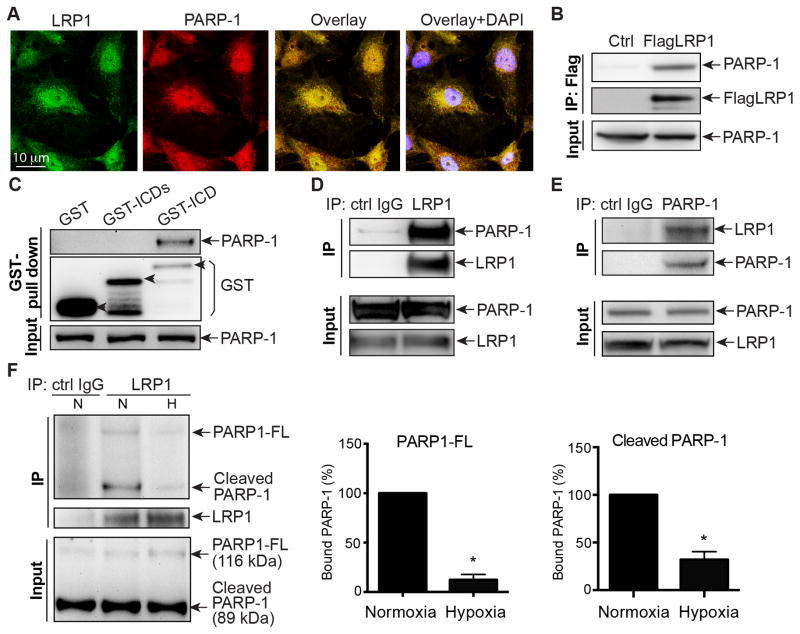
To elucidate how LRP1 regulates the cell cycle and endothelial cell proliferation, we used immunoprecipitation combined with liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) as an inductive unbiased method of identifying LRP1-associated proteins in HEK 293 cells. We identified Poly(ADP-Ribose) polymerase (PARP-1), a well defined stress sensor, as a candidate binding partner of LRP1 in HEK 293 cells (Figure SI, Table SI). PARP-1 is a nuclear enzyme that uses NAD+ as a substrate to catalyze the covalent attachment of ADP-ribose units on nuclear acceptor proteins or on PARP-1 itself30. In response to stress signals, PARP-1 is activated and plays key roles in DNA repair31–33, apoptosis34, 35, chromatin modulation and transcription36, 37 and cell cycle regulation38–41. Many reports demonstrate that PARP-1 inhibition by genetic deletion or chemical inhibitors decreases angiogenesis during melanoma tumor growth, a transplanted lung cancer model or other pathological conditions42–45. Due to its role in cell cycle control and angiogenesis, and our data suggesting PARP-1 could be a novel interactive protein of LRP1, we tested whether PARP-1 regulated the LRP1-mediated effects of endothelial cell cycle progression, proliferation and angiogenesis. First, we investigated the subcellular localization of LRP1 and PARP-1. Confocal imaging of HRECs revealed that PARP-1 and LRP1 (detected by LRP1 C-terminal antibody) substantially co-localize in the nucleus and some in the cytoplasm of HRECs (Figure 4A). Next, we confirmed their interaction by performing immunoprecipitation experiments. Immunoprecipitating for Flag-tagged LRP1 and immunoblotting for PARP-1 demonstrated that PARP-1 associates with LRP1 in HEK 293 cells (Figure 4B). We then performed GST pull down assay to compare the binding of PARP-1 with purified GST-tagged LRP1 intracellular C-terminal domain (GST-ICD; a.a. 4445-4544 of human LRP1) or the truncated intracellular C-terminal domain shortened (GST-ICDs; a.a. 4445-4511 of human LRP1). The binding of PARP-1 with GST-ICDs decreased significantly, compared to that with GST-ICD (Figure 4C). It indicates that the LRP1 C-terminal domain containing last 33 amino acids is required for its interaction with PARP-1. The interaction between endogenous LRP1 and PARP-1 was also observed in HRECs (Figure 4D–E). Next, we determined how hypoxia affects the subcellular localization of LRP1 and PARP-1 and their interaction. Interestingly, when HRECs were exposed to hypoxia for 0.5~2 hours, both LRP1 and PARP-1 signals increased in cytoplasm but decreased in nucleus, suggesting that they translocate from nucleus to cytoplasm in response to hypoxia (Figure SIIIA–C). We also observed that the interaction between LRP1 and PARP-1 dramatically decreased in response to hypoxia, compared to normoxia (Figure 4F), confirmed by our confocal imaging data (Figure SIIID). Together, these data suggest that PARP-1 directly associates with LRP1, and their association is dynamically regulated by oxygen tension.
Figure 4. LRP1 interacts with PARP-1 in HRECs.
A, HRECs were fixed for staining with anti-LRP1 (LRP1-CTD Ab, green) and PARP-1 antibodies (red) and DAPI (blue), and imaged with confocal microscopy. The test for the specificity of this LRP1-CTD antibody is shown in Figure SII. B, Lysates of HEK 293 cells with stable exogenously expressing Flag-tagged LRP1 β chain (Flag-LRP1) were immunoprecipitated with an anti-Flag resin and blotted with an anti-PARP-1 antibody. C, GST pull down assays were performed with HREC lysates. GST-fusion proteins were generated for GST-tagged LRP1 intracellular C-terminal domain (GST-ICD; a.a. 4445-4544 of human LRP1) and a truncated LRP1 intracellular C-terminal domain shortened (GST-ICDs; a.a. 4445-4511 of human LRP1) constructs, or GST as a negative control. Western blotting analysis was performed with anti-PARP-1 antibody. D, Lysates of HRECs were immunoprecipitated with anti-LRP1 antibody or control IgG and analyzed by Western blotting with an anti-PARP-1 antibody. E, Lysates of HRECs were immunoprecipitated with anti-PARP-1 antibody or control IgG and analyzed by Western blotting with an anti-LRP1 (LRP1-CTD) antibody. F, Hypoxia decreases the interaction of LRP1 and PARP-1 in HRECs. Lysates of HRECs following either normoxia (21% O2) or hypoxia (2% O2) exposure for 2 hours were immunoprecipitated with anti-LRP1 antibody or control IgG and analyzed by Western blotting with an anti-PARP-1 antibody. The associated PARP-1 full-length protein (PARP-1-FL, 116 kDa) and its cleaved form at 89 kDa (cleaved PARP-1) are quantified as percentages of total PARP-1 protein amount. *, P<0.05 via unpaired Student’s t-test, n=4.
LRP1 intracellular domain (~12 kDa) released by intramembrane proteolysis can translocate into nucleus and regulate signaling events22, 46. This processing of LRP1 is dependent on the presenillin-dependent γ-secretase activity. We asked whether hypoxia regulates the processing of this LRP1 intracellular domain. Using subcellular fractionation analysis, we observed that under normoxic condition, a fragment at ~12 kDa in the nucleus was detected with LRP1 C-terminal antibody (Figure SIV). When DAPT, an inhibitor of presenillin-dependent γ-secretase activity, was administrated, the ~25 kDa processed form of LRP1 was detected in the cytosolic fraction (Figure SIV). This confirms previous reports that the intracellular domain of LRP1 (~12 kDa) is processed from the ~25 kDa fragment by the presenillin-dependent γ-secretase activity22, 46. However, the protein level of the ~12 kDa fragment was not dramatically affected by hypoxia compared to normoxia, suggesting that hypoxia does not regulate the presenillin-dependent γ-secretase activity and the processing of the ~12 kDa LRP1 fragment. The association of LRP1 and PARP-1 is likely regulated through other unknown mechanisms.
PARP-1 is known to be associated with HIF1α47, which is a well-known regulator of hypoxia-dependent VEGF induction and angiogenesis48. Is the regulatory role of LRP1 in angiogenesis mediated through HIF1α-VEGF pathway? To answer this question, we determined the effect of LRP1 knockdown on the induction of HIF1α. As expected, hypoxia for 2 and 6 hours induced HIF1α protein in HRECs. Surprisingly, hypoxia-induced HIF1α protein decreased dramatically in LRP1 siRNA-transfected HRECs, compared to control siRNA-transfected cells (Figure SV). This observation indicates that the angiogenic effect resulted from LRP1 knockdown is not likely mediated through HIF1α-VEGF signaling pathway.
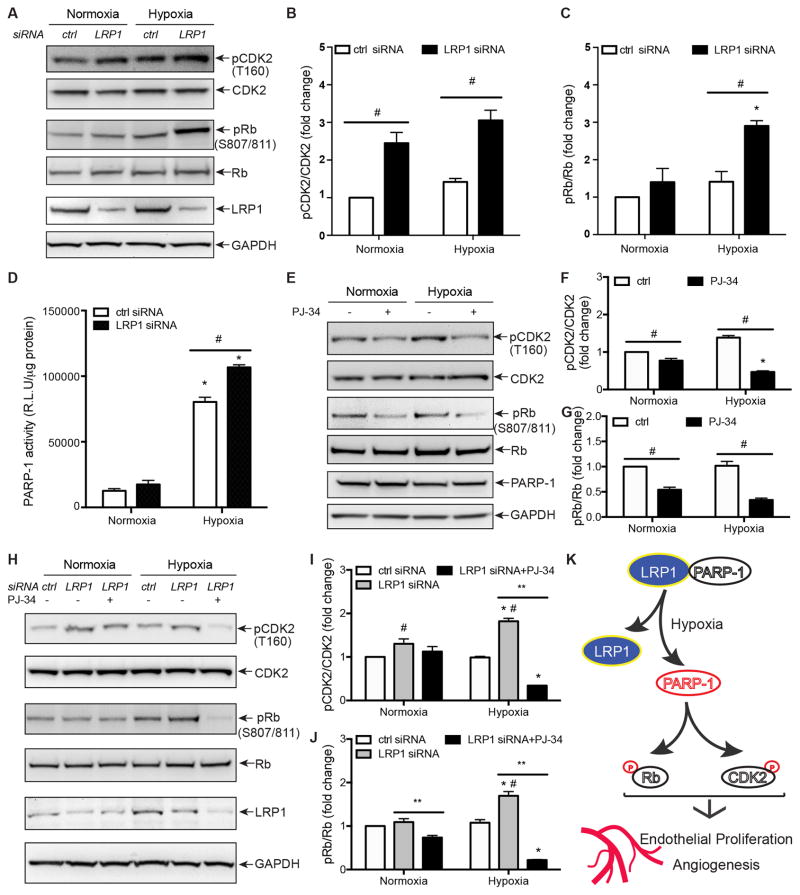
LRP1 Knockdown Increases the Phosphorylation of CDK2 and Rb by Enhancing PARP-1 Activity
Progression through the cell cycle is coordinated by the expression and activation of multiple components including cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors49. It has been reported that LRP1 depletion in MEFs increases the phosphorylation of retinoblastoma (Rb) and cyclin-dependent kinase 2 (CDK2)50, which are critical regulators for G1-S transition during cell cycle51, 52. Given that LRP1 knockdown increases the number of HRECs entering into S-phase (Figure 3G–H), we tested whether LRP1 affected cell cycle progression by modulating Rb and CDK2 activity. LRP1 knockdown in HRECs significantly increased CDK2 activity, detected by the specific phosphorylation at Thr 160 (Figure 5A and 5B). Similarly, LRP1 knockdown increased Rb phosphorylation at Ser 807/811 (Figure 5A and 5C), which represents the inactivation of Rb that is required for the release of sequestered E2F transcription factors. Given the activation of CDK2 and E2F induces the transcription of genes encoding proteins that are required for S-phase DNA synthesis51, 52, the enhanced G1-S transition and cell cycle progression due to LRP1 knockdown is likely mediated through the increase in CDK2 and Rb phosphorylation. Since PARP-1 is an important cell cycle regulator by interacting with p53 and affecting the expression and/or activation of cell cycle modulators including cyclin-dependent kinases CDKs and Rb53, 54, we tested whether PARP-1 is a mediator for LRP1 to regulate CDK2 and Rb activity. First, we determined whether PARP-1 activity is regulated by LRP1 in HRECs by performing PAR activity assay. As a stress signal, hypoxia dramatically enhanced PARP-1 activity (Figure 5D). Interestingly, LRP1 knockdown further increased PAR activity significantly (Figure 5D). Next, we tested whether the inhibition of PARP-1 enzymatic activity decreases the phosphorylation of CDK2 and Rb, and more importantly, ‘rescues’ the increase in the phosphorylation of CDK2 and Rb resulted from LRP1 knockdown. By using an inhibitor of PARP-1 activity- PJ-34 (Figure SVI), we demonstrated that the inhibition of PARP-1 activity indeed decreased phosphorylation of CDK2 and Rb at both normoxic and hypoxic conditions (Figure 5E–G). Interestingly, PJ-34 inhibited the increased phosphorylation of CDK2 and Rb induced by LRP1 knockdown (Figure 5H–J). Taken together, we conclude that LRP1 knockdown likely promotes cell cycle progression by regulating PARP-1 activity.
Figure 5. LRP1 knockdown increases PARP-1 activity-dependent phosphorylation of Rb and CDK2 in HRECs.
A–C, LRP1 knockdown increases CDK2 and retinoblastoma (Rb) phosphorylation. HRECs were transfected with LRP1 or control siRNA. Two days later, cells were incubated under normoxia (21% O2) or hypoxia (2% O2) for 2 hours. Cell lysates were immunoblotted with indicated antibodies. The ratios of the phosphorylated CDK2 and Rb to their total respective protein amounts were quantified by Image J (B and C). D, PARP-1 activity increased in LRP1 knockdown HRECs in response to hypoxia at 2% O2. ELISA enzymatic assay for PARP-1 was performed with PAR as a substrate of PARP-1 coated in the plate. Quantitative data were presented as a ratio of relative light unit to protein amount in cell lysates. E–G, PARP-1 inhibition decreases CDK2 and retinoblastoma (Rb) phosphorylation. HRECs were incubated with PARP-1 inhibitor PJ-34 at 3 μM and under normoxia (21% O2) or hypoxia (2% O2) for 2 hours. Cell lysates were immunoblotted with indicated antibodies. The ratios of the phosphorylated CDK2 and Rb to their total respective protein amounts were quantified by Image J (F and G). H–J, PJ-34 blocks the increases in CDK2 and retinoblastoma (Rb) phosphorylation following LRP1 knockdown. HRECs were transfected with LRP1 or control siRNA. Two days later, cells were treated with PARP-1 inhibitor PJ-34 at 3 μM and incubated under normoxia (21% O2) or hypoxia (2% O2) for 2 hours. Cell lysates were immunoblotted with indicated antibodies. The ratios of the phosphorylated CDK2 and Rb to their total respective protein amounts were quantified by Image J (I and J). K, Schematic illustration to shown how LRP1 regulates cell cycle progression, endothelial proliferation and retinal angiogenesis induced by hypoxia. *, P<0.05, compared to same cells under normoxia. #, P<0.05, compared to control HRECs exposed to same oxygen level. **, P<0.05, compared to LRP1 siRNA-transfected cells without treatment of PJ-34 inhibitors. n=3. All data were analyzed with two-way ANOVA analysis followed by the Fisher’s LSD or Turkey multiple comparison test.
In summary, our data indicate that LRP1 is a critical regulator of pathological angiogenesis and proliferation in the retinal endothelium. Importantly, we identify that LRP1 acts as a direct negative regulator of PARP-1, mediating CDK2 and Rb activity in the endothelium. We establish for the first time, this novel mechanism of explaining how LRP1, in part, negatively regulates endothelial cell proliferation and neovascularization in the hypoxic retina.
DISCUSSION
In this study, we have identified a regulatory role for LRP1 in pathological retinal angiogenesis. LRP1f/f;Cre+ mice with LRP1 depleted in endothelial cells display significantly more neovascularization response in the retina under hypoxic stress. This increase in vessel formation is, at least in part, contributed by the enhanced endothelial proliferative responses. We also uncover that PARP-1, a regulator of cell cycle progression, is negatively regulated by LRP1 through their dynamic interaction in response to hypoxia. These observations establish the notion that LRP1 is a critical regulator of angiogenesis and broaden our understanding of the functions that LRP1 exhibits in endothelial cells.
LRP1 is recognized as a multi-functional receptor that is involved in a variety of biological processes such as lipid metabolism, endocytosis and signal transduction6–8. In our previous study, LRP1 was discovered to be a novel regulator of zebrafish vascular development by regulating BMP signaling pathway16. BMP and BMPER are known as critical regulators of angiogenesis during development and disease conditions16, 28, 55–59. Our data demonstrate that LRP1 depletion in endothelial cells, similar to BMPER haploinsufficiency28, leads to increased retinal neovascularization in an OIR mouse model, suggesting that LRP1 might be a mediator for BMPER to regulate cell cycle progression and angiogenesis during OIR. Since multiple cellular events are involved in retinal angiogenesis, such as proliferation, apoptosis and migration, it is likely that LRP1 is also involved in other cellular processes. Our data clearly demonstrate that LRP1 negatively regulates proliferative angiogenesis in mouse retina at least in part through regulating cell cycle progression. Interestingly, the induction of HIF1α, a well-known angiogenic regulator, is not enhanced by LRP1 knockdown, suggesting that HIF1α is not likely a mediator of LRP1 depletion-dependent angiogenic effect. Further work is needed to elucidate how LRP1 regulates HIF1α-VEGF pathway and downstream effects. Although our proposed working model (Figure 5K) is oversimplified, it provides an initial framework into the role of LRP1 in endothelial function. We propose that, under normoxic conditions, LRP1 is associated with PARP-1 in endothelial cells. In response to mild hypoxia, the interaction of LRP1 and PARP-1 decreases. This dissociation, mimicked by our LRP1f/f;Tie2Cre+/− mice, may result in an increase in the PARP-1 enzymatic activity, which in turn leads to the hyperphosphorylation of Rb and activation of CDK2, and thereby promoting cell cycle progression. Combined, these molecular and cellular changes coordinately contribute to the proliferative and angiogenic effects in response to hypoxia.
PARP-1 is the founding and most studied member of the PARP family. It functions as a cellular stress sensor, directing cells to specific fates, such as DNA repair, survival, proliferation and cell death) based on the type and strength of the stress stimulus. Increasing evidence into the role of PARP-1 in the endothelium supports that PARP-1 is involved in endothelial dysfunction, atherosclerosis, restenosis and angiogenesis following stress and vascular injury30, 37, 54, 60. PARP inhibitors have been developed for therapeutic treatments of various cancers since it enhances the death of the malignant cells by interfering with cancer cell DNA repair61. Recent findings demonstrating that PARP inhibitors may inhibit angiogenesis by decreasing both growth factor expression and cell proliferation make it even more attractive cancer drug candidate42–45, 60, 62, 63. Our findings in this report provide novel mechanistic insights for PARP’s proliferative and pro-angiogenic roles via LRP1 regulation. Moreover, it indicates that the PARP-1 inhibitors can not only be applied to cancer but also therapeutically against other angiogenesis-related pathologies such as proliferative diabetic retinopathy and retinopathy of prematurity.
Poly(ADP-ribosyl)ation (or ‘PARylation’) is a chemical process that is catalyzed by PARP whereby PAR polymers are covalent attached on PARP itself and other acceptor proteins, including histones, DNA repair proteins, transcription factors, chromatin modulators30. Although we observe that LRP1 affects G1-S transition by modulating PARP-1 enzymatic activity, the detailed mechanism by which ‘PARylation’ regulates the phosphorylation of CDK2 and Rb remains elusive. One possible candidate could be p53 since it regulates CDK2 and Rb activity through the regulation of p2153 and the activity of p53 is regulated by ‘PARylation’ in response to DNA damage64. In addition, PARP-1 is proteolytically cleaved, mediating apoptosis. During apoptosis, PARP-1 is cleaved by caspase 3, and possibly other proteases, into C-terminal fragment (89 kDa) and N-terminal fragments (24 kDa)65. We observed both full length and cleaved PARP-1 at 89 kDa in HRECs in normoxia. However, 2% oxygen did not increase the amount of cleaved PARP-1 at 89 kDa (Figure 4E), indicating that PARP-1 cleavage-mediated apoptosis is not induced by hypoxia at 2% oxygen. Instead, HRECs exhibit enhanced cell growth and angiogenic responses (Figure 3B–E). Therefore, our data indicate that the PARP-1 enzymatic activity, but not the cleavage of PARP-1, is required for LRP1-dependent cell cycle regulation. In this study, we have determined the role of LRP1 in PARP-1-dependent signaling responses involved in cell cycle progression. In response to various ligands of LRP1, other PARP-1 dependent signaling pathways in endothelial cells could also be affected and lead to cellular responses such as chromatin structure remodeling and changes in DNA damage response and cell viability. The precise roles of LRP1 activity in these pathways remain to be determined. On the other hand, how PARP-1 and ‘PARylation’ affect LRP1-mediated cellular processes, including lipid metabolism, endocytosis and cellular signaling, becomes another interesting focus for the future studies.
Supplementary Material
SIGNIFICANCE.
Low density lipoprotein receptor-related protein 1 (LRP1) is a multifunctional member of the LDL receptor family, impacting a variety of biological processes such as lipid metabolism, endocytosis and signal transduction. However, the role of LRP1 in endothelium is almost unknown. Here we studied the functional roles of LRP1 in angiogenesis in oxygen-induced retinopathy mouse model. Our data reveal a critical role for LRP1 in the regulation of endothelial cell proliferation and neovascularization in the hypoxic retina. In addition, these data demonstrate for the first time a dynamic interaction of LRP1 and PARP-1 in endothelial cells. These data bring forth the possibility of novel therapeutic approaches for pathological angiogenesis such as proliferative diabetic retinopathy and retinopathy of prematurity.
Acknowledgments
We thank the Baylor College of Medicine Optical Imaging and Vital Microscopy Core, Histology Core and Mass Spectrometry Core Laboratories for their help.
SOURCES OF FUNDING
This work was supported by NIH R01s HL112890 (to X.P.) and HL061656 (to X.P.).
ABBREVIATIONS
- LRP1
low-density lipoprotein receptor related protein 1 (LRP1)
- PARP-1
poly(ADP-ribose) polymerase-1
- BMP
bone morphogenetic protein
- BMPER
BMP binding endothelial regulator
- OIR
oxygen-induced retinopathy
- GFAP
glial fibrillary acidic protein
- HREC
human retinal microvascular endothelial cells
- BrdU
5-bromo-2′-Deoxyuridine
- Rb
retinoblastoma
- CDK2
cyclin-dependent kinase 2
- PARylation
Poly(ADP-ribosyl)ation
- PJ-34
N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino)acetamide hydrochloride
Footnotes
DISCLOSURES
None.
References
- 1.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–6. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 4.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–24. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 5.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–83. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Boucher P, Herz J. Signaling through LRP1: Protection from atherosclerosis and beyond. Biochem Pharmacol. 2011;81:1–5. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Geer P. Phosphorylation of LRP1: regulation of transport and signal transduction. Trends Cardiovasc Med. 2002;12:160–5. doi: 10.1016/s1050-1738(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 9.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–21. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima C, Haffner P, Goerke SM, Zurhove K, Adelmann G, Frotscher M, Herz J, Bock HH, May P. The lipoprotein receptor LRP1 modulates sphingosine-1-phosphate signaling and is essential for vascular development. Development. 2014;141:4513–25. doi: 10.1242/dev.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–32. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 12.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RG, Herz J. LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome. PLoS One. 2007;2:e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–13. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 14.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–7. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 15.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, Fazio S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–95. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ, 3rd, Jin SW, Patterson C. LRP1-Dependent Endocytic Mechanism Governs the Signaling Output of the Bmp System in Endothelial Cells and in Angiogenesis. Circ Res. 2012;111:564–74. doi: 10.1161/CIRCRESAHA.112.274597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–41. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–7. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 19.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–82. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantuano E, Mukandala G, Li X, Campana WM, Gonias SL. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J Biol Chem. 2008;283:19904–11. doi: 10.1074/jbc.M801762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellano J, Aledo R, Sendra J, Costales P, Juan-Babot O, Badimon L, Llorente-Cortes V. Hypoxia Stimulates Low-Density Lipoprotein Receptor-Related Protein-1 Expression Through Hypoxia-Inducible Factor-{alpha} in Human Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2011;31:1411–20. doi: 10.1161/ATVBAHA.111.225490. [DOI] [PubMed] [Google Scholar]
- 24.Andras IE, Eum SY, Huang W, Zhong Y, Hennig B, Toborek M. HIV-1-induced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol Cell Neurosci. 2010;43:232–43. doi: 10.1016/j.mcn.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shimamura M, Yamashita T, Uchiyama Y, Rakugi H, Morishita R. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem. 2010;285:22091–102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.Connolly SE, Hores TA, Smith LE, D’Amore PA. Characterization of vascular development in the mouse retina. Microvasc Res. 1988;36:275–90. doi: 10.1016/0026-2862(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Miralles I, Ren R, Moser M, Hartnett ME, Patterson C. Bone morphogenetic protein endothelial cell precursor-derived regulator regulates retinal angiogenesis in vivo in a mouse model of oxygen-induced retinopathy. Arterioscler Thromb Vasc Biol. 2011;31:2216–22. doi: 10.1161/ATVBAHA.111.230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 30.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PARlaying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–67. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 31.Ding R, Pommier Y, Kang VH, Smulson M. Depletion of poly(ADP-ribose) polymerase by antisense RNA expression results in a delay in DNA strand break rejoining. J Biol Chem. 1992;267:12804–12. [PubMed] [Google Scholar]
- 32.Durkacz BW, Omidiji O, Gray DA, Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–6. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- 33.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–8. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 34.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–7. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber V, Hunting D, Trucco C, Gowans B, Grunwald D, De Murcia G, De Murcia JM. A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis, and sister chromatid exchange following DNA damage. Proc Natl Acad Sci U S A. 1995;92:4753–7. doi: 10.1073/pnas.92.11.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirier GG, de Murcia G, Jongstra-Bilen J, Niedergang C, Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982;79:3423–7. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–83. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson EL, Meadows R, Measel J. Cell cycle perturbations following DNA damage in the presence of ADP-ribosylation inhibitors. Carcinogenesis. 1985;6:711–4. doi: 10.1093/carcin/6.5.711. [DOI] [PubMed] [Google Scholar]
- 39.Nozaki T, Masutani M, Akagawa T, Sugimura T, Esumi H. Suppression of G1 arrest and enhancement of G2 arrest by inhibitors of poly(ADP-ribose) polymerase: possible involvement of poly(ADP-ribosyl)ation in cell cycle arrest following gamma-irradiation. Jpn J Cancer Res. 1994;85:1094–8. doi: 10.1111/j.1349-7006.1994.tb02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masutani M, Nozaki T, Wakabayashi K, Sugimura T. Role of poly(ADP-ribose) polymerase in cell-cycle checkpoint mechanisms following gamma-irradiation. Biochimie. 1995;77:462–5. doi: 10.1016/0300-9084(96)88161-2. [DOI] [PubMed] [Google Scholar]
- 41.Olcina M, Lecane PS, Hammond EM. Targeting hypoxic cells through the DNA damage response. Clin Cancer Res. 2010;16:5624–9. doi: 10.1158/1078-0432.CCR-10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tentori L, Muzi A, Dorio AS, Bultrini S, Mazzon E, Lacal PM, Shah GM, Zhang J, Navarra P, Nocentini G, Cuzzocrea S, Graziani G. Stable depletion of poly (ADP-ribose) polymerase-1 reduces in vivo melanoma growth and increases chemosensitivity. Eur J Cancer. 2008;44:1302–14. doi: 10.1016/j.ejca.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Albert JM, Cao C, Kim KW, Willey CD, Geng L, Xiao D, Wang H, Sandler A, Johnson DH, Colevas AD, Low J, Rothenberg ML, Lu B. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13:3033–42. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 44.Tentori L, Lacal PM, Muzi A, Dorio AS, Leonetti C, Scarsella M, Ruffini F, Xu W, Min W, Stoppacciaro A, Colarossi C, Wang ZQ, Zhang J, Graziani G. Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer. 2007;43:2124–33. doi: 10.1016/j.ejca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez MI, Peralta-Leal A, O’Valle F, Rodriguez-Vargas JM, Gonzalez-Flores A, Majuelos-Melguizo J, Lopez L, Serrano S, de Herreros AG, Rodriguez-Manzaneque JC, Fernandez R, Del Moral RG, de Almodovar JM, Oliver FJ. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013;9:e1003531. doi: 10.1371/journal.pgen.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 47.Elser M, Borsig L, Hassa PO, Erener S, Messner S, Valovka T, Keller S, Gassmann M, Hottiger MO. Poly(ADP-ribose) polymerase 1 promotes tumor cell survival by coactivating hypoxia-inducible factor-1-dependent gene expression. Mol Cancer Res. 2008;6:282–90. doi: 10.1158/1541-7786.MCR-07-0377. [DOI] [PubMed] [Google Scholar]
- 48.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–9. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 50.Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J Biol Chem. 2010;285:14308–17. doi: 10.1074/jbc.M109.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–69. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 52.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–6. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 53.Heinrichs S, Deppert W. Apoptosis or growth arrest: modulation of the cellular response to p53 by proliferative signals. Oncogene. 2003;22:555–71. doi: 10.1038/sj.onc.1206138. [DOI] [PubMed] [Google Scholar]
- 54.Kraus WL, Hottiger MO. PARP-1 and gene regulation: progress and puzzles. Mol Aspects Med. 2013;34:1109–23. doi: 10.1016/j.mam.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–12. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 56.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–79. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–53. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–82. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren R, Charles PC, Zhang C, Wu Y, Wang H, Patterson C. Gene expression profiles identify a role for cyclooxygenase 2-dependent prostanoid generation in BMP6-induced angiogenic responses. Blood. 2007;109:2847–53. doi: 10.1182/blood-2006-08-039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdallah Y, Gligorievski D, Kasseckert SA, Dieterich L, Schafer M, Kuhlmann CR, Noll T, Sauer H, Piper HM, Schafer C. The role of poly(ADP-ribose) polymerase (PARP) in the autonomous proliferative response of endothelial cells to hypoxia. Cardiovasc Res. 2007;73:568–74. doi: 10.1016/j.cardiores.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 61.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L, Pacher P. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun. 2006;350:352–7. doi: 10.1016/j.bbrc.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajesh M, Mukhopadhyay P, Godlewski G, Batkai S, Hasko G, Liaudet L, Pacher P. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem Biophys Res Commun. 2006;350:1056–62. doi: 10.1016/j.bbrc.2006.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M, Fukasawa K. Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol. 2007;9:1175–83. doi: 10.1038/ncb1638. [DOI] [PubMed] [Google Scholar]
- 65.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.