Abstract
Background
Electrical and magnetic brain stimulation can improve motor function following stroke in humans, rats and non-human primates, especially when paired with rehabilitative training (RT). Previously, we found in rodent stroke models that epidural electrical cortical stimulation (CS) of the ipsilesional motor cortex (MC) combined with motor rehabilitative training enhances motor function and motor cortical plasticity. It was unknown whether CS following experimental traumatic brain injury (TBI) would have similar effects.
Objective
To test the effects of CS combined with motor training after moderate/severe TBI on behavioral outcome and motor cortical organization.
Methods
Following unilateral controlled cortical impact (CCI) over the caudal forelimb area (CFA) of MC in adult male rats, forelimb reach training was administered daily over 9 weeks concurrently with sub-threshold 100Hz monopolar CS or no-stimulation control procedures. The rate and magnitude of behavioral improvements and changes in forelimb movement representations in the injured MC as revealed by intracortical microstimulation (ICMS) were measured.
Results
CCI resulted in severe motor impairments persisting throughout the 9 weeks of training in both groups, but CS treated animals had significantly greater behavioral improvements. CS also increased wrist motor cortical representation, one of the main movements used in the training task, compared to RT alone. However, the overall recovery level was modest, leaving animals still extremely impaired.
Conclusions
These data suggest that CS may be useful for improving rehabilitation efficacy after TBI but also raise the possibility that the CS parameters that are highly effective following stroke are suboptimal after moderate/severe TBI.
There are few well-established treatments that improve motor outcomes after TBI. In contrast, the stroke literature strongly supports that task-specific motor practice (rehabilitative training; RT) can greatly improve motor function. Adjunctive therapies, such as cortical stimulation (CS), can further enhance the efficacy of RT. In monkeys [1] and rats (e.g.[2–5]), CS of peri-infarct motor cortex (MC) coupled with impaired limb RT greatly enhances forelimb functional recovery and increases wrist representation area [3] and neuronal structural plasticity [6] in perilesion cortex.
While it seems reasonable to assume that treatments that reduce motor impairments following stroke would produce similar results following TBI, our data do not support this assumption. We and others have found that rehabilitative treatments, such as reach training, alone are insufficient to drive functional recovery following a controlled cortical impact (CCI) to MC [7, 8] and this may be, in part, due to more limited neural plasticity following CCI [4]. Thus, it was unknown if CS combined with RT would enhance behavioral function following CCI as it has following ischemia.
We investigated whether CS combined with RT would enhance the efficacy of RT and enhance the integrity of motor functioning after CCI. Seventeen male Long-Evans rats (~5 mo old) received a CCI centered over the caudal forelimb area (CFA) and then either received CS during RT (CS+RT; n=9) or RT alone (n=8) on a tray-reaching task. We used CS parameters previously found to be most effective following experimental stroke[2, 6, 9]. We also investigated whether CS+RT compared to RT alone increased cortical forelimb movement representation area, as previously found in stroke models[2, 3].
As previously described [9], rats were trained prior to surgery to criterion on the single-pellet reaching task with their preferred limb, then received a CCI of the CFA opposite the preferred reaching limb and epidural electrodes were implanted over remaining MC (see Supplemental Methods) [4, 7].
Tray reaching was used as RT, which required that animals reach for ~200 pellets placed on an inclined tray or for 20 min, whichever came first [7, 9]. All rats were attached to stimulator cables and placed into the reaching chamber. Only CS+RT animals received continuous stimulation delivered at 50% of that week’s Movement Threshold, defined as the minimal current necessary to produce visible movements of the forelimb, head or neck. No stimulation was ever delivered to the RT alone group and Movement Threshold was never assessed. Impaired forelimb function was probed with the single-pellet reaching task [6, 9] on days 8 and 9 post-CCI and then over 2 consecutive days after each week’s RT. Data were analyzed as the mean/week of the % success (pellet placed in mouth)/total reaches using repeated measures ANOVA. All probe tests were performed without CS.
After 9 weeks of RT, standard intracortical microstimulation (ICMS) mapping [2, 3, 10] was used to reveal the organization of movement representations in remaining MC of the injured hemisphere. The rostral forelimb area (RFA) and CFA were exhaustively mapped. Animals were then transcardially perfused with phosphate-buffered saline and 4% paraformaldehyde. Eight 50μ coronal sections, 600μ apart, through the cerebrum were Nissl stained for lesion volume analysis, calculated as: area of intact cortex – area of injured cortex X distance between sections.
As seen in Figure 1, CCI over CFA greatly impaired performance on the single pellet-reaching task in both groups compared to pre-injury [t(1,16) = 8.37, p<0.001]. All animals significantly improved in reaching success after 9 weeks of RT. However, the CS+RT group had a significantly greater magnitude of improvement over time compared to RT alone [DayXGroup: F(10, 150) = 2.39, p = 0.012]. There was a significant effect of Day [F(10, 150) = 18.37, p<0.001)] but no significant Group effect [F(1,15) = 1.93, p>0.05]. Post-hoc analysis indicated that there were significant differences between groups on weeks 4 and 5, p’s <0.05. Movement Thresholds significantly decreased from Week 1 (3.57±.61) compared to Week 9 (2.06±.18) in the CS+RT group [t(1,8)=2.622, p=0.013] and fell within our previously reported range following stroke [2, 6, 9].
Figure 1.
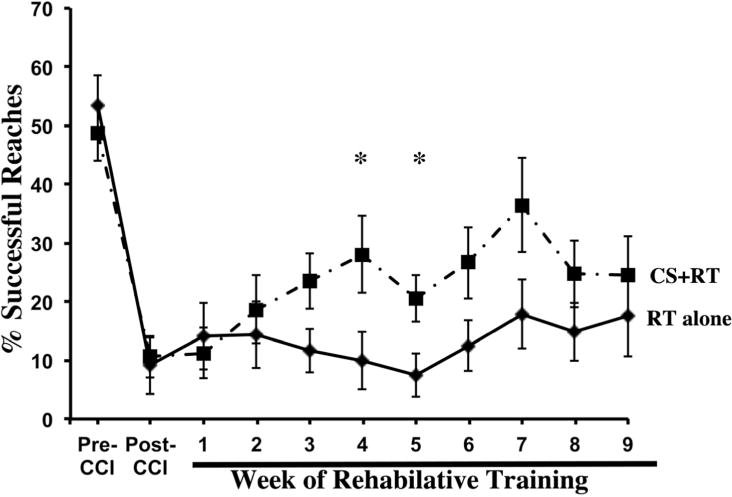
Following a unilateral CCI centered over the CFA, reaching performance with the impaired forelimb drastically declined. All animals improved after 9 weeks of RT, although far from pre-injury levels. CS+RT significantly increased reaching performance compared to RT alone. Data are means ±SEM. *p ≤ 0.05.
As seen in Figure 2, CS significantly increased the area of remaining MC (CFA+RFA) from which wrist [F(1,15) = 7.48, p=0.015], but not elbow [F(1,16)=.424, p > 0.05], movements were evoked at ≤ 100μA compared to RT alone. However, the mean wrist ICMS-evoked movement thresholds was not significantly different (p > 0.05) between CS+RT (61.1± 4.22) and RT alone (63.0±4.0). The RT alone group had no CFA and only sparse RFA wrist representation even at current levels above the standard ICMS upper boundary of 60μA, further suggestive of profoundly disrupted MC function. Only in animals that received CS+RT were we able to elicit wrist movements below 60μA and these thresholds were relatively high compared to our stroke CS+RT study [2]. There were no significant differences in injury volume between CS+RT (19.8 ± 3.85) and RT alone (22.5 ± 4.22) [F(1, 13)=.23, p=.64)].
Figure 2.
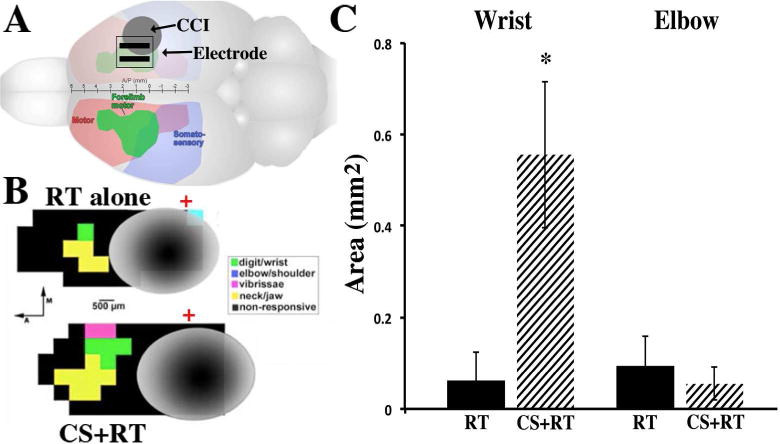
A, As seen in the rat brain schematic, CCI (grey circle) was induced over the forelimb overlap area of the sensory motor cortex (arrow). Epidural electrode contacts (parallel black bars) were placed rostral and medial to the injury over the remaining forelimb area (CFA and RFA, in green). B, Representative ICMS derived motor maps surrounding injured tissue (grey) following 9 weeks of RT alone (top) and CS+RT (bottom). Green squares are wrist, yellow are jaw, pink are whisker and black are non-responsive sites. Red crosses denote Bregma. C, CS significantly increased the total area of wrist movement representation compared to RT alone, but not elbow, movement representation area. Data are means ±SEM. *p<0.05
The organization and size of ICMS derived motor maps are thought to reflect intracortical synaptic connectivity contributing to the movement [10]. CCI profoundly diminished forelimb representations in remaining forelimb territory surrounding the contusion compared to non-injured animals in other TBI studies and following CFA focused ischemic lesions and CS+RT [2]. Although we do not have direct evidence in this study, presumably the loss of wrist representation reflects loss and dysfunction of forelimb movement circuits (i.e., [10]). This is consistent with the lack of robust improvements over 9 weeks of RT with or without CS. Following experimental stroke of CFA, robust improvements occur following 10 days–3 weeks of CS+RT compared to no-training or RT only[2, 3, 6, 9]. However, the greater wrist representation area in CS+RT compared to RT-alone likely reflects greater functional integrity of surviving circuits, and/or better interactions within these circuits, contributing to forelimb movements[2, 3, 10] consistent with its association with motor improvements.
While these findings do support that CS following TBI may be a beneficial adjunctive treatment, and CS was able to induce Movement Thresholds at the levels seen after stroke, CS+RT left considerable room for further improvement. The lack of stronger effects may be due to injury severity, as we have previously shown that CS effectiveness is reduced in severely impaired rats with ischemic CFA infarcts [6]. We have also previously found that more robust rehabilitation combinations (compared to stroke) are needed to drive motor improvements after similar CCIs [4, 7]. The CS parameters used in this study were optimized in stroke models and it is quite possible that a different frequency and/or intensity of CS, and its combination with more intense rehabilitation, would drive better motor outcomes and motor cortical reorganization.
Supplementary Material
Acknowledgments
Funding: This material is the result of work supported by NINDS NS065866 (DLA, TAJ, DAK).
References
- 1.Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurological research. 2003 Dec;25(8):801–10. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 2.Boychuk JA, Adkins DL, Kleim JA. Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil Neural Repair. 2011 Jan;25(1):88–97. doi: 10.1177/1545968310385126. [DOI] [PubMed] [Google Scholar]
- 3.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003 Dec;25(8):789–93. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 4.Jones TA, Liput DJ, Maresh EL, Donlan N, Parikh TJ, Marlowe D, et al. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma. 2012 May 1;29(7):1455–68. doi: 10.1089/neu.2011.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003 Dec;25(8):794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 6.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008 Jul;212(1):14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adkins DL, Ferguson L, Lance S, Pevtsov A, McDonough K, Stamschror J, et al. Combining Multiple Types of Motor Rehabilitation Enhances Skilled Forelimb Use Following Experimental Traumatic Brain Injury in Rats. Neurorehabil Neural Repair. 2015 Mar 11; doi: 10.1177/1545968315576577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010 Dec;27(12):2221–32. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Bryant AJ, Adkins DL, Sitko AA, Combs HL, Nordquist SK, Jones TA. Enduring Poststroke Motor Functional Improvements by a Well-Timed Combination of Motor Rehabilitative Training and Cortical Stimulation in Rats. Neurorehabil Neural Repair. 2014 Dec 19; doi: 10.1177/1545968314562112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002 Jan;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


