Abstract
Objective
Aortic valve disease, including calcification affects more than 2% of the human population and is caused by complex interactions between multiple risk factors including genetic mutations, the environment and biomechanics. At present, there are no effective treatments other than surgery and this is due to the limited understanding of the mechanisms that underlie the condition. Previous work has shown that valve interstitial cells (VICs) within the aortic valve cusps differentiate towards an osteoblast-like cell and deposit bone-like matrix that leads to leaflet stiffening and calcific aortic valve stenosis. However the mechanisms that promote pathological phenotypes in VICs are unknown.
Approach and Results
Using a combination of in vitro and in vivo tools with mouse, porcine and human tissue, we show that in VICs, reduced Sox9 expression and nuclear localization precedes the onset of calcification. In vitro, Sox9 nuclear export and calcific nodule formation is prevented by valve endothelial cells (VECs). While in vivo, loss of Tgfβ1 in the endothelium leads to reduced Sox9 expression and calcific aortic valve disease.
Conclusions
Together, these findings suggest that reduced nuclear localization of Sox9 in VICs is an early indicator of calcification and therefore pharmacological targeting to prevent nuclear export could serve as a novel therapeutic tool in the prevention of calcification and stenosis.
Introduction
Calcific aortic valve disease (CAVD) is the most prevalent valvular disorder accounting for approximately 55,000 hospitalizations and 15,000 deaths annually in the US.1 To date, no preventative medical therapies exist and valve replacement surgery remains the only effective treatment for this disease.2 Despite the high morbidity and mortality rates, the molecular mechanisms underlying CAVD remain largely unknown.1
The normal aortic valve (AoV) is composed of three cusps and function is largely achieved by a highly organized connective tissue consisting of three layers of diversified extracellular matrix (ECM) and two major cell populations. The ECM provides all the necessary biomechanical properties for coaptation during the cardiac cycle and predominantly consists of collagens, proteoglycans and elastin arranged relative to blood flow. The stratified ECM is established and maintained by valve interstitial cells (VICs) that reside within the core of mature cusps as quiescent fibroblast-like cells in the absence of disease. The valve cusps are encapsulated by a single layer of valve endothelial cells (VECs) that serve as a physical barrier between VICs and the hemodynamic environment. In addition, findings from in vitro studies suggest that VECs may influence VIC behavior and ECM production.3–5 Together, the extracellular and cellular components of the valve create an integrated and balanced connective tissue to maintain heart valve structure and function throughout life.
CAVD is a progressive disorder characterized by alterations in connective tissue homeostasis that result in valve stiffening and incomplete opening.6 While the precise etiology of CAVD remains unknown, mutations in NOTCH1 have been associated with AoV disease in humans,7 and a variety of non-genetic risk factors including diabetes, aging, hypertension, hypercholesterolemia, and smoking have been identified.8 CAVD pathogenesis is complex and although once thought of as a degenerative disorder, it is now considered an active process whereby quiescent VICs undergo phenotypic changes and ectopically express osteogenic markers including Runx2, Osteocalcin and Spp1 that facilitate deposition of mineralized ECM and formation of calcific nodules.3, 8, 9 These cellular and extracellular changes alter valve biomechanics and lead to a less flexible and more stiffened cusp that progressively results in stenosis and impaired blood flow. While the contribution of VICs in the formation of calcific nodules has been well studied in CAVD, little is known about the how this process is initiated.
We previously showed that the transcription factor Sox9 is highly expressed in VICs and plays a causative role in the onset of AoV disease.10, 11 Sox9fl/+;Col2a1-cre mice develop early onset calcification phenotypes with an associated downregulation of healthy cartilaginous ECM proteins.10, 11 These phenotypes are consistent with the diverse roles of Sox9 in positively regulating chondrogenic target genes (type II collagen, aggrecan, cartilage link protein) and repressing osteogenic markers (RUNX2, Spp1) in the developing skeletal system.9, 12–18 While our previous studies identified a causative role for reduced Sox9 function in CAVD in mice, the mechanisms of its regulation have yet to be determined. The valve endothelium has been shown to regulate VIC phenotypes in vitro 5, 19–21, and in human patients endothelial cell dysfunction accelerates the onset and progression of many cardiovascular diseases.22–25 In this current study, we examine the role of the valve endothelium in the regulation of Sox9 in CAVD and for the first time identify a hierarchical signaling pathway emanating from the VECs that is essential for maintaining Sox9 nuclear localization in VICs to prevent calcific nodule formation.
Methods
Detailed methods can be found in the online supplemental data.
Cell culture systems
Porcine AoV interstitial cells (pAVIC), valve endothelial cells (pAVEC), and murine VECs and Tgfβ1fl/fl AoV explants were isolated as described.26, 27 pAVICs were either cultured alone, co-cultured with endothelial cells, or treated with Tgfβ1, Leptomycin B, adenovirus against Cre (AdV-Cre), GFP (AdV-GFP) or Y27632 as described in the online supplemental data.
Generation of mice
Homozygotes Tgfβ1fl/fl;Nfatc1ENCre+ and Cre negative (Tgfβ1fl/fl;Nfatc1ENCre−) littermate controls were generated by breeding Tgfβ1fl/fl females (Jackson Laboratories) with Nfatc1ENCre28 males. Hypercholesterolemic Reversa mice and normocholesterolemic controls were obtained from Dr. Donald Heistad.29 Histological and molecular analyses were performed on AoVs from experimental and control mice at the indicated time points.
Human AoV specimens
Human diseased AoV specimens (n=3) were obtained from patients undergoing valve replacement surgery, while control AoVs (n=3) were collected from age-matched individuals at the time of autopsy who died of non-cardiac causes. Additional information can be found in online supplemental data.
Results
Sox9 nuclear localization is reduced in VICs and precedes calcification in vitro
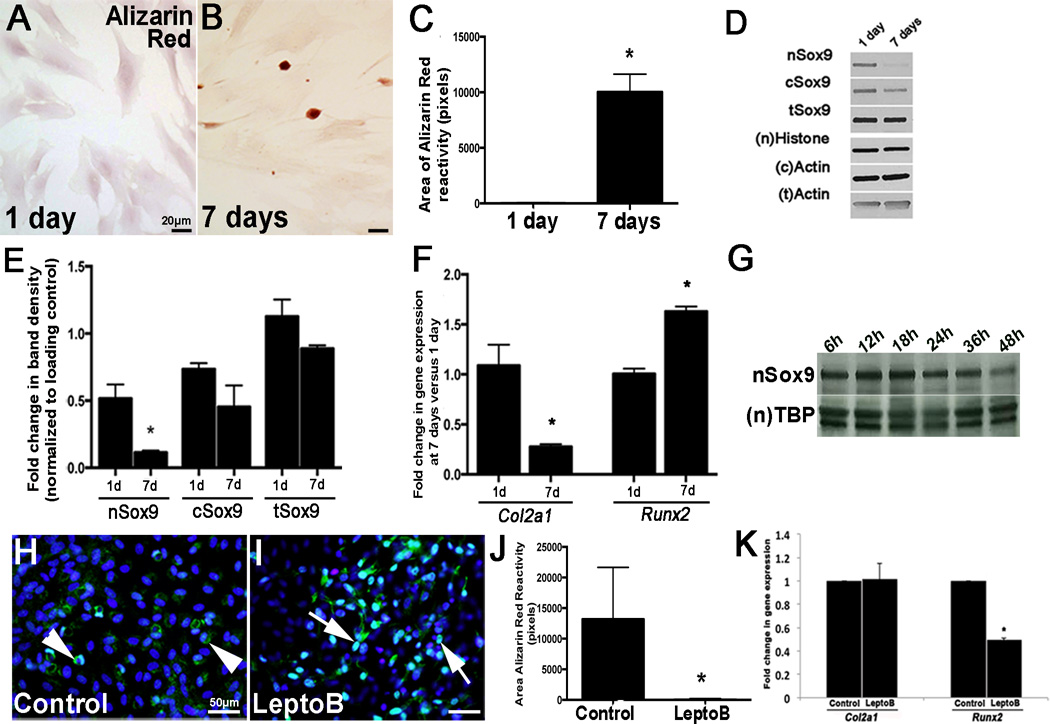
Reduced Sox9 function in vivo promotes calcific AoV phenotypes, suggesting a causative role.10, 11 To examine the regulatory mechanisms of Sox9 in the onset of calcification we utilized an in vitro porcine AoV VIC (pAVIC) calcification system. Following 7 days of culture on glass, pAVICs formed calcific nodules as detected by Alizarin Red staining (Figs. 1A–C). This was associated with reduced Sox9 expression and nuclear localization (Figs. 1D–E) and led to decreased Col2a1 (chondrogenic) and increased Runx2 (osteogenic) (Fig. 1F). To determine if these changes were associated with apoptosis, Cleaved Caspase-3 expression was examine by Western blot, but insignificant differences were observed between days 1 and 7 (Supplementary IIA). However, it should be noted that this approach using Western blot identifies Cleaved Caspase-3 expression in the cell population, and therefore associations between Alizarin Red reactivity and apoptosis cannot be made at the single cell level, but should be considered for future work. The progressive loss of Sox9 nuclear localization in cultured pAVICs began as early as 24 hours after culture (Fig. 1G), suggesting that this precedes calcific nodule formation detected at day 7. To determine if the nuclear export signal (NES) of Sox9 is required for this process, pAVICs were treated with 5ng/mL of the NES inhibitor Leptomycin B for 7 days. As shown, treatment retained Sox9 in the nucleus (Figs. 1I vs. 1H) and significantly attenuated Alizarin Red reactivity (Fig. IJ) and Runx2 expression (Fig. 1K). These studies suggest that Sox9 nuclear localization is reduced in VICs prior to the onset of calcification in vitro and this process is dependent upon the NES.
Figure 1. Nuclear Sox9 localization is reduced in pAVIC calcification assays.
(A, B) Alizarin Red staining to detect calcific nodule formation in pAVICs cultured for 1 (A) and 7 (B) days on glass. (C) Quantitation of Alizarin Red reactivity (in pixels, n=4). (D) Western blot analysis to show nuclear (n) and cytoplasmic (c) Sox9 in pAVICs cultured for 1, or 7 days. (E) Quantitation of Western blot shown in (D), normalized to respective loading controls. (F) qPCR to show changes in expression of Col2a1 and Runx2 in pAVICs cultured for 1, or 7 days. (G) Western blot analysis of nSox9 expression in pAVICs at the indicated time points. *, p<0.05 compared to 1 day cultures, n=3. (H, I) Immunohistochemistry to show Sox9 localization in control (H) or Leptomycin B treated pAVICs (I). (J) Quantitation of Alizarin Red positive nodule area. (K) qPCR to show Col2a1 and Runx2 expression in control and LeptomycinB-treated pAVICs. * p ≤ 0.05 compared to vehicle control.
Reduced Sox9 expression in VICs is associated with calcification in humans and mice
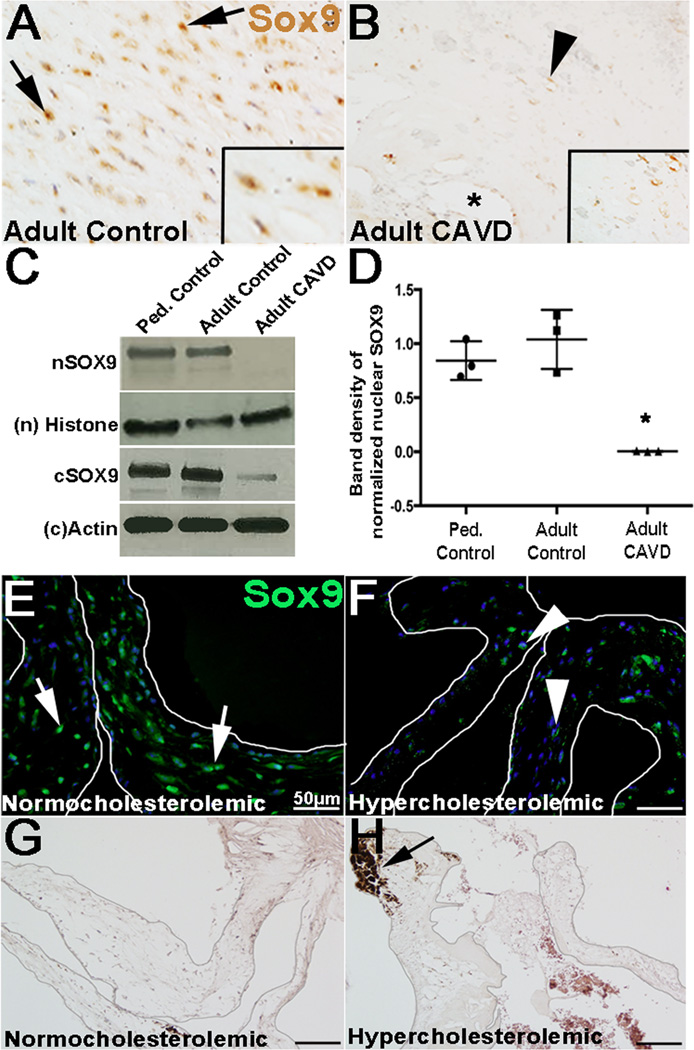
While our work has alluded to a role for Sox9 in valve calcification in mice10, 11 a similar role has not been reported in the human population. Heterozygous mutations of SOX9 in humans cause Campomelic Dysplasia and lethality is high during the neonatal period due to respiratory distress and echocardiograms are not routinely performed.30, 31 Therefore, causative links of SOX9 misexpression to human AoV disease has been challenging. Here, we examined correlations between Sox9 expression in valve tissue excised from humans (~70 years) undergoing surgical AoV replacement as a result of end-stage calcification and stenosis. As shown inII. 2B, SOX9 was significantly reduced in VICs located close to the calcific region (*, Fig. 2B) compared to age-matched non-diseased controls (arrows, Fig. 2A). Interestingly, of the remaining SOX9 expression in diseased valves, its localization was predominantly cytoplasmic (arrowhead, inset box Fig. 2B). By Western blot, nuclear SOX9 expression was undetectable in calcified adult valves while adult controls and pediatric non-calcified diseased valves expressed an abundance (Fig. 2C, D). Cytoplasmic SOX9 (cSOX9) was also reduced in CAVD patients, however this effect was not as dramatic as changes in nuclear expression. These findings suggest that reduced Sox9 expression correlates with CAVD in the human population, however as these samples were taken from end stage disease, we are unable to distinguish between cause and effect. Reversa mice serve as an established mouse model of hypercholesterolemia-induced CAVD (Figs. 2G, H), and by immunohistochemistry Sox9 expression was reduced in AoVs compared to normocholesterolemic controls (Figs. 2E, F). Interestingly, reduced Sox9 expression was noted in 11, but not 3 month old Reversa mice (Supplementary Figs. IIIC, D) prior to the onset of calcification detected at 22 months (Figs. 2G, H). Together, these findings suggest that reduced Sox9 expression is associated with AoV calcification in human patients and occurs prior to the deposition of calcified nodules in a hypercholesterolemic mouse model.
Figure 2. Sox9 expression is reduced in calcified valves from human patients and mouse models.
(A–B) Colormetric immunohistochemistry to detect Sox9 expression in VICs in AoV tissue sections isolated from non-diseased adult (~70 years of age) subjects (A) and age-matched control CAVD patients (B). Arrows indicate nuclear localization, arrowheads denote cytoplasmic expression, * shows calcific lesion. (C, D) Western blot (C) and quantitation (D, n=3) to show nuclear (n) and cytoplasmic (c) SOX9 expression in independent human samples collected from diseased, non-calcified pediatric controls (lane 1), non-diseased adult controls (lane 2) and age-matched control CAVD patients (lane 3). Immunofluorescence to detect Sox9 (green) expression (E, F) and Alizarin Red to stain calcific nodules (G, H) in AoVs isolated from Reversa hypercholesterolemic and normocholesterolemic control mice at 18 months of age. Arrow in E, F indicate nuclear localization, arrowheads denote cytoplasmic expression. Arrow in H shows calcific lesion. * p ≤ 0.05 compared to pediatric non-diseased controls.
Endothelial cells maintain Sox9 nuclear localization in VICs and attenuate calcification
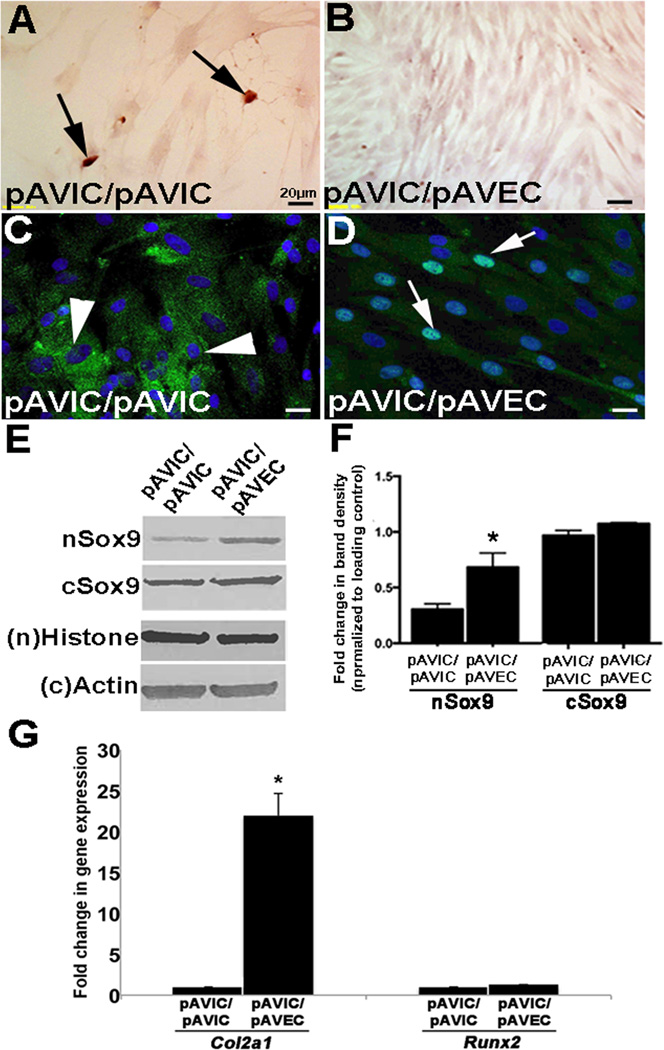
To examine the role of VECs in regulating Sox9 expression and calcification, pAVICs were co-cultured in a transwell assay in the absence (Figs. 3A, C) or presence (Figs. 3B, D) of porcine AoV endothelial cells (pAVECs). After 7 days, Alizarin Red detected calcification in pAVICs cultured alone (arrows, Fig. 3A), however in the presence of pAVECs, this was attenuated (Fig. 2B). Similar to Fig. 1D, calcification was associated with reduced Sox9 nuclear localization (Fig. 3C), however nuclear expression was retained by pAVECs (Fig. 3D). This finding was supported by Western blot showing increased Sox9 nuclear expression in pAVIC/pAVEC co-cultures (Figs. 3E, F), which was associated with increased expression of the transcriptional target gene Col2a1 (Fig. 3G). The ability of VECs to protect VICs against calcification by retaining Sox9 nuclear localization was also observed in human VIC (hVIC) co-cultures with HUVECs (Supplementary Fig. IV), suggesting conservation across species. As the transwell system is designed to prevent physical contact between endothelial cells and VICs, we hypothesized that the factor emanating from VECs to prevent calcification in VICs is secreted.
Figure 3. Endothelial cells prevent VIC-mediated calcification and promote Sox9 nuclear localization.
Alizarin Red staining to detect calcific nodules (arrows) (A, B) and immunohistochemistry to detect Sox9 expression and localization (C, D) in pAVICs cultured in the absence (A, C) or presence (B, D) of pAVECs, n=4. (E) Western blot analysis to show nuclear (n) and cytoplasmic (C) Sox9 in pAVICs co-cultured with pAVICs or pAVECs. (F) Quantitation of Western blot shown in (E), normalized to respective loading controls, based on n=3. (G) qPCR analysis of Col2a1 and Runx2 in pAVICs cultured in the absence and presence of pAVECs. *, p<0.05 compared to pAVIC/pAVIC experiments.
Tgfβ1 treatment is sufficient to promote Sox9 nuclear localization and prevent calcification of pAVICs
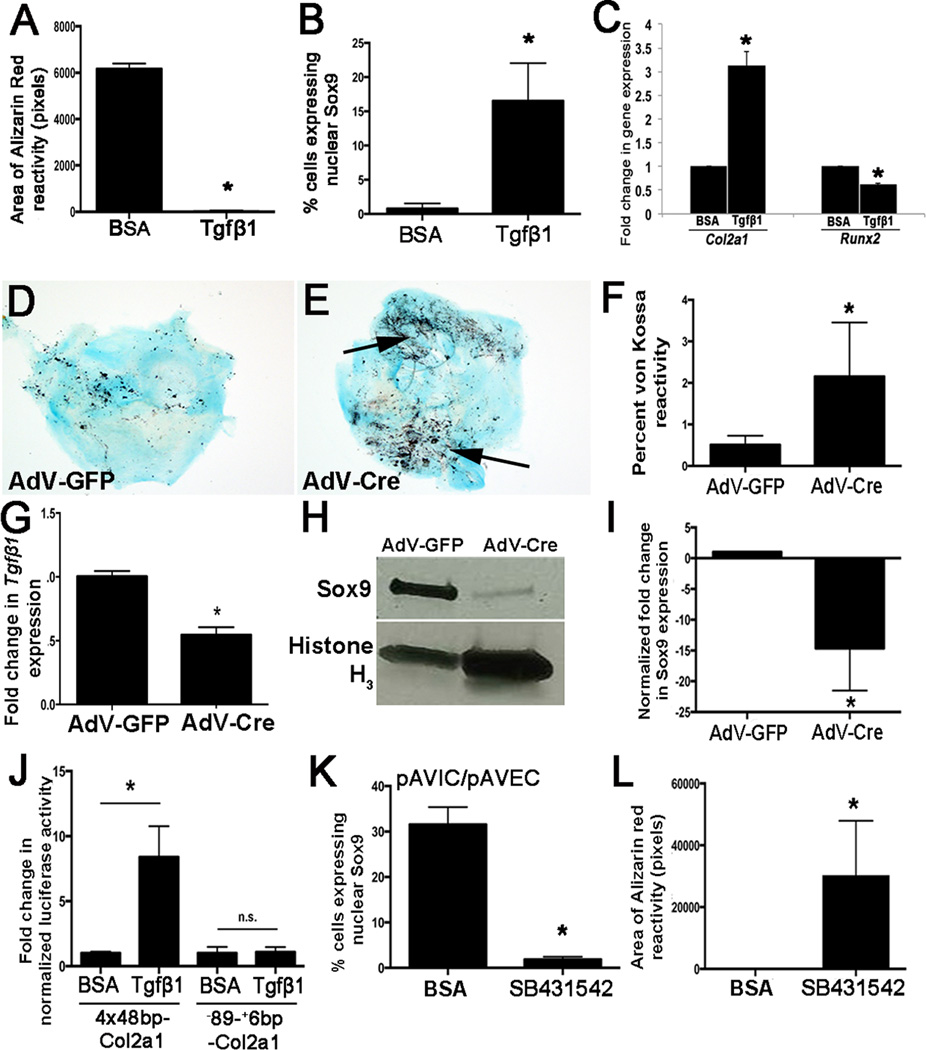
In heart valves, Sox9 expression and localization has been shown to be regulated by several signaling pathways including BMP2, Notch1 and β-catenin32–34. However based on their mechanisms of action and patterns of expression we excluded these as candidates for the VEC-mediated regulation of Sox9 in VICs. Tgfβ1 and its downstream signaling mediator pSmad2 are highly enriched in VECs compared to VICs 35(Supplementary Figs. VA–C). To determine if Tgfβ1 is sufficient to recapitulate the protective effects of VECs on VIC-mediated calcification, pAVICs were plated for 48 hours and treated with human recombinant TGFβ1 (10ng/ml) or BSA every 48 hours for an additional 5 days. As shown in Fig. 4A, TGFβ1 treatment attenuated calcification compared to BSA as indicated by Alizarin Red reactivity. In addition, TGFβ1 treatment re-established Sox9 nuclear localization in pAVICs (Figs. 4B) similar to co-culture with pAVECs, and this was associated with increased Col2a1 and decreased Runx2 (Fig. 4C). To further investigate this, whole post natal (PN) AoV explants from Tgfβ1fl/fl mice were cultured and treated with an adenovirus targeting Cre (AdV-Cre) or GFP (AdV-GFP) (Figs. 4D, E). von Kossa staining revealed that AdV-Cre treatment increased calcium deposition (Figs. 4D–F) as a result of Tgfβ1 knockdown (Fig. 4G) and decreased Sox9 expression (Figs. 4H, I). To further support a role for Tgfβ1 signaling in this process, pAVICs were co-cultured in a transwell assay with Tgfβ1fl/fl CD31+ murine cardiac endothelial cells and treated with AdV-Cre or AdV-GFP. After 48 hours co-cultured pAVICs treated with AdV-Cre similarly developed calcific nodules (Supplementary Figs. VD–F). To determine if TGFβ1 treatment affected Sox9 function, luciferase assays were performed in pAVICs using plasmids containing the minimal promoter and Sox9-responsive intron 1 of Col2a1 (4×48bp-Col2a1), or just the minimal promoter lacking SRY binding sites (−89-+6bp-Col2a1). As shown, TGFβ1 treatment increases the transcriptional activity of 4×48bp-Col2a1, and this was dependent on Sox9 response elements (Fig. 4J). To confirm that Tgfβ1 signaling emanating from VECs was responsible for maintaining Sox9 nuclear localization and preventing VIC-mediated calcification, transwell assays were repeated in the presence of the TGFβ1-receptor inhibitor, SB431542. As shown in Fig. 4K, SB431542 treatment reduced nuclear Sox9 expression (Supplementary Figs. VI, J) and enhanced calcification (Fig. 4L, Supplementary Figs. VG, H).
Figure 4. Tgfβ1 treatment prevents formation of calcific nodules by VICs and promotes Sox9 nuclear localization in VICs.
(A) Quantitation of Alizarin Red staining to detect calcific nodule formation in pAVICs treated with BSA or 10ng/mL TGFβ1 for 7 days. (B) Quantitation of the number of pAVICs expressing nuclear Sox9 over the total number of cells for each treatment. (C) qPCR of Col2a1 and Runx2 expression in BSA and TGFβ1 treated cells.*, p<0.05 compared to BSA controls, based on n=4. (D, E) von Kossa staining of AoV explants isolated from Tgfβ1fl/fl neonate pups and treated with AdV-Cre (E) or AdV-GFP (D). von Kossa reactivity is quantified in (F). (G) qPCR to show decreased Tgfβ1fl expression in AoV explant assays and Western blot to show reduced Sox9 expression relative to AdV-GFP treated controls (H, I). (J) Luciferase assay to show transcriptional activity of Col2a1 in response to BSA and TGFβ1 treatment. 4×48bp-Col2a1 contains the Col2a1 minimal mouse promoter and Sox9-responsive enhancer region of intron 1. −89-+6bp-Col2a1 contains the minimal promoter only, n=3. (K, L) Quantitation of nuclear Sox9 localization and Alizarin Red reactivity in pAVICs following co-culture with pAVECs and treated with BSA, or the TGFβ inhibitor, SB43152. *, p<0.05 compared to BSA controls, based on n=3.
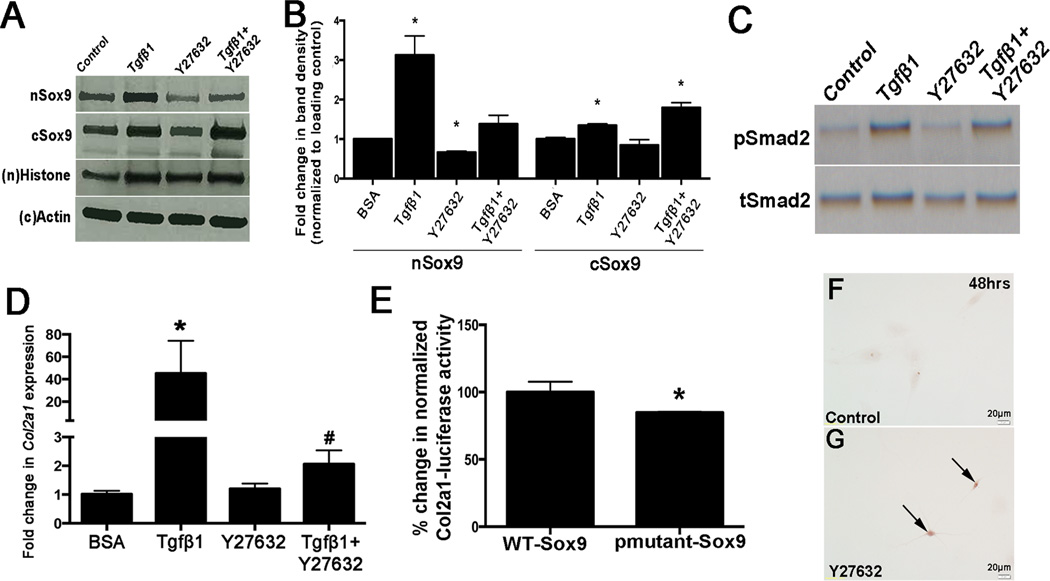
ROCK is required for Tgfβ1-mediated Sox9 regulation in pAVICs
In chondrocytes, Rho Kinase (ROCK) functions downstream of Tgfβ1,36 and therefore to determine if ROCK facilitates Tgfβ1-mediated Sox9 nuclear localization, pAVICs were pretreated with the inhibitor Y27632 prior to TGFβ1 exposure. While TGFβ1 treatment increased nuclear Sox9 expression in pAVICs (Fig. 5A, lane 2, Fig. 5B), pretreatment with Y27632 abolished this effect (Fig. VA, lane 4, Fig. VB). As expected, pSmad is increased with Tgfβ1 treatment, but the addition of the ROCK inhibitor does not significantly affect levels, suggesting that Smad and ROCK function through differential Tgfβ1-mediated signaling pathways (Fig. 5C). Col2a1 expression is increased in response to TGFβ1 and this affect appears to be dependent on ROCK activity (Fig. 5D). From these data, we speculated that ROCK might regulate Sox9 via phosphorylation and published reports have shown that phosphorylation at Serine (S) 64 and 181 drive nuclear localization.36 To test this, we performed luciferase assays and show that compared to wild-type Sox9, transactivation of Col2a1 was attenuated (~20%) when co-transfected with an S64 and S181 mutant (Fig. 5E). Interestingly, inhibition of ROCK activity alone by Y27632 was sufficient to increase Alizarin Red reactivity in cultured pAVICs after 48 hours (Figs. 5F, G). These observations suggest that Sox9 phosphorylation by ROCK may play a role VIC-mediated calcification.
Figure 5. Tgfβ1-mediated regulation of Sox9 expression requires Rho kinase.
Western blot analysis of nuclear (n) and cytoplasmic (c) Sox9 (A) and pSmad2 (C) expression in protein lysates collected from pAVICs treated with BSA (control), Tgfβ1, the ROCK inhibitor Y27632, or Tgfβ1 and Y27632. (B) Quantitation of A. (D) qPCR to detect changes in Col2a1 and Runx2 expression in treated cells. * p ≤ 0.05 compared to BSA control. (E) Luciferase assay to show Col2a1 (4×48bp-Col2a1) transcriptional activity in response to wild-type Sox9 (WT-Sox9) and a mutant Sox9 construct in which S64 and S181 phosphorylation sites have been abrogated (pmutant-Sox9). *, p<0.05 compared to WT-Sox9, based on n=3. (F, G) Alizarin Red staining to detect calcific nodule formation in pAVICs treated with BSA control or Y27632.
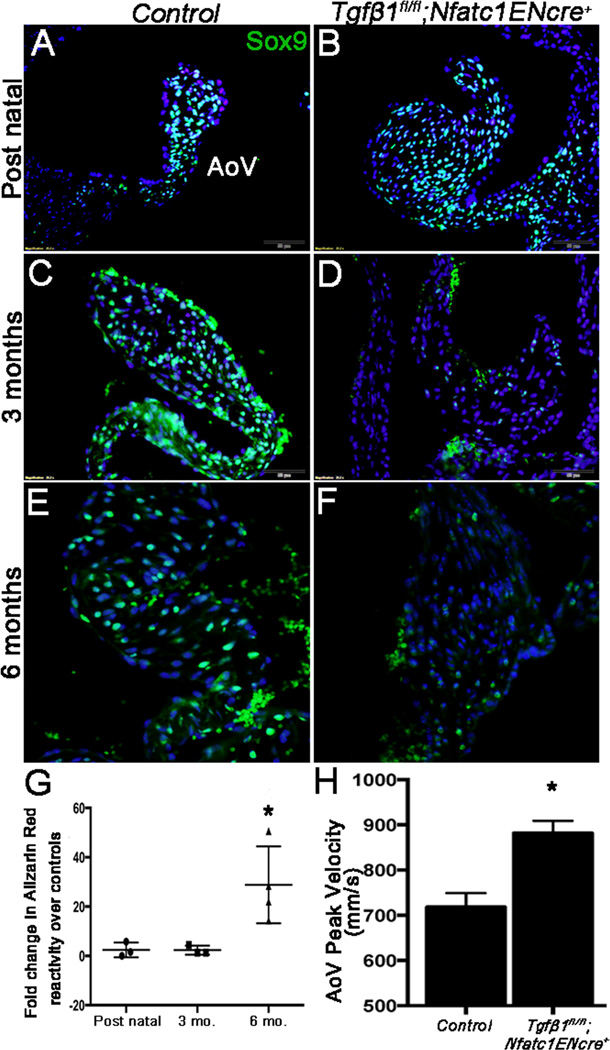
Targeted deletion of Tgfβ1 in VECs leads to aortic valve disease in vivo
To investigate if endothelial-Tgfβ1 plays a role in AoV disease in vivo, we targeted loss of function using an Nfatc1ENCre transgenic line that recombines in VECs, but not VICs or any other endothelial cells other than the early endocardium28 (Supplementary Fig. VIC). Using this approach, Tgfβ1 was ablated in VECs of Tgfβ1fl/fl;Nfatc1ENCre+ mice (Supplementary Figs. VIA, B) and subsequently pSmad2 expression was reduced (Supplementary Figs. VIIA, B) compared to littermate controls (Tgfβ1fl/fl;Nfatc1ENCre−) (Supplementary Fig. VIA). By post natal stages, AoVs from Tgfβ1fl/fl;Nfatc1ENCre+ mice were thickened and Sox9 was detected at high levels in VICs in the absence of calcification (Figs. 6A, B, G). At 3 months (Fig. 6D), Sox9 expression was dramatically reduced by immunohistochemistry (Fig. 6D) along with Col2a1 (Supplementary Fig. VIIC, D). In contrast Runx2 was increased (Supplementary Fig. VIIE, F), however Alizarin Red reactivity was still not detected (Fig. 6G). By 6 months, Sox9 remained low and positive Alizarin Red and von Kossa reactivity indicated calcific nodule formation (Figs. 6E–G, Supplementary Figs. VIE–J) in Tgfβ1fl/fl;Nfatc1ENCre+ mice. Similar to observations in vitro (Fig. 1A, B, Supplementary Figure IIIA), these phenotypes were not associated with increased cell apoptosis as determined by undetectable Cleaved Caspase-3 expression (Supplementary Figure IIIB–D). As Sox9 is a potent positive regulator of collagen, trichrome staining was performed at 6 months (Supplementary Figs. VII, J), however no significant differences were noted relative to increased thickness, although Col2a1 was reduced at 3 months (Supplementary Figs. VIIC, D). The phenotypic changes in cusp thickness and calcification at 6 months of age were associated with subtle, but significant increased AoV peak velocity (Fig. 6H) and blood flow regurgitation (Supplementary Figs. VIK, L) as determined by echocardiography. Together these data imply that loss of Tgfβ1 in VECs leads to reduced Sox9 expression in VICs at 3 months which precedes calcification onset and associated dysfunction detected at 6 months in vivo.
Figure 6. Targeted deletion of Tgfβ1 in murine VECs leads to decreased Sox9 expression, calcific nodule formation and AoV dysfunction in vivo.
(A–F) Immunohistochemistry to show Sox9 expression (green, arrows) in AoV from control (Tgfβ1fl/fl;Nfatc1ENCre) (A, C, E) and Tgfβ1fl/fl;Nfatc1ENCre+ (B, D, F) mice at post natal (A, B), 3 (C, D) and 6 months (E, F) of age. Representation images shown based on n=3. (G) Alizarin Red reactivity in Tgfβ1fl/fl;Nfatc1ENCre+ mice at each time point relative to age-matched controls, n=3. (H) Echocardiography to determine AoV peak velocity in Tgfβ1fl/fl;Nfatc1ENCre+ mice at 6 months of age compared to controls, n=6. AoV, Aortic valve. *p<0.05 compared to controls.
Discussion
Calcific aortic stenosis is the most predominant form of valve pathology affecting more than 25% of adults over the age of 65.1 At present, there are no effective treatments other than interventional surgery and pharmacological mechanistic-based therapies can only be developed if the regulatory processes that initiate CAVD onset and progression are identified. In this current study we expand on our previous work demonstrating a causative role for the transcription factor Sox9 in CAVD10, 11 and show that Tgfβ1 signaling from VECs is essential for promoting Sox9 nuclear localization in VICs to prevent calcification. In two CAVD mouse models (Tgfβ1fl/fl;Nfatc1ENCre+, hypercholesterolemia), and a calcification in vitro assay, reduced Sox9 expression in VICs preceded calcific nodule formation supporting a role during early stages of disease onset. This study directly shows that VEC dysfunction at the level of regulatory pathways is sufficient to promote CAVD and in addition highlight the potential of targeting Sox9 nuclear localization as a novel therapeutic strategy.
There is strong evidence to show that the process of calcification is mediated by VICs as a result of abnormal activation, apoptosis, ECM remodeling, and calcium deposition.37 However, based on findings from other cardiovascular diseases it has been speculated that dysfunction of the valve endothelium could also play a role. Here, we show that VECs prevent calcific nodule formation by VICs (Fig. 3, Supplementary Fig. IV) consistent with other reports demonstrating a protective role for the valve endothelium against disease processes.5, 19, 21, 38 In vivo, VECs are in direct contact with the hemodynamic environment and therefore exposed to shear stress and circulating signaling molecules, cytokines, and risk factors including cholesterol, lipids and inflammatory cells. As the VECs encapsulate the valve cusp VICs do not experience the same exposure, yet mediate pathological processes in response to abnormal mechanical stress or risk factor exposure.19, 20, 39 Therefore VECs likely serve as sensors and molecularly relay external information to underlying VICs within the leaflets during both pathological and physiological conditions and if damaged, lost or injured, these protective mechanisms are likely lost and the VICs lose their molecular communications and become directly exposed to the external environment.
In this current study we identify Tgfβ1 signaling as a critical VEC-mediated growth factor that positively regulates Sox9 expression and nuclear localization in VICs (Fig. 4) via ROCK (Fig. 5) to prevent calcification. Our findings also suggest that in addition to being anti-calcific, this pathway also promotes chondrogenic-like phenotypes (Figs. 1F, 3G, 4C) in pAVICs consistent with our previous work10 and suggesting pivotal roles in heart valve maintenance. Tgfβ1 is predominantly localized to VECs (Supplementary Fig. VIA),35 while Tgfβ2 and Tgfβ3 appear more widespread throughout the VIC population. These ligands, along with their receptors play multiple roles in valve development40, 41 and inhibition of Tgfβ signaling in mouse models of myxomatous degeneration alleviates valvular phenotypes, suggesting Tgfβ-dependency in disease states.42–49 We recognize that findings from our study are dissimilar to previous reports showing that Tgfβ1 treatment and ROCK inhibition are pro-osteogenic in cultured VICs.47, 49–51 The reasons for such disparities in response to increased Tgfβ1 signaling or Y27632 treatment in vitro are unclear, but could be dependent on the sensitivity of culture conditions including VIC passage number,52 species,53 cell contacts,54 substrate,51 endothelial cell contamination,19 Tgfβ1 dosage and endogenous Sox9 levels. In vivo, the pro-osteogenic dependency of Tgfβ1 in heart valves has not been reported, and this study is the first to suggest that regulated levels of Tgfβ1 secretion by VECs are required to maintain valve homeostasis and prevent calcification, while loss of function in VECs could be pathogenic. In vivo, the environment is very different from in vitro conditions and as Tgfβ and ROCK signaling are responsive to biomechanical cues, the hemodynamic environment or valve compliance could also influence their mechanisms of action on downstream targets and the pathological process.
While this study has shown that increased Tgfβ1 signaling in VECs prevents calcification mediated by VICs, systemic therapeutic targeting of Tgfβ1 could be problematic based on its wide-spread function in many systems and possible dosage dependency in the valves as discussed above. Therefore, nuclear retention of Sox9 in VICs would be an attractive alternative in the prevention of calcification. CRM1-dependent NES is required for nuclear export of Sox9 during the process of calcification as Leptomycin B treatment attenuated calcification in vitro (Figs. 1H–K). In addition, our findings suggest that promoting phosphorylation, or preventing phosphatase activity at S64 and S181 may be an additional mechanism to maintain Sox9 in the nuclei of VICs and prevent disease onset and progression (Fig. 5E). However, therapeutic targeting of Sox9 localization needs to be tightly regulated as we show that this transcription factor plays pivotal roles in osteogenic and chondrogenic programs. While reduced nuclear localization increases Runx2 (osteogenic), this is at the expense of Col2a1 (chondrogenic) (Fig. 1F). Similarly, nuclear retention of Sox9 in pAVICs by the presence of endothelial cells (Fig. 3G) or Tgfβ1 (Fig. 4C) attenuates calcification (Runx2), but significantly increases Col2a1. Therefore homeostatic mechanisms need to be considered.
Interestingly, while our data shows that calcification is associated with reduced Sox9 nuclear localization, we do not observe significant increases in cytoplasmic localization. This could be due to rapid degradation of cytoplasmic, or un-phosphorylated Sox9, and this may explain why an overall reduction in Sox9 expression and not nuclear localization is observed in Tgfβ1fl/fl;Nfatc1ENCre+ mice, however further work is required to test this. The relevance of nuclear Sox9 to prevent calcific nodule formation by VICs is intriguing and we, and others have shown that as a transcription factor, Sox9 binds and positively regulates cartilaginous matrix genes highly expressed in the valves and represses osteogenic gene programs associated with valve calcification including Spp1 and Runx2.9, 12–18 Therefore suggesting that in the nuclei of VICs, Sox9 plays pivotal transcriptional roles in promoting healthy (cartilaginous) phenotypes and preventing calcification, which are dysregulated in valve disease (Supplementary Fig. VIII). Interestingly, we observe Sox9 expression in valve endothelial cells of 3 month old wild type animals (Fig. 6C) and although this cell type do not typically undergo osteoblast-like changes, it is considered that Sox9 may play an additional role related to maintaining endothelial integrity, which if disrupted may have secondary effects on calcification.
Supplementary Material
Significance.
Heart valve disease is prevalent, yet the mechanisms underlying onset and progression are poorly understood. At present, there are no pharmacological therapies available to treat valve pathologies and surgical repair or replacement remains the only effective treatment. We previously showed that reduced Sox9 function in mice promotes calcific aortic valve disease (CAVD), however to date the mechanisms of this process remain elusive. Here, we demonstrate that reduced Sox9 expression and nuclear localization in VICs precedes the onset of calcification. In vitro, Sox9 nuclear export and calcific nodule formation is prevented by valve endothelial cells (VECs) and in vivo, loss of endothelial-derived Tgfβ1 signaling leads to reduced Sox9 expression and calcific aortic valve disease. Together, this work identifies a novel signaling pathway between VECs and VICs that is critical in the prevention of aortic valve disease.
Acknowledgements
We thank Scott Baldwin and Bin Zhou for sharing the Nfatc1ENCre mouse model, Michael Underhill and Martin Cheung for DNA plasmids, Kevin Bosse and Ning Zhou for technical support and Vidu Garg and Robert Weiss for intellectual input.
Sources of Funding. NIH HL091878, HL127033 (JL), NIH HL62984 (DDH), American Heart Association Grant in Aid (14GRNT19630003, JL) and Predoctoral Fellowship Awards (13PRE13930008, DJH).
Abbreviations
- AdV
Adenovirus
- AoV
Aortic valve
- CAVD
Calcific aortic valve disease
- ECM
Extracellular matrix
- FBS
Fetal bovine serum
- GFP
Green fluorescent protein
- HUVEC
Human umbilical vein endothelial cell
- hVIC
human valve interstitial cell
- mCEC
mouse cardiac endothelial cell
- NES
Nuclear export signal
- NLS
Nuclear localization signal
- pAVEC
Porcine aortic valve endothelial cell
- pAVIC
Porcine aortic valve interstitial cell
- ROCK
Rho Kinase
- Tgfβ
Transforming growth factor-β
- VEC
Valve endothelial cell
- VIC
Valve interstitial cell
Footnotes
Disclosures. The authors have nothing to disclose.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. The Journal of heart valve disease. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- 3.Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circulation research. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 4.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 5.Gould ST, Matherly EE, Smith JN, Heistad DD, Anseth KS. The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials. 2014;35:3596–3606. doi: 10.1016/j.biomaterials.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nature reviews Cardiology. 2014;11:218–231. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 8.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock JD, Huk DJ, Ediriweera HN, Lincoln J. Sox9 transcriptionally represses Spp1 to prevent matrix mineralization in maturing heart valves and chondrocytes. PloS one. 2011;6:e26769. doi: 10.1371/journal.pone.0026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Developmental biology. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circulation research. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nature genetics. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 13.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nature genetics. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 14.Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Developmental biology. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 15.Kou I, Ikegawa S. SOX9-dependent and -independent transcriptional regulation of human cartilage link protein. The Journal of biological chemistry. 2004;279:50942–50948. doi: 10.1074/jbc.M406786200. [DOI] [PubMed] [Google Scholar]
- 16.Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. The Journal of biological chemistry. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 17.Cheng A, Genever PG. SOX9 determines RUNX2 transactivity by directing intracellular degradation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:2680–2689. doi: 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19004–19009. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosse K, Hans CP, Zhao N, Koenig SN, Huang N, Guggilam A, LaHaye S, Tao G, Lucchesi PA, Lincoln J, Lilly B, Garg V. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. Journal of molecular and cellular cardiology. 2013;60:27–35. doi: 10.1016/j.yjmcc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards J, El-Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, Butcher JT. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. The American journal of pathology. 2013;182:1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circulation research. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 22.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Archives of pathology & laboratory medicine. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 23.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 24.Cavalca V, Tremoli E, Porro B, Veglia F, Myasoedova V, Squellerio I, Manzone D, Zanobini M, Trezzi M, Di Minno MN, Werba JP, Tedesco C, Alamanni F, Parolari A. Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: patterns and differences. Interactive cardiovascular and thoracic surgery. 2013;17:923–930. doi: 10.1093/icvts/ivt386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda S, Miyamoto T, Watanabe T, et al. A novel mouse model of aortic valve stenosis induced by direct wire injury. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:270–278. doi: 10.1161/ATVBAHA.113.302610. [DOI] [PubMed] [Google Scholar]
- 26.Gould RA, Butcher JT. Isolation of valvular endothelial cells. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circulation research. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN and, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 31.Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 32.Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, Merrill WH, Hinton RB, Garg V. Inhibitory role of Notch1 in calcific aortic valve disease. PloS one. 2011;6:e27743. doi: 10.1371/journal.pone.0027743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang M, Alfieri CM, Hulin A, Conway SJ, Yutzey KE. Loss of beta-Catenin Promotes Chondrogenic Differentiation of Aortic Valve Interstitial Cells. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2601–2608. doi: 10.1161/ATVBAHA.114.304579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Developmental biology. 2006;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 35.Molin DG, Bartram U, Van der Heiden K, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC. Expression patterns of Tgfbeta1–3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;227:431–444. doi: 10.1002/dvdy.10314. [DOI] [PubMed] [Google Scholar]
- 36.Haudenschild DR, Chen J, Pang N, Lotz MK, D'Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis and rheumatism. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chester AH, El-Hamamsy I, Butcher JT, Latif N, Bertazzo S, Yacoub MH. The living aortic valve: From molecules to function. Glob Cardiol Sci Pract. 2014;2014:52–77. doi: 10.5339/gcsp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue engineering. 2006;12:905–915. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 39.Arjunon S, Rathan S, Jo H, Yoganathan AP. Aortic valve: mechanical environment and mechanobiology. Annals of biomedical engineering. 2013;41:1331–1346. doi: 10.1007/s10439-013-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lincoln J, Garg V. Etiology of valvular heart disease. Circulation journal : official journal of the Japanese Circulation Society. 2014;78:1801–1807. doi: 10.1253/circj.cj-14-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnette DN, Hulin A, Ahmed AS, Colige AC, Azhar M, Lincoln J. Tgfbeta-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves. Journal of molecular and cellular cardiology. 2013;65:137–146. doi: 10.1016/j.yjmcc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. The Journal of clinical investigation. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munjal C, Opoka AM, Osinska H, James JF, Bressan GM, Hinton RB. TGF-beta mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease. Disease models & mechanisms. 2014;7:987–996. doi: 10.1242/dmm.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovascular research. 2013;99:175–184. doi: 10.1093/cvr/cvt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne JO, Nusgens BV, Radermecker MA, Colige AC. Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovascular research. 2012;93:480–489. doi: 10.1093/cvr/cvr337. [DOI] [PubMed] [Google Scholar]
- 46.Kim L, Kim do K, Yang WI, Shin DH, Jung IM, Park HK, Chang BC. Overexpression of transforming growth factor-beta 1 in the valvular fibrosis of chronic rheumatic heart disease. Journal of Korean medical science. 2008;23:41–48. doi: 10.3346/jkms.2008.23.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. The Annals of thoracic surgery. 2007;83:946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circulation research. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 50.Das D, Holmes A, Murphy GA, Mishra K, Rosenkranz AC, Horowitz JD, Kennedy JA. TGF-beta1-Induced MAPK activation promotes collagen synthesis, nodule formation, redox stress and cellular senescence in porcine aortic valve interstitial cells. The Journal of heart valve disease. 2013;22:621–630. [PubMed] [Google Scholar]
- 51.Gu X, Masters KS. Role of the Rho pathway in regulating valvular interstitial cell phenotype and nodule formation. American journal of physiology Heart and circulatory physiology. 2011;300:H448–H458. doi: 10.1152/ajpheart.01178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pho M, Lee W, Watt DR, Laschinger C, Simmons CA, McCulloch CA. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. American journal of physiology Heart and circulatory physiology. 2008;294:H1767–H1778. doi: 10.1152/ajpheart.01305.2007. [DOI] [PubMed] [Google Scholar]
- 53.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. The Annals of thoracic surgery. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-6. [DOI] [PubMed] [Google Scholar]
- 54.Bowen CJ, Zhou J, Sung DC, Butcher JT. Cadherin-11 coordinates cellular migration and extracellular matrix remodeling during aortic valve maturation. Developmental biology. 2015;407:145–157. doi: 10.1016/j.ydbio.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.