Abstract
The long non-coding RNA CDKN2B-AS1, commonly referred to as the Antisense Non-coding RNA in the INK4 Locus (ANRIL), is a 3.8-kb-long RNA transcribed from the short arm of human chromosome 9 on p21.3 that overlaps a critical region encompassing three major tumor suppressor loci juxtaposed to the INK4b-ARF-INK4a gene cluster and the methyl-thioadenosine phosphorylase (MTAP) gene. Genome-wide association studies have identified this region with a remarkable and growing number of disease-associated DNA alterations and single nucleotide polymorphisms, which corresponds to increased susceptibility to human disease. Recent attention has been devoted on whether these alterations in the ANRIL sequence affect its expression levels and/or its splicing transcript variation, and in consequence, global cellular homeostasis. Moreover, recent evidence postulates that ANRIL not only can regulate their immediate genomic neighbors in cis, but also has the capacity to regulate additional loci in trans. This action would further increase the complexity for mechanisms imposed through ANRIL and furthering the scope of this lncRNA in disease pathogenesis. In this chapter, we summarize the most recent findings on the investigation of ANRIL and provide a perspective on the biological and clinical significance of ANRIL as a putative biomarker, specifically, its potential role in directing cellular fates leading to cancer and cardiovascular disease.
1 The DNA and RNA Landscape Overlapping Chr9p21 Loci
A growing number of genome-wide association studies (GWASs) have identified specific regions of the human genome with a strong non-random correlation to complex human traits with predisposition to disease (de los Campos et al. 2010). Indeed, several single nucleotide polymorphisms (SNPs) have been identified on the INK4b-ARF-INK4a locus located on the human chromosome 9p21 that are tightly related with the increase of cardiovascular disease (CVD) (de los Campos et al. 2010; Gschwendtner et al. 2009) ischemic stroke (Gschwendtner et al. 2009; Matarin et al. 2008), aortic aneurysm (Helgadottir et al. 2008), type II diabetes (Zeggini et al. 2007; Scott et al. 2007), glioma (Shete et al. 2009; Wrensch et al. 2009), and cancer predisposition (Shete et al. 2009; Wrensch et al. 2009; Cunnington et al. 2010; Bishop et al. 2009), among other conditions.
The INK4b-ARF-INK4a locus encodes three critical tumor suppressor genes, p14ARF (p19ARF in mice), p15INK4b, and p16INK4a, all of which play a central role in cell-cycle arrest, thus affecting key cellular processes such as senescence, apoptosis, and stem cells self-renewal by triggering the activities of both retinoblastoma (Rb) and p53 pathways (Gil and Peters 2006; Popov and Gil 2010). Specifically, p15INK4b and p16INK4a target cyclin-dependent kinases CDK4 and CDK6, preventing the binding of these proteins to D-type cyclins and, as a consequence, inhibiting CDK4/6-mediated phosphorylation (inactivation) of retinoblastoma (RB1) family members. In contrast, the unrelated p14ARF protein acts primarily by binding to the E3 ubiquitin-protein ligase MDM2, promoting its degradation, and therefore abrogating MDM2 inhibition of the TRP53 activity (Popov and Gil 2010). The locus contains a fourth gene, methylthioadenosine phosphorylase (MTAP), which has annotated exons overlapping the INK4b-ARF-INK4a locus (Nobori et al. 1996). MTAP catalyzes the phosphorylation of 5′methyladenosine (MTA) in the polyamine pathway, and it has also been associated with cancerogenesis (Behrmann et al. 2003; Schmid et al. 1998).
The long non-coding RNA ANRIL (Antisense Non-coding RNA in the INK4 Locus) was first identified within the 403-kb germ-line deletion of a French family with a history of melanoma and neural system tumors (Pasmant et al. 2007). ANRIL is transcribed as a 3,834-bp lncRNA in the opposite direction from the INK4b-ARF-INK4a cluster (Yu et al. 2008), and it shares a bidirectional promoter with p14ARF, as the 5′ end of the first exon of ANRIL is located 300 bp upstream of the transcription start site (TSS) of the p14ARF gene. Hence, the expression of both genes is coordinated, and reporter assays have shown a transcriptional activation of this divergent promoter by E2F1 and the insulator CTCF (Sato et al. 2010; Rodriguez et al. 2010). Specifically, CTCF binding is required to maintain the INK/ARF locus in an inducible conformation, which is abrogated upon DNA methylation, having consequences in cancer progression (Rodriguez et al. 2010).
ANRIL transcript contains 20 exons, many of them consisting of LINE, SINE, and Alu repetitive elements (Jarinova et al. 2009), that can be alternatively spliced. ANRIL transcripts are expressed at very low levels, and the two short forms, both terminating with polyadenylated exon 13, EU741058 (exons 1, 5, 6, 7, 13) and DQ485454 (exons 1–13), and the long form NR_003529 that lacks the exon 13 and terminates with polyadenylated exon 20 (exons 1–20), are the most abundant transcripts. Circular ANRIL (cANRIL) isoforms have also been described (Burd et al. 2010), which result from exon skipping events occurring during RNA splicing. Thus, cANRIL show non-sequential linkages between various ANRIL exons, appearing species like exons 4–6 and 14–5, to name some examples. A fusion transcript between the MTAP gene and the 3′ end of ANRIL has also been identified in cell lines with 9p21 deletion but not in normal cell lines (Burd et al. 2010; Schmid et al. 2000). Many of the ANRIL isoforms can coexist in the same cell type although others are tissue-specific (Burd et al. 2010; Folkersen et al. 2009), increasing the complexity of its regulatory mechanism. These alternative splicing events might modify ANRIL structure leading to changes not just in Polycomb group (PcG) proteins-mediated INK4b-ARF-INK4a locus regulation. In fact, the overexpression of exons 13–19 in HeLa cells resulted in the repression of a wide set of genes involved in chromatin architecture remodeling, being Centrosomal protein 290 kDa (CEP290), E1 A binding protein p300 (EP300), and transcription factor 7-like 1 (TCF7L1) the most repressed proteins (Sato et al. 2010). Interestingly, Ras responsive element binding protein 1 (RREB1) and Zinc finger and BTB domain containing 32 (ZBTB32) were upregulated upon ANRIL 13–19 overexpression (Sato et al. 2010).
2 ANRIL and Polycomb Group Proteins
The PcG proteins were originally identified in Drosophila melanogaster, as transcriptional repressors of homeotic (Hox) genes, required for the correct spatiotemporal expression of developmental regulators along the body axis (Lewis 1978). In most metazoan species, the PcG proteins form two macromolecular repressive complexes named polycomb repressive complex-1 (PRC1) and polycomb repressive complex-2 (PRC2) (Levine et al. 2002). The PRC2 complex consists of three subunits: embryonic ectoderm development (EED), suppressor of zeste 12 (SUZ12), and enhancer of zeste 2 or 1 (EZH2/1), which catalyze the mono-, di-, and trimethylation of lysine 27 of histone H3 (H3K27me1, H3K27me2, and H3K27me3) (Margueron et al. 2008; Shen et al. 2008). H3K27me3 is a signature for chromobox-domain (CBX) protein recognition and PRC1 recruitment. The PRC1 composition is heterogeneous, depending on the cellular context, and contains several PcG proteins, including one member of the PCGF family (PCGF1-PCGF6) and of the HPH family (HPH1-HPH3), together with chromobox-domain (CBX) protein and RING1a/1b, which catalyze the monoubiquitination of H2a on K119 (H2AK119ub1) for the maintenance of silent chromatin (Cao et al. 2005; Wang et al. 2004).
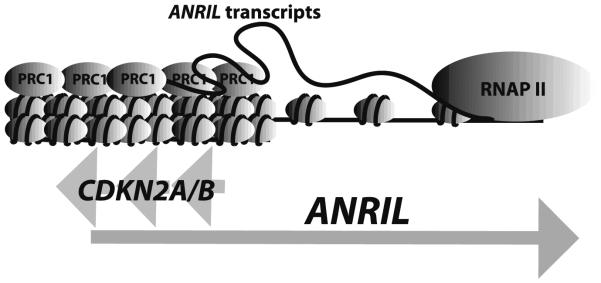
Several long non-coding RNAs have a direct role in recruiting PcG proteins to specific loci to modify the epigenetic chromatin state and thereby to repress gene expression. Some documented examples include XIST RNA (Mak et al. 2002; Zhao et al. 2008), KCNQTLOT1 (Fitzpatrick et al. 2002; Pandey et al. 2008), HOTAIR (Rinn et al. 2007) and ANRIL (Kotake et al. 2011; Yap et al. 2010). Indeed, ANRIL specifically associates with the chromodomain of chromobox homolog 7 (CBX7), a subunit of the PRC1 complex, and participates in CBX7 recognition of H3K27me3 to silence the INK4b-ARF-INK4a cluster (Yap et al. 2010). This interaction is abolished after treatment of cell nuclei with the transcriptional inhibitor α amanitin, indicating that ANRIL is stably associated with CBX7 as a nascent transcript generated by the RNA polymerase II. Moreover, knockdown of ANRIL decreases H3K27me3 levels and it is associated with increased p16INK4a expression, which coincides with a reduction in CBX7 and EZH2 binding at the p16INK4a TSS (Yap et al. 2010). Overall, this mechanism is important for the INK4b-ARF-INK4a locus repression in order to control senescence [reviewed by (Aguilo et al. 2011)] (Fig. 1).
Fig. 1.
Illustration of how the ANRIL transcript may facilitate polycomb repressive complex 1 to compact chromatin structure of the INK4b-ARF-INK4a locus
On the other hand, ANRIL can also interact with the PRC2 component SUZ12 and influence SUZ12 binding to the p15INK4b locus. Thus, depletion of ANRIL increases the expression of p15INK4b, but not p16INK4a or p14ARF, and inhibits cellular proliferation, thereby influencing human disease progression (Aguilo et al. 2011). Recently, RIP sequence (RIP-seq) experiments performed in MonoMac cells in which two specific exon-combinations of ANRIL were overexpressed, showed a binding of ANRIL with CBX7 and RING1B from PRC1, a binding with the PRC2 subunits EED, JARID2, RBAP46, and SUZ12, and PRC-associated proteins RYBP and YY1 (Holdt et al. 2013).
3 ANRIL and Cardiovascular Disease
CVD covers a wide array of disorders, including diseases of the cardiac muscle and of the vascular system supplying the heart, brain, and other vital organs.
ANRIL locus has been highlighted as the strongest genetic susceptibility locus for CVD, being numerous polymorphisms located in this locus directly associated with increased risk of developing CVD (Cunnington et al. 2010; Folkersen et al. 2009; Holdt et al. 2010; Holdt and Teupser 2012; Liu et al. 2009). In particular, the coronary artery disease (CAD)-associated SNPs are located on chromosome 9p21.3, specifically in a linkage disequilibrium block that does not contain known protein-coding genes, spanning a region of 58-kb named the CAD interval (Guttman et al. 2009). For example, the SNP rs496892-G is linked to atherosclerotic stroke, whereas the rs10757276-G is the lead SNP for CVD risk. These polymorphisms affect the expression of ANRIL (Holdt et al. 2010; Congrains et al. 2012), which in turn regulates the expression of downstream genes involved in several atherogenic pathways and/or inflammation response. For example, decreased expression of ANRIL transcripts containing exon 13 correlates with decreased expression of adiponectin receptor 1 (ADIPOR1), vesicle-associated membrane protein 3 (VAMP3), and transmembrane protein 258 (C11ORF10) (Bochenek et al. 2013).
Another possibility is that the polymorphism in the CAD interval may affect ANRIL splicing and, as a consequence, ANRIL structure. Specifically, two SNPs (rs.7341786 and rs7341791) identified in the exon 15, from where most of the cANRIL transcripts arise, were shown to be in linkage disequilibrium with the ASVD-associated SNP rs1075728 and were predicted to increase the ability of exon 15 of acting as splice acceptor. Furthermore, individuals harboring the casual variants mentioned above exhibit a derepressed INK4b-ARF-INK4a expression (Burd et al. 2010), indicating that the alteration of ANRIL structure may affect the efficiency of ANRIL at repressing the INK4b-ARF-INK4a locus.
Additionally, many of the polymorphisms in the 9p21 locus can also disrupt predicted transcription factor binding sites (Harismendy et al. 2011). For instance, rs564398, one of the SNPs most strongly correlated with ANRIL expression, disrupts ‘Ras Responsive Element Binding Protein 1’ (RREB1) binding site, and the SNP (rs10757278) disrupts the binding of the STAT1 (Signal-transducer and activator of transcription) transcription factor, increasing CVD susceptibility (Harismendy et al. 2011).
The presence of multiple enhancers in this region suggests that the expression of the INK4b-ARF-INK4a locus is regulated in a temporal and tissue-specific manner. Thus, some enhancers in the CAD interval appear functional in certain cell types and have cell-type-specific effects. An example is given from the transcription factor STAT1. In physiological conditions, activation of the JAK-STAT pathway is triggered when type II interferons (IFN) bind to their receptor, inducing Janus kinase (JAK) phosphorylation, which in turn phosphorylates STAT family of transcription factors. Upon tyrosine phosphorylation, STAT dimerizes and translocates to the nucleus where it modulates a number of target genes. Although the STATs are generally associated with transcriptional activation, examples of STAT-dependent transcriptional repression have also been reported (Aaronson and Horvath 2002). Thus, studies in limphoblastoid cell lines (LCL) show that there is a correlation between the CAD risk variants and CDKN2A/B and ANRIL expression in lymphocytes (Helgadottir et al. 2008), and that the CAD risk alleles preclude binding of STAT1 at the enhancer ECAD9. STAT1 occupancy on this enhancer correlates with the repression of ANRIL expression. On the other hand, IFNγ treatment of endothelial cells (HUVEC) induces STAT1 binding to the same enhancer, which in turn results in increased ANRIL expression (Helgadottir et al. 2007).
The Ras/Raf/Mitogen-activated protein kinase/ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) cascade is another essential signaling transduction pathway involved in INK4b-ARF-INK4a locus regulation (Malumbres et al. 2000). In fact, it has been shown that the pro-oncogenic Ras protein inhibits ANRIL expression and activates p15INK4b, suggesting a potential negative regulation of p15INK4b by ANRIL (Kotake et al. 2011). Ras has an important role in atherosclerosis progression, promoting vascular senescence and inducing the expression of pro-inflammatory cytokines. In particular, a constitutive activation of Ras is involved in atherogenesis by inducing vascular smooth muscle cell (VSMC) senescence and expression of proinflammatory cytokines (Minamino et al. 2003). Furthermore, activation of ERK and vascular inflammation is associated with VSMC senescence in human atherosclerosis, which suggests that the Ras/Raf/MEK/ERK signaling cascade plays an important role in regulating VSMC lifespan and function in vivo (Minamino et al. 2003).
Previous mechanistic studies postulated that ANRIL serve as a scaffold for the chromatin modifying complexes PRC1 and PRC2, mediating the repression in cis of the INK4b-ARF-INK4a locus (Kotake et al. 2011; Yap et al. 2010). Nonetheless, a recent study revealed that ANRIL association with CVD susceptibility can be related to its capability of regulating gene expression in trans (Holdt et al. 2013), leading to decreased apoptosis and increased cell proliferation and cell adhesion, characteristic and essential alterations of atherogenesis (Lusis 2000). In particular, ANRIL-regulated genes contain an Alu repeat motif in their promoters, and the occupancy of CBX7 and SUZ12 is highly enriched ~150 bp downstream of this Alu motif. Alu repeats are a family of primate-specific short interspersed repeat elements (SINEs) with more than one million copies in the human genome (Lander et al. 2001; Dewannieux et al. 2003) and have been linked with genetic disease (Burns and Boeke 2012). Interestingly, the Alu motif is also present in the ANRIL transcript and it is predicted to locate in a central stem-loop-like structure (Holdt et al. 2013), pointing to RNA-chromatin interactions as an effector mechanism (Mercer et al. 2009).
4 ANRIL and Cancer Predisposition
Cancer is a group of more than 100 diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body.
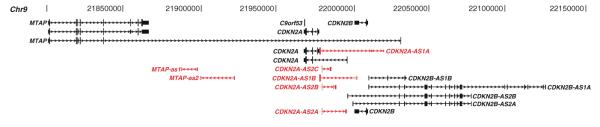
The INK4b-ARF-INK4a gene cluster is homozygously deleted or silenced in approximately 40% of human cancers (Iacobucci et al. 2011), being ANRIL one of the most frequently altered lncRNAs in cancer development and progression, including ovarian cancers, breast cancer, lymphoblastic leukemia, nasopharyngeal carcinoma, basal cell carcinoma, and gliomas (Shete et al. 2009; Stacey et al. 2009; Turnbull et al. 2010; Pasmant et al. 2011). Moreover, several polymorphisms identified in the ANRIL locus show a significant correlation with tumor development (Shete et al. 2009; Wrensch et al. 2009; Bishop et al. 2009). To name a few examples, the SNP rs1063192-C is highly correlated with glioma, the SNP rs1011970-T with melanoma susceptibility (Cunnington et al. 2010), and the SNP Rs564398 increases the risk of lymphoblastic leukemia development (Iacobucci et al. 2011). These polymorphisms alter the expression pattern of ANRIL splice variants (Fig. 2), and in consequence, dysregulate the INK4b-ARF-INK4a locus expression. A hypothesis for ANRIL function is that this ncRNA is composed of several RNA transcript variants such that the accumulation of transcript variants may focalize PRC1 via CBX7 in proximity to the p16INK4a gene promoter to selectively silence p16INK4a (Aguilo et al. 2011). As sequence technology evolves to incorporate higher resolution, we predict that novel isoforms will emerge and specific diseased states will be represented by the presence (or absence) of the transcript variants for ANRIL (Fig. 2).
Fig. 2.
Comprehensive transcript map overlapping the human INK/ARF locus determines the assembly of transcripts by long read- and strand-specific RNA sequencing by ISO-Seq. Samples taken for ISO-Seq analysis are from a single prostate invasive carcinoma specimen and compared with the paired normal prostate duct epithelium. Highlighted in red are novel transcript isoforms identified in the tumor specimen when compared to the normal duct epithelium of the prostate
Although the underlying molecular mechanism by which ANRIL increases the risk of cancer progression remains ambiguous, it is believed that high expression levels may lead to cancer predisposition. Indeed, it has been reported that ANRIL is overexpressed in preneoplastic and neoplastic epithelial tissues (Yap et al. 2010), gastric cancer tissues (Zhang et al. 2014), esophageal squamous cell carcinoma (ESCC) (Chen et al. 2014), and leukemia leukocytes (Cunnington et al. 2010; Yu et al. 2008) compared with the non-tumor tissues. In normal cells, induction of ANRIL transcript levels by E2F1 is required for the suppression of p14ARF, p15INK4b, and p16INK4a expression at the late stage of DNA damage response, in order to return to physiological cellular levels after the completion of the DNA repair. However, in cancerous cells, aberrant expression of ANRIL would cause a blockage of the control of the DNA damage response mechanism, leading to genomic instability, and therefore, tumor progression (Wan et al. 2013).
ANRIL also influences cell proliferation by regulating target genes in trans. Hence, in gastric cancer tissues, ANRIL cooperates with microRNAs in the epigenetic level by binding to EZH2. Specifically, ANRIL silences miR-99a/miR-449a, therefore up-regulating the miR-99a/miR-449a target genes mTOR and CDK6, and as a consequence, up-regulating the CDK6 target gene E2F1 (Zhang et al. 2014). This positive feedback loop could in part account for ANRIL-mediated cell growth regulation. On the other hand, in esophageal squamous cell carcinoma tissues, ANRIL influences cell growth by repression of the TGFβ/Smad signaling pathway (Chen et al. 2014), although the exact molecular mechanisms of interaction between ANRIL and TGFβ1 remain elusive.
Collectively, ANRIL could serve as a candidate biomarker for cancer detection, and novel cancer therapies should consider ANRIL depletion to specifically target highly proliferative cells. However, despite growing knowledge about ANRIL function in cancer and other disease models, a broader understanding of the molecular mechanism of action, and the regulatory pathways, hierarchies and networks in which ANRIL and other lncRNA operate, is the essential first step for its therapeutic manipulation.
Acknowledgments
F.A. was supported by the Catalan Agency for Administration of University and Research (AGAUR) under a Beatriu de Pinos postdoctoral fellowship. S.D.C was supported by the European School of Molecular Medicine (SEMM) and CEINGE-Biotecnologie Avazate s.c. a.r.l, Napoli, under a PhD student fellowship. M.J.W. was supported by a Senior Scholar Award in Aging (AG-SS-2482-10) from the Ellison Medical Foundation and Public Health Service Awards HL103967, HL067099, and CA154903 from the NIH.
Contributor Information
Francesca Aguilo, Departments of Structural and Chemical Biology, Genetics and Genomic Sciences and Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Serena Di Cecilia, Departments of Structural and Chemical Biology, Genetics and Genomic Sciences and Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY, USA; European School of Molecular Medicine, CEINGE—Biotecnologie Avanzate, Naples, Italy.
Martin J. Walsh, Departments of Structural and Chemical Biology, Genetics and Genomic Sciences and Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY, USA, Martin.walsh@mssm.edu
References
- de los Campos G, Gianola D, Allison DB. Predicting genetic predisposition in humans: the promise of whole-genome markers. Nat Rev Genet. 2010;11(12):880–886. doi: 10.1038/nrg2898. [DOI] [PubMed] [Google Scholar]
- Gschwendtner A, et al. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol. 2009;65(5):531–539. doi: 10.1002/ana.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarin M, et al. Whole genome analyses suggest ischemic stroke and heart disease share an association with polymorphisms on chromosome 9p21. Stroke. 2008;39(5):1586–1589. doi: 10.1161/STROKEAHA.107.502963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgadottir A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40(2):217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- Zeggini E, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shete S, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41(8):899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrensch M, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington MS, et al. Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with ANRIL Expression. PLoS Genet. 2010;6(4):e1000899. doi: 10.1371/journal.pgen.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DT, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet. 2009;41(8):920–925. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7(9):667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- Popov N, Gil J. Epigenetic regulation of the INK4b-ARF-INK4a locus: in sickness and in health. Epigenetics. 2010;5(8):685–690. doi: 10.4161/epi.5.8.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobori T, et al. Genomic cloning of methylthioadenosine phosphorylase: a purine metabolic enzyme deficient in multiple different cancers. Proc Natl Acad Sci U S A. 1996;93(12):6203–6208. doi: 10.1073/pnas.93.12.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann I, et al. Characterization of methylthioadenosin phosphorylase (MTAP) expression in malignant melanoma. Am J Pathol. 2003;163(2):683–690. doi: 10.1016/S0002-9440(10)63695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, et al. Homozygous deletions of methylthioadenosine phosphorylase (MTAP) are more frequent than p16INK4A (CDKN2) homozygous deletions in primary non-small cell lung cancers (NSCLC) Oncogene. 1998;17(20):2669–2675. doi: 10.1038/sj.onc.1202205. [DOI] [PubMed] [Google Scholar]
- Pasmant E, et al. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67(8):3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- Yu W, et al. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451(7175):202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, et al. ANRIL is implicated in the regulation of nucleus and potential transcriptional target of E2F1. Oncol Rep. 2010;24(3):701–707. doi: 10.3892/or_00000910. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, et al. CTCF is a DNA methylation-sensitive positive regulator of the INK/ARF locus. Biochem Biophys Res Commun. 2010;392(2):129–134. doi: 10.1016/j.bbrc.2009.12.159. [DOI] [PubMed] [Google Scholar]
- Jarinova O, et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol. 2009;29(10):1671–1677. doi: 10.1161/ATVBAHA.109.189522. [DOI] [PubMed] [Google Scholar]
- Burd CE, et al. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6(12):e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, et al. A methylthioadenosine phosphorylase (MTAP) fusion transcript identifies a new gene on chromosome 9p21 that is frequently deleted in cancer. Oncogene. 2000;19(50):5747–5754. doi: 10.1038/sj.onc.1203942. [DOI] [PubMed] [Google Scholar]
- Folkersen L, et al. Relationship between CAD risk genotype in the chromosome 9p21 locus and gene expression. Identification of eight new ANRIL splice variants. PLoS ONE. 2009a;4(11):e7677. doi: 10.1371/journal.pone.0007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Levine SS, et al. The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol Cell Biol. 2002;22(17):6070–6078. doi: 10.1128/MCB.22.17.6070-6078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32(4):503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32(4):491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20(6):845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431(7010):873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Mak W, et al. Mitotically stable association of polycomb group proteins eed and enx1 with the inactive x chromosome in trophoblast stem cells. Curr Biol. 2002;12(12):1016–1020. doi: 10.1016/s0960-9822(02)00892-8. [DOI] [PubMed] [Google Scholar]
- Zhao J, et al. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick GV, Soloway PD, Higgins MJ. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet. 2002;32(3):426–431. doi: 10.1038/ng988. [DOI] [PubMed] [Google Scholar]
- Pandey RR, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32(2):232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Rinn JL, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30(16):1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap KL, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38(5):662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilo F, Zhou MM, Walsh MJ. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. 2011;71(16):5365–5369. doi: 10.1158/0008-5472.CAN-10-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9(7):e1003588. doi: 10.1371/journal.pgen.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersen L, et al. Relationship between CAD risk genotype in the chromosome 9p21 locus and gene expression. Identification of eight new ANRIL splice variants. PLoS ONE. 2009b;4(11):e7677. doi: 10.1371/journal.pone.0007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol. 2010;30(3):620–627. doi: 10.1161/ATVBAHA.109.196832. [DOI] [PubMed] [Google Scholar]
- Holdt LM, Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol. 2012;32(2):196–206. doi: 10.1161/ATVBAHA.111.232678. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS ONE. 2009;4(4):e5027. doi: 10.1371/journal.pone.0005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congrains A, et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. 2012;220(2):449–455. doi: 10.1016/j.atherosclerosis.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Bochenek G, et al. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 2013;22(22):4516–4527. doi: 10.1093/hmg/ddt299. [DOI] [PubMed] [Google Scholar]
- Harismendy O, et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature. 2011;470(7333):264–268. doi: 10.1038/nature09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Ortega S, Barbacid M. Genetic analysis of mammalian cyclin-dependent kinases and their inhibitors. Biol Chem. 2000;381(9–10):827–838. doi: 10.1515/BC.2000.105. [DOI] [PubMed] [Google Scholar]
- Minamino T, et al. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation. 2003;108(18):2264–2269. doi: 10.1161/01.CIR.0000093274.82929.22. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35(1):41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- Burns KH, Boeke JD. Human transposon tectonics. Cell. 2012;149(4):740–752. doi: 10.1016/j.cell.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- Iacobucci I, et al. A polymorphism in the chromosome 9p21 ANRIL locus is associated to Philadelphia positive acute lymphoblastic leukemia. Leuk Res. 2011;35(8):1052–1059. doi: 10.1016/j.leukres.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Stacey SN, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. 2009;41(8):909–914. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull C, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42(6):504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasmant E, et al. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25(2):444–448. doi: 10.1096/fj.10-172452. [DOI] [PubMed] [Google Scholar]
- Zhang EB, et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget. 2014;5(8):2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, et al. ANRIL inhibits p15(INK4b) through the TGFbeta1 signaling pathway in human esophageal squamous cell carcinoma. Cell Immunol. 2014;289(1–2):91–96. doi: 10.1016/j.cellimm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Wan G, et al. Long non-coding RNA ANRIL (CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cell Signal. 2013;25(5):1086–1095. doi: 10.1016/j.cellsig.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]