Abstract
Alterations in hypothalamic-pituitary-adrenal (HPA) axis function contribute to many of the adverse behavioral effects of chronic voluntary alcohol drinking, including alcohol dependence and mood disorders; limbic brain structures such as the bed nucleus of the stria terminalis (BNST) may be key sites for these effects. Here, we measured circulating levels of several steroid hormones and performed whole-cell electrophysiological recordings from acutely-prepared BNST slices of male rhesus monkeys allowed to self-administer alcohol for 12 months or a control solution. Initial comparisons revealed that BNST neurons in alcohol-drinking monkeys had decreased membrane resistance, increased frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) with no change in spontaneous excitatory postsynaptic currents (sEPSCs). We then used a combined variable cluster analysis and linear mixed model statistical approach to determine whether specific factors including stress and sex hormones, age, and measures of alcohol consumption and intoxication are related to these BNST measures. Modeling results showed that specific measures of alcohol consumption and stress-related hormone levels predicted differences in membrane conductance in BNST neurons. Distinct groups of adrenal stress hormones were negatively associated with the frequency of sIPSCs and sEPSCs, and alcohol drinking measures and basal neuronal membrane properties were additional positive predictors of inhibitory, but not excitatory, PSCs. The amplitude of sEPSCs was highly positively correlated with age, independent of other variables. Together, these results suggest that chronic voluntary alcohol consumption strongly influences limbic function in non-human primates, potentially via interactions with or modulation by other physiological variables, including stress steroid hormones and age.
Keywords: bed nucleus of the stria terminalis, electrophysiology, rhesus monkey, stress hormones, HPA axis, glucocorticoids, synaptic transmission
Introduction
Chronic alcohol consumption increases the risk for a number of long-term negative health outcomes, including alcohol dependence and anxiety. A growing body of evidence suggests that chronic alcohol exposure-induced alterations in hypothalamic-pituitary-adrenal (HPA) axis function contribute to these pathological behaviors. While the ability of acute alcohol to engage the HPA axis to stimulate the release of adrenal hormones is variable (Mick et al., 2012; Richardson et al., 2008; Thayer et al., 2006), it is well established that chronic alcohol exposure causes aberrant plasticity in the HPA axis that may result in dysregulated release of adrenal hormones in alcohol-dependent humans (Beresford et al., 2006; Dai et al., 2007; Stalder et al., 2010; Thayer et al., 2006; Tirabassi et al., 2013), rhesus macaques (Helms et al., 2014a), and rodents (Adinoff et al., 1990; Rasmussen et al., 2000; Richardson et al., 2008; Zorrilla et al., 2001). Consistent with this, humans with hypercortisolism have increased risk for the development of alcohol dependence (Besemer et al., 2011; Tirabassi et al., 2013), and blocking glucocorticoid receptors (GRs) in rodents prevents escalation of alcohol drinking to dependence (Vendruscolo et al., 2012). Further, longitudinal studies in macaque monkeys have revealed that changes in circulating levels of stress hormones depend on access to ethanol and the degree to which monkeys engage in heavy alcohol drinking behavior (Helms et al., 2012; Helms et al., 2014a).
Altered stress hormone activity due to alterations in the HPA axis may contribute to persistent adaptations in central circuits that underlie reward-seeking behavior and stress responsivity, leading to an increased negative affective state and alcohol-seeking behavior (Funk et al., 2006; Koob and Kreek, 2007; Koob, 2003; Santibanez et al., 2005; Valdez et al., 2002). The bed nucleus of the stria terminalis (BNST) is a major component of the central extended amygdala that plays a critical role of integration of stress and reinforcement and also mediates the negative affective state associated with chronic alcohol and drug use (Eiler et al., 2003; Erb et al., 2001; Fox et al., 2010; Francesconi et al., 2009; Harris and Aston-Jones, 2007; Hyytia and Koob, 1995; Kash, 2012; Sahuque et al., 2006). Imaging studies have identified the BNST as a site of aberrant plasticity and increased amygdalar connectivity in human alcoholics (O’Daly et al., 2012). Pharmacological manipulations in the rodent BNST can alter alcohol drinking behaviors; for example, blockade of dopamine or GABA receptors in the BNST reduces alcohol drinking behavior (Eiler and June, 2007; Eiler et al., 2003). In addition, chronic alcohol exposure and withdrawal have been shown to increase intrinsic excitability, alter modulation of synaptic transmission by neuropeptides,, and produce aberrant plasticity in BNST neurons (Kash, 2012; Kash et al., 2009; McElligott et al., 2013; McElligott and Winder, 2009; Olive et al., 2002; Pleil et al., 2015; Silberman et al., 2013). Neurons in the BNST express both glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) (de Kloet et al., 2005; Pietranera et al., 2001; Vendruscolo et al., 2012; Watters et al., 1996), which are bound by stress-related hormones, including the adrenal steroid hormones cortisol/corticosterone (CORT) (McEwen, 2007; McEwen et al., 1968; Watters et al., 1996), aldosterone (ALD) (Lienard et al., 1996; Watters et al., 1996), and deoxycorticosterone (DOC) (Pietranera et al., 2001). Chronic alcohol exposure causes functional alterations in GR expression in the BNST and other limbic brain areas (Vendruscolo et al., 2012), suggesting that stress hormones may mediate or interact with the effects of chronic alcohol on BNST function (Garcia-Perez et al., 2012). In addition to being a site of action for stress hormones, the BNST is a critical site for central feedback of the HPA axis (Ulrich-Lai and Herman, 2009), suggesting it may play a particularly important role in chronic alcohol-induced plasticity of adrenal hormone secretion.
While neuroimaging studies in humans have identified the BNST as a critical region of plasticity in alcoholic individuals (O’Daly et al., 2012), and rodent models of chronic voluntary alcohol use and alcohol dependence have provided some circuit-specific mechanistic insight about how the BNST may play a role in these processes (Kash et al., 2015; Pleil et al., 2015; Silberman et al., 2013), to date there have been no studies combining the use of mechanistic tools in primates. Here, we use a chronic voluntary alcohol consumption paradigm in non-human primates that produces similar drinking patterns as those observed in humans, including a large subset of monkeys classified as exhibiting chronic binge drinking behavior associated with alcohol use disorder (AUD) in humans, combined with ex vivo electrophysiological recordings of BNST neurons. We directly compare BNST measures in alcohol drinkers and controls to assess the effects of long-term alcohol self-administration on BNST function. We then employ a linear mixed model statistical approach to characterize the effects of age, alcohol consumption and intoxication levels, and stress and sex steroid hormone levels on synaptic function in the BNST.
Methods and Materials
Subjects
Subjects were 18 male rhesus macaques (Macaca mulatta) acquired from the breeding program of the Oregon National Primate Research Center; 14 were given long-term continuous access to ethanol and four were ethanol-naive controls. Monkeys were singly housed in a colony room in which they could interact with one another, trained to present their legs for femoral blood sampling, and habituated to this procedure prior to ethanol access (Helms et al., 2014a). All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center IACUC.
Ethanol access paradigm
Monkeys were trained to operate a panel to self-administer water, and were then gradually induced to self-administer 0.5, 1.0, and 1.5 g/kg ethanol (4% w/v) over three consecutive 30 day epochs, as previously described (Grant et al., 2008; Helms et al., 2014a). After training, they were given free access to 4% ethanol in water (w/v) 22 hours per day (termed “open-access”) for at least 12 calendar months (depicted in Figure 1A; detailed time line information is available at www.MATRR.com). Using this protocol, monkeys with average body mass of 9.42 ± 0.31 kg (mean ± SEM) consumed an average of 2.5 ± 0.3 g/kg per day during the open-access period and average lifetime ethanol intake (LEI) levels of 1244.5 ± 107.0 g/kg. Blood samples were collected every 5–7 days, consistently at seven hours into the daily 22 hr ethanol access period, to assess blood ethanol content (BEC) across the open-access period. Patterns of ethanol intake and intoxication observed in these monkeys were variable, as in the human literature, such that monkeys in this study represented the full spectrum of previously identified categories, including low, binge, high, and very high ethanol drinkers (Helms et al., 2014a). Control monkeys were in similar housing conditions for at least 10 months and were given open access to an isocaloric 10% maltose dextrin solution in water for seven weeks prior to the necropsy but never received access to ethanol.
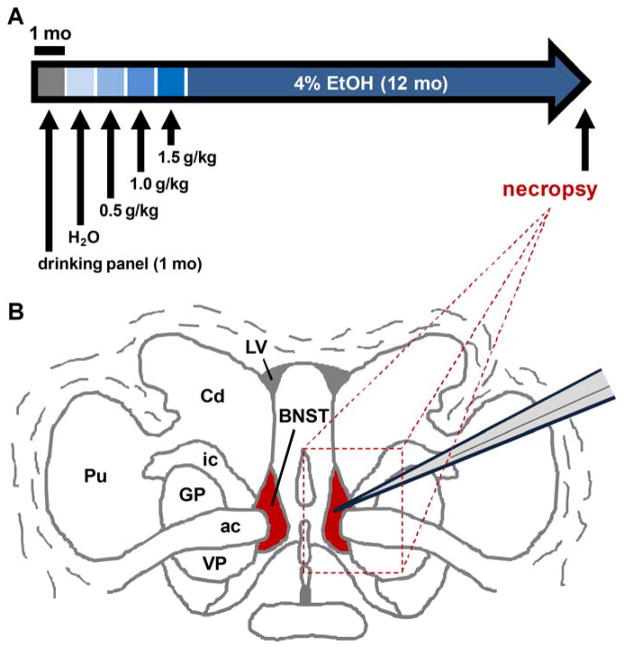
Figure 1.
Experimental design for assessing bed nucleus of the stria terminalis (BNST) function in alcohol-exposed rhesus monkeys. (a) Ethanol induction paradigm in which male rhesus monkeys were trained to use an operant panel in their home cages and then trained to self-administer increasing daily amounts of ethanol across the 4-month induction phase of the paradigm. Monkeys were then allowed to self-administer 4 percent (w/v) ethanol for 22 hours/day for 12 months and then went to necropsy. (b) Representation of a coronal section of rhesus monkey brain tissue with BNST in red and dashed line square indicating a typical block of tissue sliced for electrophysiological recordings
Necropsy and slice preparation
The necropsy procedure was conducted as previously described (Davenport et al., 2014). Briefly, at the time a daily drinking session would typically begin, monkeys were instead sedated with ketamine (10 mg/kg), and isofluorane was used at a dose to maintain a deep surgical plane of anesthesia. Trunk blood samples were collected prior to transcardial perfusion with ice-cold oxygenated artificial cerebrospinal fluid (aCSF) to remove blood and any ethanol from the brain tissue. A craniotomy was conducted and brains were rapidly removed and an approximate 4mm × 6 mm × 8 mm block of tissue containing the bed nucleus of the stria terminalis (BNST) and adjacent sections of the caudate was isolated (shown in Figure 1B). The tissue block was placed in a conical tube of ice-cold oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 4.5 KCl, 1MgCl2, 26 NaHCO3, 1.2 NaH2PO4, 10 glucose, and 2 CaCl2, continuously aerated with a mixture of 95% O2/5% CO2 gas and transported on ice for slicing. Tissue was then transferred to ice-cold cutting solution containing (in mM) 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 glucose, aerated with a mixture of 95% O2/5% CO2 gas. Coronal slices at a thickness of 300 μm were obtained with a ceramic blade (Camden Instruments Limited) attached to a VT 1200S vibratome tissue slicer (Leica Biosystems, Wetzlar, Germany). Slices were equilibrated in aCSF at a temperature of 33 C for one hour and then transferred to room temperature until experimental use.
Blood hormone analysis
Trunk blood collected during necropsy was assayed for plasma levels of the following sex and stress hormones by the Oregon National Primate Research Center Endocrine Technology Services Laboratory using standardized procedures previously described (Helms et al., 2014a): the pituitary stress hormone adrenocorticotropic hormone (ACTH), the glucocorticoid/mineralocorticoid adrenal hormones CORT, ALD, and DOC, the adrenal sex steroid hormone/neurosteroid dehydroepiandrosterone sulfate (DHEAS), and the gonadal sex steroid hormone testosterone (T). Given the intimate interactions between the HPA and hypothalamic-pituitary-gonadal (HPG) axis (Viau, 2002) and the ability of several of these hormones to be converted into/serve as precursors for others, the impact of these hormones on BNST function was determined using a multi-step statistical approach, described below.
Slice Electrophysiology
Slices were transferred to a recording chamber fixed to the stage of an upright microscope, stabilized by an overlying platinum ring and continuously perfused with solution maintained at a temperature of 28–32 C with the temperature not varying more than 1 C during a given experiment. Individual neurons in the BNST were identified under infrared optics using a 40x water immersion objective and images were displayed on a computer monitor, which aided the navigation and placement of the recording pipette. Patch pipettes were pulled from borosilicate glass capillaries (1.5 mm outer diameter, 0.86 mm inner diameter, Sutter Instruments Co., Novato, CA) and filled with either potassium-gluconate (K-Gluc) or cesium-chloride (Cs-Cl)-based intracellular solutions (pH 7.25, 290–295 mOsmol) for excitatory and inhibitory synaptic transmission recordings, respectively. The Cs-Cl solution contained (in mM) 150 CsCl; 10 HEPES, 2 MgCl2; 0.3 Na-GTP, 3 Mg-ATP, 0.2 BAPTA-4K, and 2 QX-314, and the K-Gluc solution contained (in mM) K-126 gluconate, 4 KCl, 10 HEPES, 0.3 Na-GTP, 4 Mg-ATP, and 10 phosphocreatine. Bicuculline (30μM) was added to aCSF when recording spontaneous excitatory postsynaptic currents (sEPSCs), and 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX; 5μM) was added to aCSF to isolate spontaneous inhibitory postsynaptic currents (sIPSCs). Recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA). Whole-cell membrane currents were digitized at 20 kHz, filtered at 2 kHz, and analyzed with pClamp software (Molecular Devices, Sunnyvale, CA).
Data Analysis
Excitatory and inhibitory synaptic transmission were measured using different intracellular recording solutions (K-Gluc and Cs-Cl, respectively), and therefore analyses were performed separately. In total, eight separate dependent variables were evaluated. Because dependent measure data sets were normally distributed in natural log space, statistical analyses were performed with log-transformed values; data are presented in natural space to indicate values measured during experiments. First, we performed unpaired two-tailed t-tests in Graphpad Prism (GraphPad Software, San Diego, CA) to directly compare dependent measures from individual BNST neurons between EtOH and CON monkeys in order to assess whether chronic voluntary alcohol drinking altered cell membrane properties and excitatory and inhibitory synaptic transmission in the BNST (Figure 2). Effects of alcohol exposure were then further characterized by incorporating age, specific measures of alcohol consumption and levels of intoxication, and hormone measurements taken at necropsy into statistical models of BNST measures. Specifically, SAS statistical software (v. 9.3, SAS Institute, Cary, NC) was used to explore the relationships between in vivo predictor variables of ethanol drinking, age, and sex and stress hormone levels and ex vivo dependent physiological measures of membrane properties and synaptic function of neurons in the rhesus monkey BNST; MATLAB (MathWorks, Natick, MA) was used to create heat maps to visualize these effects.
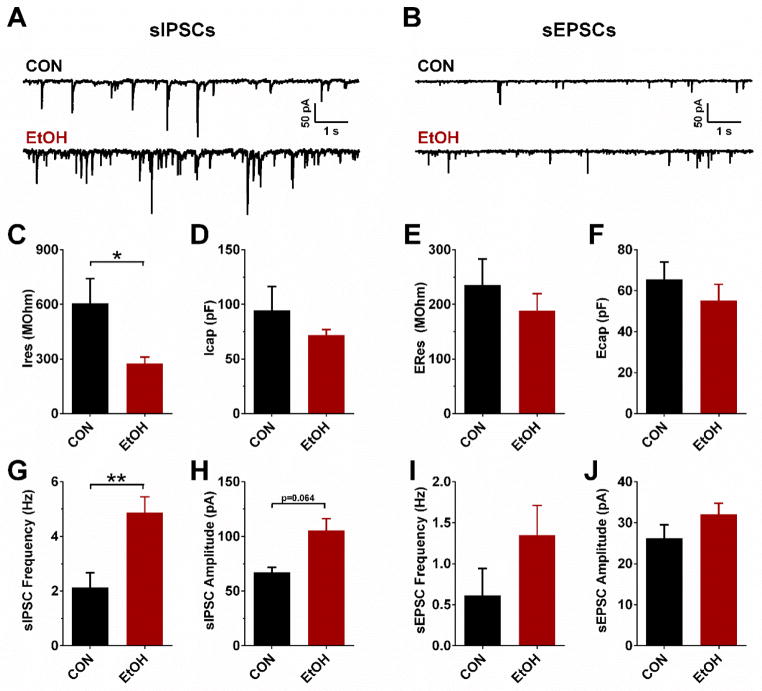
Figure 2.
Effects of chronic voluntary alcohol exposure on cell membrane properties and synaptic transmission in the rhesus monkey bed nucleus of the stria terminalis (BNST). (a) and (b) Representative traces of spontaneous inhibitory postsynaptic currents (sIPSCs; a) and excitatory postsynaptic currents (sEPSCs; b) in BNST neurons from CON and EtOH monkeys. (c) and (d) BNST neurons patched with a Cs-Cl interacellular recording solution had significantly lower membrane resistance (IRes) in EtOH monkeys than in CONs (c; t(38) = 2.11, *P = 0.041), but neuron capacitance (ICap) did not differ between groups (d; P>0.45). (e) and (f) Neurons patched with a K-Gluc intracellular recording solution did not significantly differ in membrane resistance (ERes; e) or capacitance (ECap; f; P>0.25). (g) sIPSC frequency was significantly higher in BNST neurons from EtOH monkeys than CON monkeys (t(38) = 3.38, **P = 0.002). (h) There was a non-significant trend toward increased sIPSC amplitude in BNST neurons of EtOH monkeys compared with CONs (t(38) = 1.91, P = 0.064). (i) and (j) Neither sEPSC frequency (i) nor amplitude (j) significantly differed between CON and EtOH monkey BNST neurons (P>0.10). All data in panels B-J are presented in natural space as mean ± SEM; because these data.
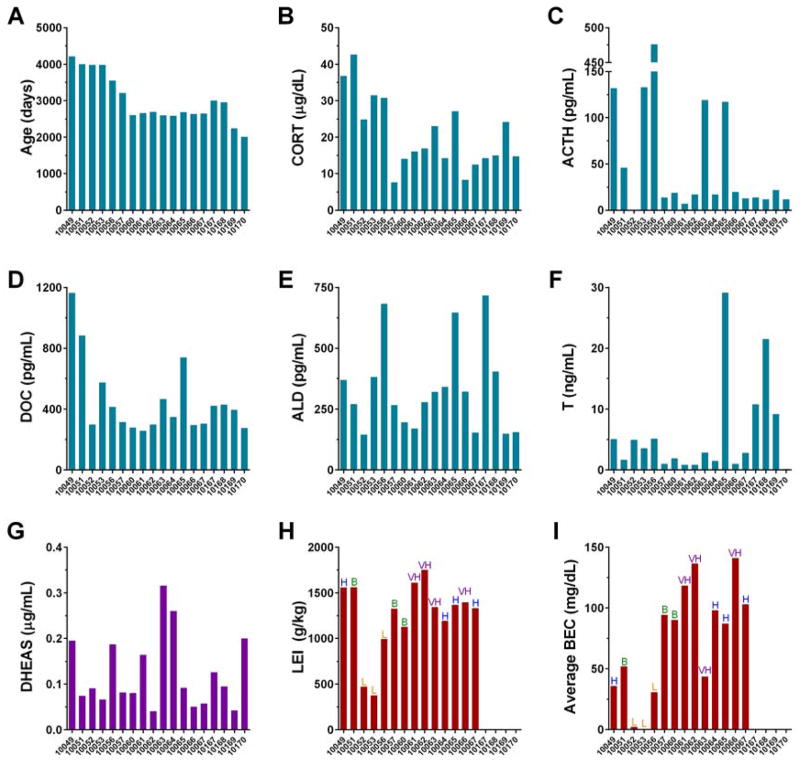
Similar to dependent measures of cell membrane properties and synaptic transmission described above, values for physiological measures of hormone levels were normally distributed in natural log space; thus, log-transformed values for all physiological variables were used for all statistical analyses. The dependent measures included here were restricted to those measured during electrophysiological recordings (cell membrane properties and measures of excitatory and inhibitory synaptic transmission). In vivo demographic, behavioral, and physiological measures were included as predictor (independent) variables in these analyses and raw values for all variables are included in Figure 3. They include: 1) age at necropsy—all monkeys that self-administered ethanol underwent the same behavior/necropsy timeline but were given ethanol access starting at a range of ages between adolescence and middle adulthood (5.5 and 11.5 years of age). Because control monkeys were ethanol-naive and thus do not have an “age of onset of ethanol drinking”, age at necropsy is used as a predictor variable in the models for all monkeys; 2) cumulative lifetime ethanol intake (LEI)—recent research has indicated that the amount of ethanol consumed across the lifespan in humans is highly correlated with many negative health outcomes associated with alcohol use, including risk for cancers, cardiovascular disease, and neuropsychiatric conditions (Chen et al., 2011; Corrao et al., 2000; Smith and Riechelmann, 2004). Thus, we used the cumulative amount of ethanol consumed as a predictor variable for BNST function; 3) average blood ethanol concentration (BEC) across the 12-mo open access period, as described above, as a measure of average intoxication. Previous human and non-human primate research shows that individuals not exposed to ethanol do not have detectable BECs, so these levels were considered to be zero in our analyses; 4) plasma levels of stress and sex hormone taken at necropsy, as described above, as measures of the relative availability of these hormones in the brain during the time of electrophysiological recordings. Importantly, thresholds for the LEI and BEC measures described are consistently used to categorize ethanol drinking patterns for monkeys in this model as low, binge, high, or very high drinkers (e.g., Helms et al., 2014a); thus the use of these continuous variables in our statistical analyses provides a representative metric of ethanol drinking pattern. We observed a full spectrum of these categorized ethanol drinking patterns (labeled above bars in panels H and I in Figure 3), which allowed us to closely examine how ethanol dose mediates the effects of chronic ethanol exposure on BNST function.
Figure 3.
Values of all in vivo predictor variables for all monkeys used for electrophysiological recordings, color-coded by the variable clusters determined in the variable cluster analysis shown in Figure 4. Ethanol drinking pattern category for each monkey according to its ethanol intake and average blood ethanol content (BEC), as defined in this monkey model, is indicated above bars in panels H and I: very high drinker (VH) has average ethanol intake ≥3 g/kg AND ≥10 percent of days with intake over 4 g/kg; high drinker (H) has ethanol intake above ≥3 g/kg for ≥ 20 percent of days; binge drinker (B) has ethanol consumption ≥2 g/kg for ≥55 percent of days AND at least one recorded BEC ≥80 mg/dL; and low drinker (L) does not meet criteria for any other categorization
Linear mixed effects models (SAS proc MIXED) were used for this analysis because ex vivo whole-cell patch-clamp electrophysiology was performed in several neurons from each monkey BNST (Rappaport and Kupper, 2008). Given multiple measures of specific variables for individual monkeys, estimates were made of both within- and between-monkey measurement variance. Here, within-monkey variance represents the spread of measurements across neurons for any given monkey, and between-monkey variance represents the spread of average measures across all monkeys. For any variable with repeated measures (i.e., measures of cell membrane properties and excitatory and inhibitory synaptic transmission), the sum of the within- and between-monkey variance equals the total measurement variance. The intraclass correlation coefficient (ICC) is then the ratio of the between-monkey variance to the total variance. This value is useful for gauging the proportion of total measurement variance that can be attributed to differences across individual monkeys.
Due to the relatively small number of monkeys in the study, and the potential for collinearity between predictor variables that can lead to model instability, we limited the number of predictor variables in the initial models. We identified which variables to include in initial models for basal membrane properties using a three-step process. First, a variable cluster analysis (VCA) was performed (SAS proc VARCLUS) using all possible predictor variables (i.e., Age, LEI, BEC, CORT, ACTH, DOC, ALD, T, DHEAS), a maximum Eigenvalue of 1.0, and a threshold of 0.70 for proportion of total variance explained. Linear mixed models for dependent measures of synaptic transmission also included basal membrane properties of those cells as predictor variables. Next, Pearson correlation coefficients were estimated (SAS proc CORR) across all variable pairs (both within and across clusters). Finally, correlation coefficient estimates were used to select one representative variable from each cluster that would be examined in the initial models - selected variables had strong within-cluster correlations, and weak across-cluster correlations. As there is no automated procedure for elimination of insignificant variables in SAS proc MIXED, final models were ultimately determined using a manual backwards stepwise elimination procedure. We set a significance level of α = 0.10 in order to identify potentially meaningful associations between variables from a relatively small number of monkeys in our study.
Notably, because slice electrophysiological recordings could only be performed at necropsy, our statistical analyses were used to evaluate associations between in vivo predictor and ex vivo dependent measures of BNST function. Observed associations do not necessarily indicate directionality, and therefore do not imply causality. However, a major strength in using this statistical approach is that it allowed evaluation of individual predictor variables while controlling for other covariates. Importantly, because we experimentally manipulated whether each individual monkey received access to ethanol or served as an ethanol-naive control, effects of ethanol variables in our models likely indicate effects of chronic ethanol exposure on BNST function, and we were able discretely evaluate the predictive contributions of level of alcohol consumption and intoxication, age, and hormone measures to our dependent measures of BNST neuronal function. Thus, we were able to include all available data into one analysis to fully characterize the contributions of variables as they relate to BNST function in the primate.
To visually illustrate effects revealed by mixed models, heat maps containing all individual data points used for analyses are presented as a proportion of the range of the variable, with PROPX = (X − MIN)/(MAX − MIN), where X is the current value, MIN is the smallest value in the dataset, and MAX is the largest value in the dataset. Heat maps are presented by organizing cells based on the response variable, from lowest to highest value. This illustrates both the dynamic range of values for each variable across neurons from which measurements were taken as well as the nature of the linear relationship between the response variable presented in the top row of the heat map and predictor variables in subsequent rows. To gauge measurement variance within and between monkeys, and the ability of predictor variables to explain components of measurement variance, ICC estimates are given based on null models (ICCnull; containing a model intercept as the only fixed effect) and final models (ICCfinal; containing all significant predictor variables).
Results
Effects of chronic alcohol exposure on BNST function
Whole-cell voltage-clamp electrophysiological recordings were performed in individual BNST neurons from acutely prepared coronal BNST slices from rhesus monkeys that received open access to and voluntarily consumed alcohol (EtOH) and control monkeys that received open access to a control maltrose dextrin solution (CON; representative traces in Figure 2A & B). Because brains were perfused with ice-cold aCSF during necropsy, no ethanol was present during electrophysiological recordings. Response variables evaluated were membrane capacitance in neurons recorded using a Cs-Cl intracellular solution (ICap) or K-Gluc solution (ECap), a membrane property that indicates cell size and modulates both synaptic efficacy and the velocity of action potential propagation, and membrane resistance (ERes and IRes), the inverse of the total conductance across the cell membrane. Direct comparisons of cell membrane properties between groups (Figure 2C–F) revealed that BNST neurons from EtOH monkeys had lower membrane resistance than CONs when a Cs-Cl solution intracellular solution was used (t(38) = 2.11, p = 0.041; Figure 2C), suggesting that the conductance of ion channels on the cell membrane was increased by chronic alcohol exposure. However, the capacitance of BNST neurons did not differ between groups (p’s > 0.25; Figure 2D & F). The frequency of sIPSCs in BNST neurons was significantly higher in EtOH monkeys than CONs (t(38) = 3.38, p = 0.002; Figure 2G) and there was a trend toward increased amplitude in EtOH mice (t(38) = 1.91, p = 0.064; Figure 2H), but there were no effects of alcohol drinking on the frequency or amplitude of sEPSCs (p’s > 0.10; Figure 2I & J). This suggests that basal inhibitory tone was increased by chronic alcohol exposure.
Predictor variable clustering
To determine whether specific measures of alcohol intake contributed to the effects of chronic alcohol exposure on BNST neuronal function, or whether other physiological measures might mediate, interact with, or have independent effects from chronic alcohol exposure on BNST neuronal function, we employed statistical modeling to our BNST data. Raw values for all in vivo variables used to model electrophysiology data for all monkeys are presented in Figure 3 and can also be accessed through the Monkey Alcohol Tissue Research Resource (MATRR) website, www.MATRR.com. First, a VCA of the predictor variables was performed to determine which variables were statistically clustered together to identify candidate variables to be included in statistical models for response variables measured during electrophysiological recordings (depicted in Figure 4).
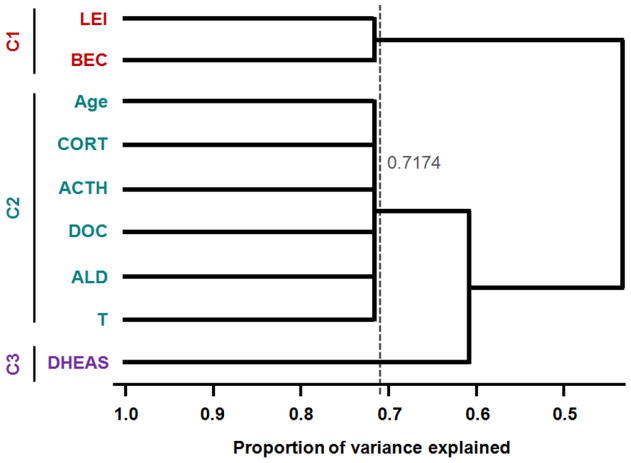
Figure 4.
Dendrite diagram for variable cluster analysis (VCA) of in vivo predictor variables used in mixed models for cell membrane properties, showing that three clusters of variables explain more than 70 percent of the total variance: lifetime ethanol intake (LEI), average blood ethanol concentration (BEC), age at necropsy (Age), cortisol (CORT), adrenocorticotropic hormone (ACTH), deoxycortisol (DOC), aldosterone (ALD), testosterone (T) and dehydroepiandrosterone sulfate (DHEAS)
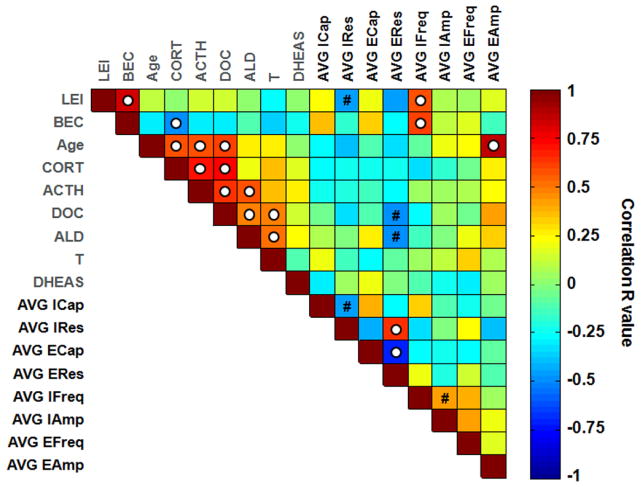
This analysis revealed that variables were grouped into three clusters, which explained greater than 70% of the total variance. Cluster 1 included LEI and BEC; Cluster 2 included Age, CORT, ACTH, DOC, ALD, and T; and Cluster 3 included only DHEAS. The clustering of these variables suggests that both measures of ethanol intake (i.e., LEI and BEC) were highly associated with one another, and that age, T, and all stress hormones were highly associated. Surprisingly, DHEAS was not associated with Cluster 2 variables, even though it is an abundant adrenal hormone and one of its physiological roles is to serve as a precursor for the gonadal hormones T and estradiol (Soma et al., 2014). A correlation matrix, presented in Figure 5, illustrates the pairwise relationships between variables measured in monkeys used for electrophysiological recordings, confirming significant associations between variables within clusters.
Figure 5.
Correlation matrix heat map indicating Pearson’s R values for correlations between all predictor variables (labeled in gray) and response variables [labeled in black: average neuronal capacitance and resistance per monkey using Cs-Cl intracellular solution (AVG ICap, AVG IRes) and K-Gluc solution (AVG ECap, AVG ERes), average sIPSC and sEPSC frequency (AVG IFreq, AVG EFreq) and amplitude (AVG IAmp, AVG EAmp) per monkey] across all 18 monkeys used for electrophysiological recordings. White dot indicates statistically significant correlation with P<0.05; # indicates 0.05<P<0.10. Note that for all electrophysiological measures, one value per monkey representing the average value from all neurons recorded was used
Basal membrane properties
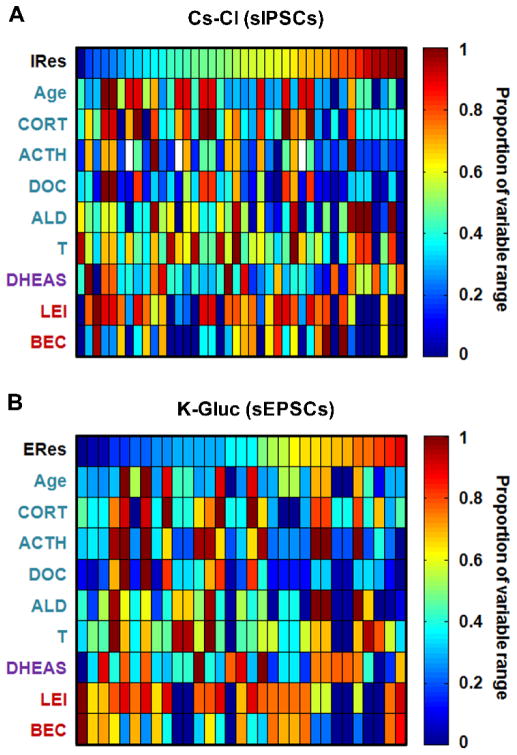
We evaluated whether the in vivo predictor variables of voluntary ethanol self-administration, age, and the plasma levels of stress and sex hormones were associated with the basal membrane properties of BNST neurons from which we recorded using Cs-Cl or K-Gluc intracellular recording solutions. One representative variable from each cluster was used in each linear mixed model; each selected variable was highly correlated with all other variables in its cluster but not with variables from other clusters (see Figure 5). This led to the inclusion of LEI from Cluster 1, DOC from Cluster 2, and DHEAS from Cluster 3 for all predictive models of neuronal membrane properties. Mixed model results showed no significant effects of LEI, DOC, or DHEAS on either ICap or ECap. However, there was a trend for LEI to predict IRes (p = 0.092) with a negative association, explaining 8.2% of the total measurement variance, and DOC was a significant predictor of ERes (p = 0.034) with a negative association, explaining 13.9% of the measurement variance (Table 1). Despite these observed associations, approximately 90% of the variability in IRes and ERes could not be explained using the predictor variables included in our analyses. ICC estimates for capacitance suggest that about 30–65% of the measurement variance was observed between monkeys and that 35–70% of the measurement variance was observed across neurons within individual monkeys. ICC estimates for IRes were in between those for ICap and ECap; the similarity between ICCnull and ICCfinal for IRes suggests that including LEI as a model fixed effect had little impact on variance estimates. The estimate of ICCnull for ERes indicates that the majority of measurement variance (80%) was observed across neurons within individual monkeys. In fact, after adjusting for DOC in the final model, all residual variance was observed within-monkeys (i.e., ICCfinal = 0). As these basal membrane properties have been shown to indicate aspects of neuronal phenotype, ICC results indicate that there are many different cell types expressed in the monkey BNST that may be differentially modulated by predictor variables.
Table 1.
Results from final mixed models for basal membrane properties of rhesus monkey BNST neurons.
| Response Variable | Fixed Effect | Coefficient (SE) | p-value | Model R2 | ICCnull | ICCfinal |
|---|---|---|---|---|---|---|
| Cs-Cl (sIPSCs) 1 | ||||||
| Capacitance (pF) | intercept | 4.24 (−0.089) | <0.0001 | 0 | 0.294 | 0.294 |
| Resistance (MΩ) | intercept | 6.02 (−0.324) | <0.0001 | 0.082 | 0.574 | 0.541 |
| LEI (g/kg) | −4.9 × 10−4 (2.8 × 10−4) | 0.092 | ||||
| K-Gluc (sEPSCs) 2 | ||||||
| Capacitance (pF) | intercept | 3.93 (0.147) | <0.0001 | 0 | 0.645 | 0.645 |
| Resistance (MΩ) | intercept | 8.80 (1.64) | 0.0001 | 0.139 | 0.188 | 0 |
| DOC (μg/dL) | −0.624 (0.271) | 0.034 | ||||
I=17 monkeys, N=40 measurements
I=14 monkeys, N=32 measurements
Together, mixed model results in Table 1 show that Cluster 1 and 2 variables were most influential on the input resistance of BNST neurons. Heat maps illustrating the relationship between predictor variables and Res are shown in Figure 6. While ethanol consumption and sex and stress hormones appear to have a modest positive association with the intrinsic excitability/conductance of BNST neurons (as measured by Res), our overall results indicate that predictor variables included here may not be the most important factors regulating the basal membrane properties of BNST neurons.
Figure 6.
Heat maps of variables ordered by cell membrane resistance in neurons patched with a Cs-Cl-based intracellular solution (IRes; A) or a K-Gluc-based intracellular solution (ERes; B), illustrating results from correlations and mixed models
Synaptic transmission measures
We next examined which variables were significant predictors of synaptic transmission measures (sIPSC and sEPSC frequency and amplitude). We examined a fourth variable cluster (in addition to those shown in Figure 4), which included Cap and Res, because these measures have previously been described to influence synaptic transmission in rodents. Notably, Cap and Res were inversely correlated with one another for neurons patched with either Cs-Cl or K-Gluc internal solution (Supplementary Figure 1A). Because ICap and ECap were less associated with the other predictor variables (LEI, DOC, and DHEAS) than IRes and ERes (as shown in Figure 5), they were selected to represent the cell membrane properties in the mixed models.
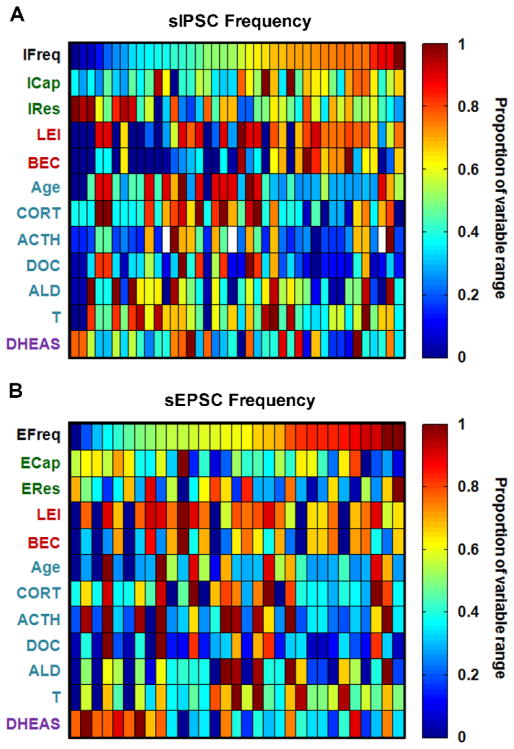
Significant or trend effects of DOC (p = 0.058; negative association), LEI (p = 0.003; positive association), and ICap (p = 0.001; positive association) were observed on sIPSC frequency (IFreq). These three predictor variables collectively explained 42.4% of the variance in IFreq data, as detailed in Table 2 and illustrated in Figure 7A. In contrast, there was a trend for DHEAS to predict sEPSC frequency (EFreq; p = 0.080; negative association), explaining 12.4% of the variance (Table 2; Figure 7B). These results suggest that inhibitory and excitatory synaptic transmission in BNST neurons are differentially modulated by variables included in our analyses; inhibitory tone in the BNST is highly downregulated by stress hormones enhanced by a history of high ethanol drinking, and also dependent on the basal membrane properties of the neurons patched, while excitatory synaptic input to BNST neurons is not modulated by these factors but in part suppressed by the complex adrenal hormone DHEAS.
Table 2.
Results from final mixed models for measures of synaptic transmission in BNST neurons.
| Response Variable | Fixed Effect | Coefficient (SE) | p-value | Model R2 | ICCnull | ICCfinal |
|---|---|---|---|---|---|---|
| sIPSCs 1 | ||||||
| Frequency (Hz) | intercept | 0.346 (1.83) | 0.852 | 0.424 | 0.324 | 0.152 |
| DOC (μg/dL) | −0.524 (0.262) | 0.058 | ||||
| LEI (g/kg) | 6.41 × 10−4 (1.9 × 10−4) | 0.003 | ||||
| ICap (pF) | 0.804 (0.218) | 0.001 | ||||
| Amplitude (pA) | intercept | 4.43 (0.093) | <0.0001 | 0 | 0.268 | 0.268 |
| sEPSCs 2 | ||||||
| Frequency (Hz) | intercept | −2.81 (1.15) | 0.031 | 0.124 | 0.477 | 0.389 |
| DHEAS | −0.949 (0.510) | 0.08 | ||||
| Amplitude (pA) | intercept | 3.34 (0.094) | <0.0001 | 0 | 0.704 | 0.704 |
I=17 monkeys, N=40 measurements
I=14 monkeys, N=31 measurements
Figure 7.
Heat maps of values for all neurons patched with variables ordered by IFreq (A) and EFreq (B), illustrating mixed model results that IFreq was predicted by variables from most variable clusters (except the DHEAS cluster), and EFreq was predicted by only DHEAS
There was no correlation between frequency and amplitude for either sIPSCs or sEPSCs (Supplementary Figure 1B), suggesting that these measures were independent. In addition, final models showed no significant predictors of sIPSC or sEPSC amplitude (IAmp or EAmp, respectively; Table 2), suggesting that postsynaptic modulation of synaptic transmission is not highly modulated by variables measured here. However, while no significant predictors of EAmp were detected in the final models, correlational analysis showed that it had a significant positive association with Age (as illustrated in Figure 5). This was particularly intriguing because age was significantly correlated with many other hormones in its cluster that were not correlated with EAmp, suggesting a potentially unique and specific role for age in the regulation of excitatory transmission in BNST neurons.
A large difference in the partitioning of measurement variance was observed when comparing results for amplitude. The ICC estimate for EAmp suggests that 70% of the measurement variance was observed between monkeys, whereas the estimate for IAmp suggests that only about 30% of the measurement variance was observed between monkeys. Estimates of ICCnull for IFreq and EFreq suggest that between-monkey variance comprised 30% and 50%, respectively, of the total measurement variance. After adjusting significant fixed effects, estimates of ICCfinal were reduced below those of ICCnull, suggesting that predictor variables were able to explain measurement difference across monkeys for both of these variables.
Discussion
Here, we used a non-human primate model to examine the effects of chronic alcohol exposure on the function of the BNST, a limbic structure integral to the regulation of emotional and reward-related behaviors that has never been previously characterized in primates. The ethanol self-administration paradigm employed here induced an average LEI equivalent to approximately nine alcoholic drinks per day, everyday. This drinking behavior models chronic binge drinking in humans with AUD, characterized by an average daily BEC above 80 mg/dl over one year of drinking. Direct comparisons between EtOH and CON monkeys revealed robust effects of chronic alcohol exposure on cell membrane conductance and inhibitory synaptic transmission in the BNST. The specificity of these identified effects was intriguing, because in conjunction with mixed modeling results discussed below, our results suggest that increased inhibitory tone in BNST neurons may be an adaptive homeostatic mechanism compensating for increased membrane-bound ion channel conductance and intrinsic neuronal excitability, as we have previously reported in a mouse model of binge ethanol drinking (Pleil et al., 2015). We have also reported similar effects of chronic voluntary alcohol drinking on upregulation of neuropeptide Y signaling that serves as a homeostatic mechanism to further increase inhibition in BNST neurons in both rhesus monkeys and mice produces an (Pleil et al., 2015). Future studies examining more specific measures of BNST excitability following chronic alcohol exposure will be able to further characterize these effects.
We further examined how a number of in vivo demographic, physiological, and behavioral measures are related to these effects of alcohol exposure. Results from linear mixed models of eight dependent measures of neuronal and synaptic function in the BNST showed that measures of the level of alcohol consumption/intoxication and stress steroid hormones accounted for a small but significant proportion of variance in neuronal conductance in the BNST, as measured by resistance, without being associated with neuronal size, as measured by capacitance (Table 1). Notably, measures of inhibitory and excitatory synaptic transmission were predicted by different clusters of variables; DHEAS was negatively associated with sEPSC frequency, while the other three clusters of variables, represented by LEI (positive association), DOC (negative association), and ICap (positive association), significantly predicted sIPSC frequency and explained greater than 40% of the total variance in this measure (Table 2). These results suggest that greater ethanol intake, lower stress hormone levels, and high capacitance/low resistance neuronal phenotype were associated with greater inhibitory synaptic transmission, while the complex neurosteroid DHEAS may suppress excitatory synaptic transmission in the monkey BNST. Mixed modeling revealed no significant effects of any variables on sIPSC or sEPSC amplitude, however Age was highly positively correlated with sEPSC amplitude (Figure 5). These results demonstrate that a number of variables predict different aspects of BNST function.
Because we experimentally manipulated whether and at what age monkeys gained access to alcohol, we are able to reveal a causal effect of chronic alcohol exposure on membrane conductance and inhibitory synaptic transmission and an effect of Age on postsynaptic excitatory synaptic transmission in the primate BNST. However, our study is unable to identify a directional relationship between specific in vivo hormone and alcohol consumption/intoxication physiological predictor measures and dependent measures of BNST function, because these predictor measures were not experimentally controlled and were assessed at necropsy, along with BNST recordings. The inclusion of ethanol-naive monkeys in the study to control for hormone levels and age, however, leads us to hypothesize that there is a causal role for alcohol drinking, age, and hormones in BNST function. Further, results suggest that while chronic alcohol drinking may be an important modulator of limbic circuit function, many other factors including age and stress and sex hormones affect BNST function, either independently or by mediating some of the effects of chronic alcohol consumption. Importantly, in vivo predictor variables in this study have been shown to modulate each other and likely interact to influence BNST function, as implicated by our mixed modeling results. While in vivo variables were used as predictors in this study, it is also possible that there is a bidirectional relationship between them and BNST function, such that intrinsic BNST function modulates HPA axis function and alcohol drinking behavior (Herman et al., 2003).
Interestingly, the identified roles of alcohol consumption/intoxication measures (LEI, BEC) in the mixed models for membrane resistance and sIPSC frequency in the BNST were consistent with differences between CON and EtOH monkeys initially identified in this study. This suggests that the degree of excessive alcohol drinking may mediate the effects of chronic alcohol exposure on BNST function, rather than alcohol exposure per se simply regulating function. This is consistent with the human literature suggesting that the volume and pattern of alcohol drinking are extremely influential on negative health outcomes associated with chronic alcohol use, including risk for cancers, cardiovascular disease, and importantly neuropsychiatric conditions, which the BNST has been associated with (Chen et al., 2011; Corrao et al., 2000; Smith and Riechelmann, 2004). As the predictor variables used here (LEI, BEC) have been used to categorize the functional ethanol drinking patterns of monkeys in this study and other cohorts of monkeys in this model of chronic alcohol drinking (see Figure 3) that are similar to patterns observed in humans, our results may have important implications for the effects of chronic alcohol drinking on limbic function in humans with AUDs. In addition, we found mixed model effects for sEPSC frequency when no differences were found in initial direct comparisons, highlighting the importance of including specific measures of alcohol consumption and intoxication when evaluating the effects of chronic alcohol on neural function.
Relationships between in vivo predictor variables
Cumulative lifetime ethanol consumption and average concentrations of blood ethanol during the 12-month open access were strongly positively correlated and comprised one of the variable clusters in the VCA. This was an expected association given the established positive relationship between ethanol consumption and BECs observed across many cohorts of monkeys in this non-human primate model of chronic alcohol exposure (Grant et al., 2008), and it additionally indicates that monkeys that acquired ethanol drinking thresholds rapidly across the induction phase of ethanol access also consumed high levels of ethanol during the open access period. Most of the adrenal and pituitary hormones measured here were clustered together with each other and with age; interestingly, T was also in this cluster of variables, while DHEAS was not significantly associated with any hormones in that cluster and instead was the lone measure in a separate variable cluster. Given the broad spectrum of ages of subjects used in this study and the strong correlations between age and many stress and sex hormones observed here and reported in other studies, future studies with larger cohorts of monkeys will be important for teasing apart the specific contributions of age and hormones in this cluster to the effects on BNST neuronal function. The highly-significant association between Age, but not other Cluster 2 variables, and sEPSC amplitude observed here suggests that the effects of these variables may be independent in some cases. This is described in more detail below.
While the exclusion of DHEAS from this cluster was somewhat surprising given that DHEAS is synthesized in the adrenals of primates, DHEAS responds to chronic restraint stress differently than CORT in rhesus monkeys (Maninger et al., 2010). In addition, it has a myriad of functions—from directly modulating GABA and NMDA receptor function (Compagnone and Mellon, 2000; Demirgoren et al., 1991; Majewska et al., 1990; Tanaka and Sokabe, 2012) to serving as a precursor to the sex hormones T and estradiol (Soma et al., 2014)—indicating the complexity of its actions as a unique neuromodulator. This suggests that DHEAS may be a critical factor in alcohol-induced plasticity and should be explored further in rodent models using mechanistic tools.
Modulation of membrane conductance
Neuronal ion channels have been shown to be dynamically modulated by a number of molecules and plasticity-related events. For example, the effects of chronic alcohol exposure on small-conductance calcium-activated potassium channels and GABAA receptor chloride channels in limbic and cortical brain regions have been well-characterized (Botting et al., 2003; Hopf et al., 2011a; Hopf et al., 2011b; McCool et al., 2003; Mulholland, 2012; Mulholland et al., 2011; Mulholland et al., 2009). Further, chronic high levels of stress hormones such as CORT have been found to bidirectionally modulate neuronal calcium channel function (Nair et al., 1998). Our finding that ethanol consumption and stress hormones significantly predicted cell membrane conductance, as well as inhibitory synaptic transmission, in the rhesus BNST suggests that the BNST may be a key site for the actions of chronic alcohol exposure. An interesting question raised by our results is why different variables were found to contribute to the membrane resistance when different intracellular recording solutions were used. One possibility is that steroid hormones and chronic alcohol exposure modulate distinct types of ion, as indicated by previous reports; the high chloride intracellular solution that was used to record sIPSCs is more sensitive to changes in chloride conductances, such as those that arise from tonically-active GABA neurons as well as calcium-activated chloride channels (Botting et al., 2003; McCool et al., 2003; Nair et al., 1998). Further, the intracellular solution used to record EPSCs contained cesium, which blocks a subset of potassium channels and could potentially mask any effects mediated by them. The lack of strong modulation of cell membrane capacitance by in vivo variables in our study was not surprising given that several studies in mouse and cell culture have indicated that membrane capacitance is not influenced by biological processes and alterations in channel functions (Gentet et al., 2000). Together, our results support the notion that chronic alcohol consumption alters the signaling within and between neurons.
Differential modulation of inhibitory and excitatory neurotransmission
Adrenal hormones were negative predictors of frequency of both excitatory and inhibitory synaptic transmission, suggesting that local interneurons and/or neurons from other brain regions that synapse onto BNST neurons are suppressed by this hormone millieu. sIPSC frequency was also positively predicted by ethanol consumption levels, suggesting that there are effects of chronic alcohol exposure on BNST function that occur via actions in brain regions that have dense GABAergic projections to the BNST, such as the central amygdala (Li et al., 2012; Oler et al., 2012; Sun et al., 1991). These network-dependent effects on synaptic transmission in the BNST may have broad effects on anxiety and reward-related behaviors, as the BNST projects to many brain areas directly involved in the regulation of stress and alcohol-related behaviors, (Dong et al., 2000; Dong et al., 2001; Dong and Swanson, 2004; Kash, 2012; Silberman et al., 2013). Interestingly, the amplitude of inhibitory and excitatory postsynaptic currents in the BNST was not predicted by circulating stress and sex hormones, even though the BNST is densely populated with many receptors for these hormones (Pietranera et al., 2001; Vendruscolo et al., 2012; Williamson and Viau, 2007). However, ICC results demonstrated that there was a large amount of intra-monkey variance in basal membrane properties, which were shown to highly predict the frequency of inhibitory synaptic transmission in the BNST. As these properties can indicate aspects of neuronal phenotype, our results speak to the heterogeneity of cell types in the rhesus monkey BNST and suggest that there may be some postsynaptic cell-type specificity for the GABAergic inputs modulated by ethanol consumption and steroid hormones. For example, neuropeptide Y modulation of GABAergic synaptic transmission is specific to corticotropin-releasing factor neurons in the mouse BNST, which regulate alcohol drinking behavior, and this modulatory role is altered after chronic voluntary alcohol exposure (Pleil et al., 2015). In addition, recent rodent studies have attempted to categorize BNST neurons into three types based on specific electrophysiological properties (Hammack et al., 2007; Silberman et al., 2013). However, even individual neurons in some discrete genetically-defined or circuit-specific neuronal populations do not all fit into the same cell-type category (Silberman et al., 2013). This suggests that categorization of neurons based on these properties is not necessarily indicative of cell type or projection target. In addition, because this is the first study to examine the primate BNST, it is unclear whether BNST neurons from naive primates can be categorized into the same subtypes as those in rodents. Thus, because we were attempting to evaluate the involvement of many in vivo variables on the net function of the primate BNST, our results suggest that the identified relationships between variables in our study are extremely robust because they were detected in the absence of defined neuronal subpopulations. They further demonstrate the necessity of recording from several neurons in each monkey to accurately assess the impact of and interaction between all variables on overall BNST function.
Also of note was that while there were no significant predictors of sIPSC or sEPSC amplitude in mixed models for these measures, age was highly positively related to the amplitude of sEPSCs in BNST neurons according to pairwise correlations, regardless of ethanol intake. While this has not been directly examined in the BNST of monkeys, there are reports of age-related reductions in AMPA receptor expression that may underlie changes in learning and memory (Gocel and Larson, 2013). Interestingly, DHEAS levels decline across adulthood (Sorwell et al., 2014) and were observed to negatively influence sEPSC frequency in our study, suggesting that age and DHEAS may have complex oppositional roles in the modulation of excitatory synaptic transmission. Because ethanol intake did not appear to modulate sEPSC amplitude, our results suggest that age of onset of ethanol drinking is not likely a risk factor for the development of aberrant synaptic function in the BNST. Interestingly, we have previously shown that age of onset is a risk factor for heavy drinking in this translational model (Helms et al., 2014b), suggesting that alterations in excitatory synaptic transmission in the BNST are more likely related to the consequences, rather than the risk, of chronic ethanol consumption.
Conclusions
This study is the first to characterize the impact of chronic alcohol consumption on neuronal function in the extended amygdala of non-human primates. In addition, we examined the interaction between chronic alcohol exposure and other physiological variables shown to impact neuronal function, including age and steroid hormones. While many of the stress/sex hormones in this study were associated with one another, our results indicate that DHEAS may play a complex but unique role in the BNST. Finally, these results implicate a critical role for adrenal hormones in the regulation of BNST function and suggest a direction forward for the mechanistic assessment of the interactions between chronic alcohol exposure and hormone signaling in the BNST.
Supplementary Material
Acknowledgments
We would like to thank Matthew Stiegel and Joachim Pleil for assistance with statistical analyses. NIH grant support for this research was provided by F32 AA021043 and K99 AA023599 (KEP), U01 AA020911 (TLK), the Bowles Center for Alcohol Studies (KEP & TLK), U01 AA013510 (KAG & CMH), R24 AA019431 (JBD, KAG & CMH), and the Oregon National Primate Research Center grant P51 OD011092 (KAG & CMH).
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or conflicts of interest.
References
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Beresford HF, Du Y, Liu D, Shen D, Davatzikos C, Laudenslager ML. Hypercortisolism in alcohol dependence and its relation to hippocampal volume loss. J Stud Alcohol. 2006;67:861–867. doi: 10.15288/jsa.2006.67.861. [DOI] [PubMed] [Google Scholar]
- Besemer F, Pereira AM, Smit JW. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med. 2011;69:318–323. [PubMed] [Google Scholar]
- Botting SK, Frye GD, Pulido MD, McCool BA. Effects of chronic alcohol ingestion on rat lateral/basolateral amygdala ligand-gated chloride channels. Ann N Y Acad Sci. 2003;985:479–480. doi: 10.1111/j.1749-6632.2003.tb07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Davenport AT, Grant KA, Szeliga KT, Friedman DP, Daunais JB. Standardized method for the harvest of nonhuman primate tissue optimized for multiple modes of analyses. Cell Tissue Bank. 2014;15:99–110. doi: 10.1007/s10561-013-9380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Demirgoren S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience. 1991;45:127–135. doi: 10.1016/0306-4522(91)90109-2. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, June HL. Blockade of GABA(A) receptors within the extended amygdala attenuates D(2) regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O’Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez D, Laorden ML, Milanes MV, Nunez C. Glucocorticoids regulation of FosB/DeltaFosB expression induced by chronic opiate exposure in the brain stress system. PLoS One. 2012;7:e50264. doi: 10.1371/journal.pone.0050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocel J, Larson J. Evidence for loss of synaptic AMPA receptors in anterior piriform cortex of aged mice. Front Aging Neurosci. 2013;5:39. doi: 10.3389/fnagi.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp Res. 2012;36:995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Park B, Grant KA. Adrenal steroid hormones and ethanol self-administration in male rhesus macaques. Psychopharmacology (Berl) 2014a doi: 10.1007/s00213-014-3590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rau A, Shaw J, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology (Berl) 2014b;231:1853–1861. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Bonci A. The SK channel as a novel target for treating alcohol use disorders. Channels (Austin) 2011a;5:289–292. doi: 10.4161/chan.5.4.16577. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2011b;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46:303–308. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13. doi: 10.14348/molcells.2015.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard F, Thornton SN, Martial FP, Mousseau MC, Galaverna O, Meile MJ, Nicolaidis S. Effects of DOCA pretreatment on neuronal sensitivity and cell responsiveness to angiotensin II, in the bed nucleus of the stria terminalis in the rat. Regul Pept. 1996;66:59–63. doi: 10.1016/0167-0115(96)00060-2. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgoren S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35:1055–1062. doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL, Wightman RM. Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology. 2013;38:1665–1673. doi: 10.1038/npp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Mick I, Spring K, Uhr M, Zimmermann US. Alcohol administration attenuates hypothalamic-pituitary-adrenal (HPA) activity in healthy men at low genetic risk for alcoholism, but not in high-risk subjects. Addict Biol. 2012;18:863–871. doi: 10.1111/j.1369-1600.2011.00420.x. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ. K(Ca)2 channels: novel therapeutic targets for treating alcohol withdrawal and escalation of alcohol consumption. Alcohol. 2012;46:309–315. doi: 10.1016/j.alcohol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69:625–632. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, Bonci A, Treistman SN, Chandler LJ. Sizing up ethanol-induced plasticity: the role of small and large conductance calcium-activated potassium channels. Alcohol Clin Exp Res. 2009;33:1125–1135. doi: 10.1111/j.1530-0277.2009.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SM, Werkman TR, Craig J, Finnell R, Joels M, Eberwine JH. Corticosteroid regulation of ion channel conductances and mRNA levels in individual hippocampal CA1 neurons. J Neurosci. 1998;18:2685–2696. doi: 10.1523/JNEUROSCI.18-07-02685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA, Stodola DE, Essex MJ, Davidson RJ, Kalin NH. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage. 2012;61:1059–1066. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietranera L, Saravia FE, McEwen BS, Lucas LL, Johnson AK, De Nicola AF. Changes in Fos expression in various brain regions during deoxycorticosterone acetate treatment: relation to salt appetite, vasopressin mRNA and the mineralocorticoid receptor. Neuroendocrinology. 2001;74:396–406. doi: 10.1159/000054706. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, Kash TL. NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci. 2015;18:545–552. doi: 10.1038/nn.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM, Kupper LL. Quantitative Exposure Assessment. Stephen M. Rappaport; 2008. [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl) 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez M, Gysling K, Forray MI. Adrenalectomy decreases corticotropin-releasing hormone gene expression and increases noradrenaline and dopamine extracellular levels in the rat lateral bed nucleus of the stria terminalis. J Neurosci Res. 2005;81:140–152. doi: 10.1002/jnr.20538. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Riechelmann H. Cumulative lifelong alcohol consumption alters auditory brainstem potentials. Alcohol Clin Exp Res. 2004;28:508–515. doi: 10.1097/01.alc.0000117870.11317.92. [DOI] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE. DHEA effects on brain and behavior: Insights from comparative studies of aggression. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Testosterone increases circulating dehydroepiandrosterone sulfate levels in the male rhesus macaque. Front Endocrinol (Lausanne) 2014;5:101. doi: 10.3389/fendo.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol. 2010;85:357–360. doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull. 1991;27:651–662. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sokabe M. Continuous de novo synthesis of neurosteroids is required for normal synaptic transmission and plasticity in the dentate gyrus of the rat hippocampus. Neuropharmacology. 2012;62:2373–2387. doi: 10.1016/j.neuropharm.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, 3rd, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Tirabassi G, Boscaro M, Arnaldi G. Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine. 2013 doi: 10.1007/s12020-013-0112-y. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Wilkinson CW, Dorsa DM. Glucocorticoid regulation of vasopressin V1a receptors in rat forebrain. Brain Res Mol Brain Res. 1996;38:276–284. doi: 10.1016/0169-328x(95)00345-s. [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.