Abstract
The paraventricular nucleus of the thalamus (PVT) appears to participate in drug addiction. Recent evidence in rats shows that ethanol drinking is increased by orexin/hypocretin (OX) afferents from the hypothalamus, acting specifically in the anterior (aPVT) rather than posterior (pPVT) PVT subregion. The present study sought to identify neuropeptides transcribed within the PVT, which themselves might contribute to ethanol drinking and possibly mediate the actions of OX. We discovered that substance P (SP) in the aPVT can stimulate intermittent access ethanol drinking, similar to OX, and that SP receptor (NK1R) antagonists in this subregion reduce ethanol drinking. As with OX, this effect is site-specific, with SP in the pPVT or dorsal third ventricle having no effect on ethanol drinking, and it is behaviorally specific, with SP in the aPVT reducing the drinking of sucrose and stimulating it in the pPVT. A close relationship between SP and OX was demonstrated by a stimulatory effect of local OX injection on SP mRNA and peptide levels, specifically in the aPVT but not pPVT, and also a stimulatory effect of OX on SP expression in isolated thalamic neurons, reflecting postsynaptic actions. A functional relationship between OX and SP in the aPVT is suggested by our additional finding that ethanol drinking induced by OX is blocked by a local NK1R antagonist administered at a subthreshold dose. These results, suggesting that SP in the aPVT mediates the stimulatory effect of OX on ethanol drinking, identifies a new role for SP in the control of this behavior.
Keywords: neurokinin 1 receptor, posterior paraventricular thalamus, tachykinin receptor 1
INTRODUCTION
The paraventricular nucleus of the thalamus (PVT) has been suggested to participate in the neurocircuitry of addiction (Matzeu et al., 2014). This nucleus, the only area of the thalamus that projects to limbic regions involved in drug-seeking (Vertes and Hoover, 2008), affects functions related to addiction, including arousal, nociception, stress, and homeostasis (Kolaj et al., 2014). The PVT appears to have a particular relationship with ethanol drinking, with its neurons activated under conditions of both ethanol seeking (Dayas et al., 2008; Hamlin et al., 2009) and intake (Barson et al., 2015; Ryabinin et al., 1997), and with lesions of the PVT preventing the reinstatement of beer seeking (Hamlin et al., 2009).
The anterior (aPVT) and posterior (pPVT) subregions of the PVT may differ in their role in addiction (Barson et al., 2015; Flagel et al., 2011), possibly due to their connectivity to other brain regions (Vertes and Hoover, 2008). While the aPVT sends stronger projections than the pPVT to certain brain areas implicated in drug seeking, such as the nucleus accumbens core, medial prefrontal cortex, and ventral subiculum, the pPVT has a larger projection to areas implicated in anxiety, including the central nucleus of the amygdala (Li and Kirouac, 2008; Vertes and Hoover, 2008). Support for this differential role of the PVT subregions in addiction comes from evidence obtained with orexin/hypocretin (OX), an orexigenic neuropeptide transcribed almost exclusively within neurons of the hypothalamus, with a few additional cells at the junction of the hypothalamus and thalamus (Peyron et al., 1998; Sakurai et al., 1998). This neuropeptide sends one of its densest projections to the PVT subregions (Kirouac et al., 2005; Peyron et al., 1998) where both of its receptors, OX1R and OX2R, are expressed (Marcus et al., 2001). With OX2R targeted by both OX isoforms, OX-A and OX-B, while OX1R is primarily targeted by OX-A (Sakurai et al., 1998), our findings in the aPVT, that both OX-A and OX-B promote ethanol drinking and that an OX2R but not OX1R antagonist suppresses it (Barson et al., 2015), support the specific involvement of OX2R in the aPVT in stimulating ethanol drinking. This is in contrast to the pPVT, where OX does not affect ethanol drinking but instead promotes palatable food intake (Barson et al., 2015; Choi et al., 2012). This evidence, supporting a role for hypothalamic OX projections in promoting ethanol drinking through their actions at OX2R in the aPVT, has led us to investigate neuropeptides transcribed within the PVT itself that may also be involved in this behavior and possibly in mediating the actions of OX.
Based on limited published evidence, we focused on the neuropeptide, substance P (SP), as a possible candidate involved in the PVT promotion of ethanol drinking. Studies have shown that systemic injection of antagonists of the SP receptor, neurokinin 1 receptor/tachykinin receptor 1 (NK1R), can reduce ethanol intake and seeking as well as the motivation to obtain ethanol (Schank et al., 2011; Schank et al., 2013; Steensland et al., 2010) and that mice genetically deficient in NK1R drink less ethanol at high concentrations than do wild-type controls (George et al., 2008). Also, in alcohol-preferring P rats, whereas SP injection at moderate-to-high levels in the amygdala reduces the self-administration of ethanol (Yang et al., 2009), the finding that an NK1R antagonist injected into this nucleus leads to the same suppression of ethanol self-administration (Schank et al., 2013) supports the idea that endogenous SP functions normally in the amygdala to stimulate ethanol intake. This neuropeptide may have similar actions in the PVT, where it has been detected in perikarya and fibers (Battaglia et al., 1992; McLean et al., 1985) and NK1R has also been described (Mantyh et al., 1984). The idea that SP in the PVT may function in close relation to OX is suggested by the finding that expression of tachykinin precursor 1 which encodes SP is enhanced in the amygdala of dogs with a mutation of OX2R (Lindberg et al., 2007) and that OX1R in rats is expressed on SP-containing dorsal root ganglion neurons (Colas et al., 2014). These studies hint at the possibility that SP may function in close relation to OX, acting in the PVT to stimulate ethanol drinking.
To investigate the role of SP in the PVT, we first examined the impact of this neuropeptide in the PVT subregions on a preclinical model of alcohol abuse (Carnicella et al., 2014), intermittent-access ethanol drinking, using microinjections of SP and NK1R antagonists. Then, with OX afferents also found to alter ethanol intake, we explored the relationship between OX and SP in the PVT through measurements of gene and protein expression as well as ethanol drinking. Together, these experiments tested the hypothesis that SP transcribed within the PVT influences high ethanol intake and that this occurs specifically in the aPVT in response to stimulation by OX.
MATERIALS AND METHODS (see Appendix for further details)
Subjects
Adult, male Long-Evans rats (N = 178; 250 - 275 g, Charles River Laboratories International, Inc., Kingston, NY, USA) and timed-pregnant female Long-Evans rats (embryonic day 9, N = 10; Charles River Laboratories International) were individually housed in an AAALAC-accredited facility, on a 12-hour reversed light/dark cycle (lights off at 0900 h for males; lights off at 1200 h for females). All rats were given at least one week after arrival to acclimate to the facility prior to testing. They received ad libitum chow (PicoLab Rodent Diet 20 5053, Lab Diet, St. Louis, MO, USA) and filtered water unless otherwise specified. Males received water via two plastic 8 oz bottles (PETCO Animal Supplies, Inc, San Diego, CA, USA). Experiments were approved by the Institutional Animal Care and Use Committee of The Rockefeller University and followed the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Protocol
Seven experiments were performed to investigate the role of SP in the PVT (Figure S1). Experiments 1 and 2 examined the impact of SP in the PVT on intermittent-access 20% ethanol drinking; Experiments 3-5 determined if SP gene expression and peptide levels in the PVT are affected by OX; Experiment 6 directly compared the effects of the OX isoforms in the PVT on ethanol drinking; and Experiment 7 tested if the integrity of the SP system is necessary for OX to exert this effect on ethanol drinking.
Experiment 1
To examine the impact of SP in the PVT on ethanol drinking, rats (N = 30) were trained to drink 20% ethanol over two weeks and then cannulated in the aPVT or pPVT or in the adjacent dorsal third ventricle (d3v), which served as an anatomical control against leakage into the ventricle (n = 10/area). After anesthesia with 75 mg/kg ketamine and 10 mg/kg xylazine (i.p.), 10 mm guide shafts (21-gauge stainless steel) were implanted perpendicularly, aimed at the aPVT (Posterior (P) −1.7 mm, Lateral (L) ±0.2 mm, Ventral (V) −4.6 mm), pPVT (P −3.4 mm, L ±0.2 mm, V −4.6 mm), or d3v (P −2.5 mm, L ±0.2 mm, V −3.9 mm), relative to bregma (P), the midsaggital sinus (L), and level skull surface (V) (Paxinos and Watson, 2005). Prior to lowering the guide shaft into place, the midsaggital sinus was gently moved to the side. Injectors protruded 1 mm beyond the guide shafts, and to prevent occlusion, stainless steel stylets were left in the guide shafts between injections. Rats were given at least one week of recovery after surgery before receiving their first set of injections (described below) and then at least one additional week of recovery before SP injections. For these tests, they were injected with SP (0.075, 0.750, 7.500 nmol; American Peptide, Sunnyvale, CA, USA), counterbalanced against saline vehicle (0.3 μl; Hospira Inc., Lake Forest, IL, USA), in a within-subject Latin square design across four ethanol access days. These doses of SP were chosen based on prior studies showing the middle and high doses to reduce ethanol drinking after injection in the amygdala but not caudate putamen (Yang et al., 2009). To determine if the effects of SP were specific to ethanol intake, a different set of rats (N = 20) was trained over two weeks to drink 2% sucrose, which is consumed at comparable levels to 20% ethanol when measured in g/kg/24h (Barson et al., 2015; Li et al., 2011). They were then cannulated in the aPVT or pPVT (n = 10/area) and injected with the middle dose of SP (0.750 nmol; American Peptide) counterbalanced against saline vehicle (0.3 μl; Hospira Inc.) in a within-subject Latin square design across two sucrose days. Following injections, intake of ethanol or sucrose as well as simultaneously-available food and water was measured at 0.5 h, 1 h, 2 h, and 4 h. (See Appendix for details of ethanol and sucrose drinking and microinjections.)
Experiment 2
To determine if the effects of SP injection on ethanol drinking reflect endogenous SP activity, the ethanol-drinking rats from Experiment 1 which had cannulas in the aPVT or pPVT (n = 10/area), were tested one week after surgery by being injected with the NK1R antagonist, L-733,060 hydrochloride (0.5, 5.0 nmol; Tocris Bioscience, Minneapolis, MN, USA), counterbalanced against saline vehicle (0.3 μl; Hospira Inc.) in a within-subject Latin square design across three ethanol days. These antagonist doses were selected based on prior studies showing their central injection to antagonize the effects of SP and to alter pain processing (Chai et al., 2012; Hamity et al., 2010). Then, two weeks after recovery from SP injections (see Experiment 1), they were injected with the specific NK1R antagonist RP 67580 (0.5, 5.0 nmol; Tocris) counterbalanced against dimethyl sulfoxide vehicle (0.3 μl; Sigma-Aldrich, St. Louis, MO, USA) in a within-subject Latin square design across three ethanol days. Following injections, intake of ethanol, food, and water was measured at 0.5 h, 1 h, 2 h, and 4 h.
Experiment 3
To determine if SP is affected by OX which in the PVT alters ingestive behavior, a new set of ethanol-naïve rats (N = 48) was cannulated in the aPVT or pPVT (n = 24/area) and given one week of recovery before testing. They were then injected with OX-A (1.0 nmol; American Peptide), OX-B (1.0 nmol; American Peptide), or saline vehicle (0.3 μl; Hospira Inc.), in a between-subject design (n = 8/treatment/area). These OX doses are high enough to effectively promote ethanol drinking (Barson et al., 2015) and are the same or lower than those used to induce feeding (Sweet et al., 1999) and emotional behaviors (Li et al., 2009; Li et al., 2010). Following microinjection, food was removed, and rats were sacrificed sixty minutes later via rapid decapitation. The area injected was then dissected out and stored at −40°C in RNAlater (Sigma–Aldrich) until mRNA extraction and analysis with quantitative real time polymerase chain reaction (qRT-PCR). (See Appendix for details of brain dissections and qRT-PCR.)
Experiment 4
To determine if SP peptide levels in the PVT are also affected by OX, an additional set of ethanol-naïve rats (N = 30) was cannulated in the aPVT or pPVT (n = 15/area) and injected as in Experiment 3 (n = 5/treatment/area). Following injection, food was removed, and rats were sacrificed ninety minutes later via rapid decapitation. The area injected was then dissected out, flash-frozen in cold isopentane (−25°C), and stored at −80°C until analysis of SP peptide levels with enzyme-linked immunosorbent assay (ELISA). (See Appendix for details of ELISA.)
Experiment 5
To determine if the effect of OX is postsynaptic and involves neurons expressing SP, primary thalamic neurons were isolated from embryonic day 19 embryos of pregnant dams (N = 10) and were treated with OX-A (50 nM, 100 nM; American Peptide), OX-B (50 nM, 100 nM; American Peptide), or untreated (control group) in a within-subject design, with three to four wells of neurons from a dam used per treatment sample. These doses of OX are close to the half-maximal concentration and double the half-maximal concentration required for increasing cytoplasmic Ca2+ levels by both OX-A and OX-B acting at the OX2R and by OX-A acting at the OX1R (Sakurai et al., 1998). Sixty minutes following the start of treatment, samples were collected with RNAprotect Cell Reagent (Qiagen, Valencia, CA, USA) for immediate mRNA extraction and later analysis with qRT-PCR. (See Appendix for details of cell culture.)
Experiment 6
To directly compare the effects of the OX isoforms in the PVT on ethanol drinking and ensure that the effects are not due to leakage into the adjacent ventricle, rats (N = 20) were trained to drink 20% ethanol over two weeks and then cannulated in the aPVT, or pPVT (n = 10/area). After recovery from surgery, the rats were injected with OX-A (1.0 nmol; American Peptide) counterbalanced against OX-B (1.0 nmol; American Peptide) and saline vehicle (0.3 μl; Hospira Inc.) in a within-subject Latin square design across three ethanol access days. These OX doses are high enough to effectively promote ethanol drinking (Barson et al., 2015). Rats with cannulas in the d3v, described in Experiment 1, were tested after one week of recovery from surgery (n = 10). Following injections, intake of ethanol, food, and water was measured at 0.5 h, 1 h, 2 h, and 4 h.
Experiment 7
To determine if the integrity of the SP system is necessary for OX to affect ethanol drinking, rats were trained to drink 20% ethanol and then cannulated in the aPVT or pPVT (N = 20). After recovery, they were injected with: 1) the NK1R antagonist L-733,060 hydrochloride (0.5 nmol; Tocris) followed 15 minutes later by OX-B (1 nmol; American Peptide); 2) saline (0.3 μl; Hospira Inc.) followed by OX-B (1 nmol); or 3) saline followed by saline, in a within-subject Latin square design across three ethanol days. Following injections, intake of ethanol, food, and water was measured at 0.5 h, 1 h, 2 h, and 4 h.
RESULTS
Histology, ethanol drinking, and blood ethanol concentration
Histological examination showed that aPVT injections were made between bregma −1.44 and −1.92 mm, pPVT injections were between −3.00 and −3.48 mm, and d3v injections were between −2.16 and −2.64 mm (Figure S2). Analysis of the diffusion of 0.3 μl methylene blue dye after injection in the aPVT showed that it never reached the pPVT, and vice versa, and that it had a radial spread of approximately 0.5 mm.
Under the 20% ethanol intermittent-access, two-bottle-choice paradigm, the rats (N = 67) drank an average of 4.0 ± 0.4 g/kg of ethanol in 24 hours and 0.7 ± 0.1 g/kg of ethanol in the first 30 minutes of daily access. When measured after 30 minutes of access, their blood ethanol concentrations averaged 53.4 ± 4.5 mg/dl and went as high as 121.2 mg/dl. These concentrations were predicted significantly by the 30-minute intake prior to blood collection (R2 = +0.89, P < 0.0001) (Figure S3). Under the 2% sucrose intermittent-access paradigm, the rats (N = 20) drank an average of 3.6 ± 0.3 g/kg of sucrose in 24 hours and 0.6 ± 0.1 g/kg of sucrose in the first 30 minutes of daily access.
Experiment 1: Substance P specifically in the anterior PVT selectively stimulates ethanol drinking
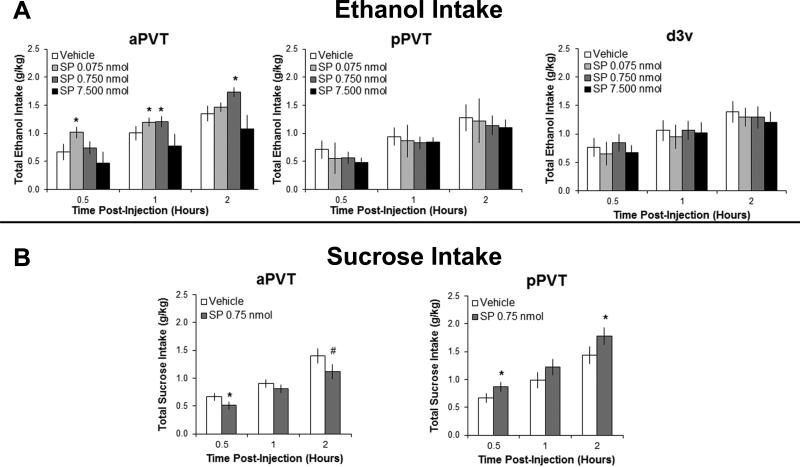
In rats trained to drink ethanol, results with injection of SP into the aPVT revealed a significant main effect of drug on ethanol intake [F(3,27) = 3.87, P < 0.05] and a significant interaction effect between drug and time [F(6,54) = 7.33, P < 0.001] (Figure 1A). Pairwise comparisons demonstrated that both the low (0.075 nmol) and middle (0.750 nmol) doses of SP in the aPVT compared to vehicle significantly increased ethanol drinking (P < 0.05) while the highest dose (7.500 nmol) had no effect, and tests of the simple effects showed that the low dose stimulated intake at 30 minutes and 1 hour post-injection (P < 0.01 and P < 0.05, respectively) and the middle dose stimulated intake at 1 and 2 hours post-injection (P < 0.05 and P < 0.01, respectively). Analysis of the intake of simultaneously-available water and food showed this SP effect to be specific to ethanol, with no main effects observed on the intake of either water [F(3,27) = 1.10, NS] or food [F(3,27) = 2.60, NS] (data not shown). In contrast to the aPVT, results from injection of SP in the pPVT showed no significant main effects on ethanol [F(3,27) = 0.92, NS] (Figure 1A), water [F(3,27) = 0.12, NS] or food intake [F(3,27) = 0.49, NS]. Results with SP injection in the d3v, while showing no main effect on ethanol intake [F(3,24) = 0.21, NS] (Figure 1A), revealed a significant suppression of water intake [F(3,24) = 4.11, P < 0.05] and a trend toward suppressing food intake [F(3,24) = 2.85, P = 0.06] (Table S2). These results confirm that the increase in ethanol intake induced by injection in the aPVT reflects a local response, one that is not due to the spread of SP to nearby brain regions.
Figure 1.
Injection of substance P (SP) into the subregions of the paraventricular thalamus significantly affects ingestive behavior (Experiment 1). A SP (0.075, 0.750, 7.500 nmol in 0.3 μl) compared to saline vehicle significantly increases intermittent-access 20% ethanol drinking with injection into the anterior paraventricular thalamus (aPVT, n = 10), but not into the posterior paraventricular thalamus (pPVT, n = 10) or the adjacent dorsal third ventricle (d3v, n = 9). B SP (0.750 nmol in0.3 μl) compared to saline vehicle significantly decreases intermittent-access 2% sucrose drinking with injection into the aPVT (n = 10), but increases sucrose drinking with injection into the pPVT (n = 10). Data are mean ± S.E.D.; *p < 0.05, #p = 0.06 vs. vehicle.
To determine if effects were specific to ethanol, analysis of SP in the aPVT of rats trained to drink sucrose demonstrated that this injection significantly reduced the drinking of sucrose, leading to a significant main effect on sucrose intake [F(1,9) = 5.61, P < 0.05], with no significant interaction effect between drug and time [F(2,18) = 1.78, NS] (Figure 1B). This is in contrast to the pPVT, where results demonstrated that SP enhanced sucrose drinking, leading to a significant main effect on sucrose intake [F(1,9) = 4.78, P < 0.05], with no significant interaction effect between drug and time [F(2,18) = 1.34, NS] (Figure 1B). In these sucrose-drinking rats, there were no significant main effects of SP injection in the aPVT on intake of water [F(1,9) = 0.74, NS] or food [F(1,9) = 0.08, NS] or in the pPVT on intake of water [F(1,9) = 0.27, NS] or food [F(1,9) = 0.46, NS] (data not shown). These results reveal a double dissociation in the subregion-specific effects of SP on consummatory behavior, with SP in the aPVT selectively stimulating intake of the drug ethanol and SP in the pPVT selectively stimulating intake of the palatable food, sucrose.
Experiment 2: Substance P receptor antagonists in the anterior PVT reduce ethanol drinking
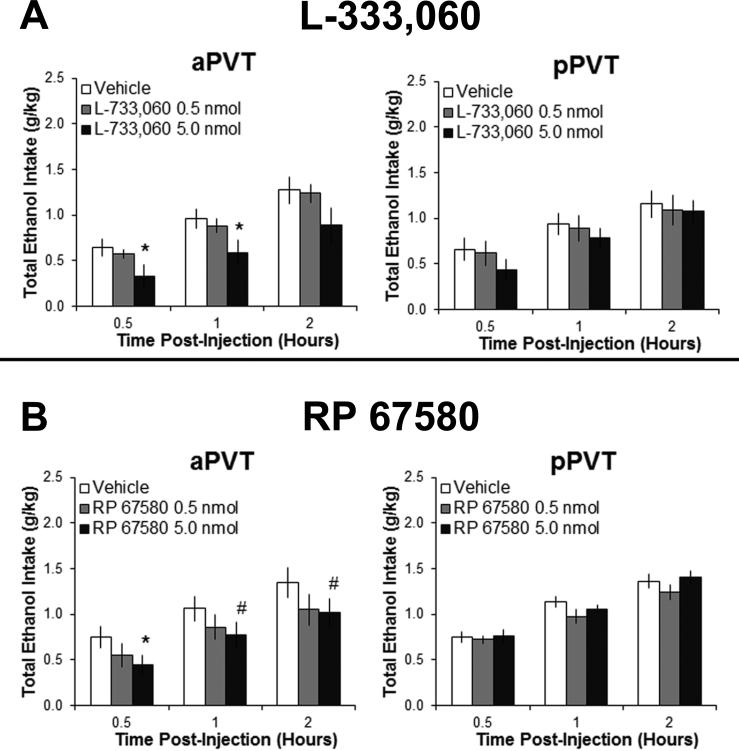
To determine if the effects of SP on ethanol drinking reflect endogenous SP activity, rats trained to drink ethanol were injected with NK1R antagonists. The results showed that L-733,060 in the aPVT led to a significant main effect on ethanol intake [F(2,18) = 5.67, P < 0.05], with no significant interaction effect between drug and time [F(4,36) = 0.41, NS] (Figure 2A). Pairwise comparisons revealed a significant decrease in ethanol drinking at the high dose of this NK1R antagonist (5.0 nmol) in the aPVT as compared to vehicle (P < 0.05), opposite to that induced by SP in Experiment 1, with no significant effect at the low dose (0.5 nmol) (NS). There were no main effects of this antagonist on the intake of water [F(2,18) = 1.57, NS] or food [F(2,18) = 3.17, NS] (data not shown). In contrast to the aPVT, results from injections of L-733,060 in the pPVT showed no significant main effects on the intake of ethanol [F(2,18) = 0.89, NS] (Figure 2A), water [F(2,18) = 0. 02, NS] or food [F(2,18) = 0.03, NS]. Tests with injection of the NK1R antagonist RP 67580 revealed similar results. In the aPVT, RP 67580 had a significant main effect on ethanol intake [F(2,18) = 3.66, p < 0.05], with no significant interaction effect between drug and time [F(4,36) = 0.46, NS] (Figure 2B). Pairwise comparisons revealed a significant decrease in ethanol drinking with RP 67580 in the aPVT at the high dose (5.0 nmol) compared to vehicle (P < 0.05). Again, in the pPVT, there were no significant main effects of this antagonist on ethanol intake [F(2,18) = 1.59, NS] (Figure 2B) or in either PVT subregion on intake of water (aPVT: [F(2,18) = 0.91, NS]; pPVT: [F(2,18) = 0.07, NS]) or food (aPVT: [F(2,18) = 1.54, NS]; pPVT: [F(2,18) = 1.23, NS]). These findings with two different NK1R antagonists support the idea that SP in the aPVT acts endogenously to promote the drinking of ethanol.
Figure 2.
Injection with neurokinin 1 receptor antagonists into the anterior paraventricular thalamus (aPVT) significantly reduces ethanol drinking (Experiment 2). A L-733,060 (0.5, 5.0 nmol in 0.3 μl) compared to saline vehicle significantly reduces intermittent-access 20% ethanol drinking with injection into the aPVT (n = 10), but not into the posterior paraventricular thalamus (pPVT, n = 10). B Injection of RP 67580 (0.5, 5.0 nmol in 0.3 μl) compared to dimethyl sulfoxide vehicle significantly reduces intermittent-access 20% ethanol drinking with injection into the aPVT (n = 10), but not into the posterior paraventricular thalamus (pPVT, n = 10). Data are mean ± S.E.D.; *p < 0.05, #p = 0.06 vs. vehicle.
Experiment 3: Substance P gene expression in the PVT subregions is affected by orexin
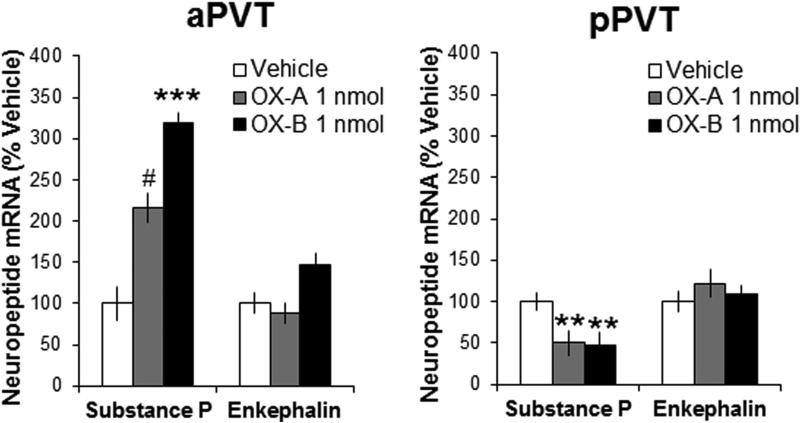
To determine if SP gene expression in the PVT is affected by OX, which has previously been found to affect ethanol drinking, analysis of the aPVT using qRT-PCR revealed a significant main effect of OX on local levels of SP mRNA [F(2,21) = 10.41, P < 0.001], with post-hoc tests showing a significant increase in the gene expression of SP after injection of OX-B (+219%, P < 0.001) and a trend for an increase after injection of OX-A (+116%, P = 0.06) (Figure 3). In contrast, in the pPVT, the significant main effect of OX injection on SP mRNA [F(2,21) = 10.19, P < 0.01] reflected a significant decrease in mRNA levels in response to both OX-B (−54%, P < 0.01) and OX-A (−50%, P < 0.01) (Figure 3). Results also showed a main effect of OX on mRNA levels of the opioid enkephalin (ENK) in the aPVT [F(2,21) = 4.17, P < 0.05]; however, post-hoc tests failed to reveal a significant change in ENK in response to either OX-A or OX-B (NS), and there were no significant main effects on ENK in the pPVT [F(2,21) = 0.49, NS] (Figure 3). Also, there was no significant main effect of OX on mRNA levels of the opioid dynorphin (DYN) in the aPVT [F(2,21) = 2.62, NS] or pPVT [F(2,21) = 2.30, NS] (data not shown), and ΔΔCT values for this neuropeptide were an order of magnitude lower than for SP and ENK, indicating that DYN was expressed only at low levels. These results demonstrate that local gene expression of SP, but not the opioids, is significantly affected by OX, with opposite effects in the aPVT and pPVT
Figure 3.
Injection of orexin into the subregions of the paraventricular thalamus significantly affects local gene expression of substance P (Experiment 3). Local administration of orexin-A (OX-A, 1 nmol in 0.3 μl) or orexin-B (OX-B, 1 nmol in 0.3 μl) compared to saline vehicle increases gene expression of substance P in the anterior paraventricular thalamus (aPVT, n = 8) but decreases it in the posterior paraventricular thalamus (pPVT, n = 8), as assessed by quantitative real-time polymerase chain reaction 60 minutes after injection. Data are mean ± S.E.M.; ***p < 0.001, **p < 0.01, #p = 0.06 vs. vehicle.
Experiment 4: Substance P peptide levels in the anterior PVT are increased by orexin-B
Using ELISA to examine SP peptide levels affected by OX injection in the PVT subregions, analysis of the aPVT revealed that OX compared to vehicle produced significant changes [F(2,12) = 8.57, P < 0.01], with post-hoc tests showing this to be due specifically to OX-B, which produced a small but significant increase in SP peptide levels (P < 0.01) while OX-A produced no significant change (NS) (Table 1). This effect in the aPVT contrasts with that in the pPVT, where no main effect of OX on SP levels was observed [F(2,12) = 0.65, NS] (Table 1). These results demonstrate that OX-B but not OX-A in the aPVT increases local SP peptide levels, with neither OX isoform having a significant effect in the pPVT.
Table 1.
Local levels of substance P after injection of orexin into the anterior or posterior paraventricular thalamus.
| Injection | aPVT Levels of SP (pg/ml) | pPVT Levels of SP (pg/ml) |
|---|---|---|
| Vehicle | 3532.6 ± 42.2 | 3620.0 ± 23.7 |
| Orexin-A 1 nmol | 3564.0 ± 23.5 | 3687.4 ± 48.1 |
| Orexin-B 1 nmol | 3699.4 ± 27.7* | 3657.1 ± 48.7 |
Injections were 0.3 μl of orexin-A (1 nmol), orexin-B (1 nmol), or saline vehicle (Experiment 4, n = 5/group). Data are mean ± S.E.M.
p < 0.05 vs. vehicle.
aPVT, anterior paraventricular thalamus; pPVT, posterior paraventricular thalamus; SP, substance P.
Experiment 5: Increase in substance P expression induced by orexin-B is postsynaptic
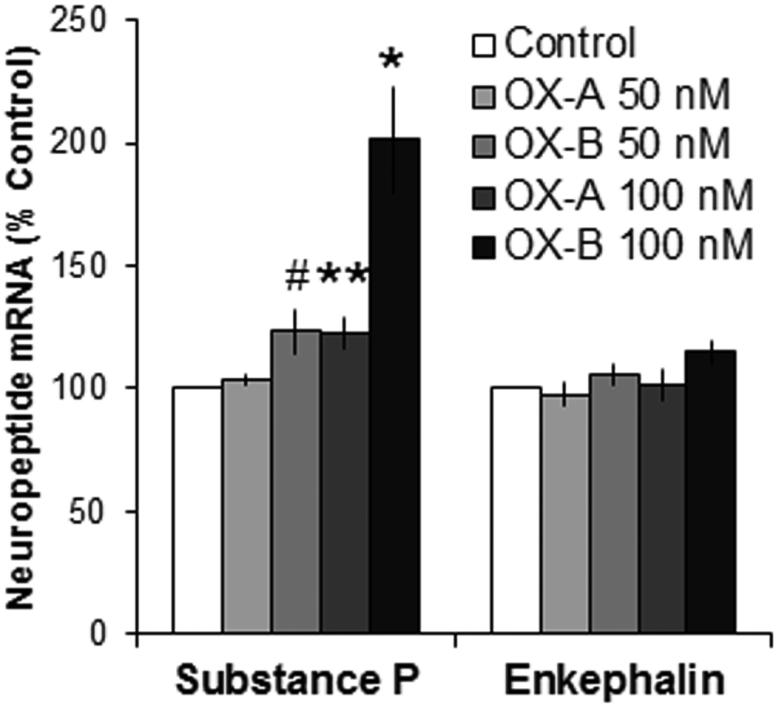
To ensure that the effects of OX on SP are direct postsynaptic neuronal effects, isolated primary thalamic neurons were treated with OX and examined for changes in mRNA using qRT-PCR. Analysis revealed a significant main effect of OX on levels of SP [F(4,12) = 14.51, P < 0.01]. Treatment with OX-B increased gene expression of SP to a greater extent than OX-A, as revealed by a significant linear trend from within-subject contrasts [F(1,3) = 41.10, P < 0.01] (Figure 4). Follow-up tests showed that the OX-B doses significantly stimulated SP mRNA [F(2,8) = 14.07, P < 0.01] and that this varied by dose [F(1,4) = 21.94, P < 0.01], with paired comparisons revealing a significant increase at the higher dose (+101%, P < 0.01) and a trend for an increase at the lower dose (+23%, P = 0.06). A similar but smaller effect was seen with the OX-A doses, which significantly stimulated SP [F(2,6) = 8.06, P < 0.05] according to dose [F(1,3) = 14.21, P < 0.05], with only the higher but not lower dose significantly stimulating gene expression (+23%, P < 0.05 and +4%, NS, respectively). As in Experiment 3, there were no significant main effects of OX on gene expression of ENK [F(4,12) = 1.33, NS] (Figure 4) or DYN [F(4,12) = 1.96, NS]. These results suggest that the increase in levels of SP in the aPVT in response to OX, as observed in Experiments 3 and 4, reflects direct postsynaptic actions of OX on local thalamic neurons.
Figure 4.
Treatment with orexin of isolated thalamic neurons significantly stimulates gene expression of substance P (Experiment 5). Treatment of cultured primary thalamic neurons with orexin-A (OX-A; 50, 100 nM) or orexin-B (OX-B; 50, 100 nM) compared to no-treatment control significantly increases gene expression of substance P, as assessed by quantitative real-time polymerase chain reaction 60 minutes after application (N = 10 litters, n = 4-5 litters/treatment). Data are mean ± S.E.M.; **p < 0.01, *p < 0.05, #p = 0.06 vs. control.
Experiment 6: Ethanol drinking is stimulated by orexin-B in the anterior PVT more than orexin-A
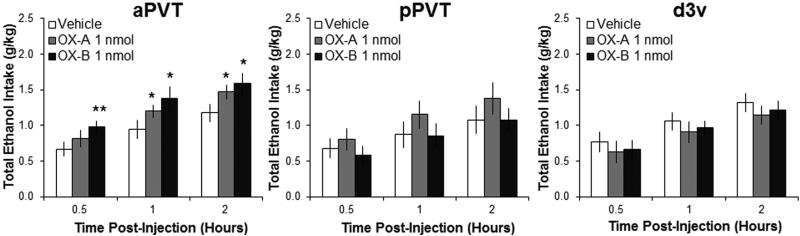
This experiment directly compared the effects on ethanol drinking of OX-A and OX-B in the PVT subregions, as well as in the d3v to ensure that effects were not due to leakage. In ethanol-drinking rats, results with injection in the aPVT of the OX isoforms revealed a significant main effect on ethanol intake [F(2,18) = 5.86, P < 0.05], with no significant interaction effect between drug and time [F(4,36) = 0.61, NS]. While pairwise comparisons showed both OX-A and OX-B compared to vehicle in the aPVT to stimulate ethanol drinking (P = 0.05 and P < 0.01, respectively), the effect size from OX-B was greater than from OX-A (η2P = 0.55 vs. η2P = 0.47), and within-subject contrasts revealed a significant linear trend [F(1,9) = 10.86, P < 0.01], demonstrating that drinking after OX-B was significantly greater than after OX-A (Figure 5). Measurements of the intake of water and food showed that there were no main effects of OX agonists on water [F(2,18) = 2.82, NS] or food intake [F(2,18) = 0.16, NS] (data not shown), confirming the substance specificity of this effect. These results contrast with those in the pPVT, where the OX agonists had no significant main effects on intake of ethanol [F(2,18) = 1.26, NS] (Figure 5), water [F(2,18) = 0.62, NS], or food [F(2,18) = 0.25, NS]. The results observed with injection in the d3v, showing no main effect of OX on intake of ethanol [F(2,16) = 1.32, NS] (Figure 5) or water [F(2,16) = 1.32, NS] (Table S2), further confirm that the increase in ethanol intake after injection in the aPVT was not due to the spread of OX to nearby brain regions. Instead, injection in the d3v led to a significant main effect on food intake [F(2,16) = 5.98, P < 0.05], which reflected a decrease in feeding that was similarly induced by injection of both OX-A and OX-B (P < 0.01 and P < 0.05, respectively) (Table S2). These findings show that OX increases ethanol drinking in a site-specific manner, with OX-B having stronger effects than OX-A, and that these effects are similar to those observed with SP.
Figure 5.
Injection of orexin into the anterior paraventricular thalamus (aPVT) significantly stimulates ethanol drinking, with orexin-B (OX-B) having larger effects than orexin-A (OX-A) (Experiment 6). Injection of OX-A (1 nmol in 0.3 μl) or OX-B (1 nmol in in 0.3 μl) compared to saline vehicle significantly increases intermittent-access 20% ethanol drinking with injection into the aPVT (n = 10), but not the posterior paraventricular thalamus (pPVT, n = 10) or adjacent dorsal third ventricle (d3v, n = 9) Data are mean ± S.E.D.; **p < 0.01, *p < 0.05 vs. vehicle.
Experiment 7: Substance P receptor antagonist blocks the effects of orexin-B in the anterior PVT
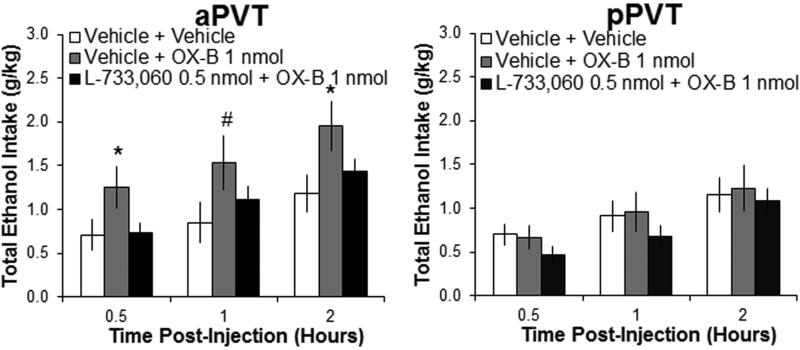
To determine if the integrity of the SP system is necessary for the changes in behavior produced by OX, ethanol-drinking rats were first injected in the aPVT or pPVT with the lower dose of the NK1R antagonist, L-733,060 (0.5 nmol), which in Experiment 2 did not affect ethanol intake, and were then injected with OX-B, which generally showed stronger effects than OX-A on both ethanol drinking and SP expression. With injection in the aPVT, results revealed a significant main effect of drug on ethanol intake [F(2,16) = 4.03, P < 0.05]. Pairwise comparisons showed that pretreatment with the sub-threshold dose of the NK1R antagonist completely blocked the stimulatory effect of OX-B in the aPVT on ethanol drinking (P < 0.05), with intake after the combined injection of these two drugs showing no difference from intake after vehicle (NS) (Figure 6). Tests of the simple effects in the aPVT showed that the significant interaction effect between drug and time F(4,32) = 3.64, P < 0.05] was due to a significant stimulatory effect of OX-B on ethanol intake at 30 minutes and 2 hours post-injection (P < 0.05), with a trend for increased intake at 1 hour post-injection (P = 0.06). In contrast, drug injections in the pPVT of OX-B alone or in combination with L-733,060 produced no significant main effect on ethanol intake [F(2,16) = 0.61, NS] (Figure 6), consistent with the lack of effect in this subregion of OX-B or this antagonist on ethanol drinking. There were also no significant main effects of these drugs on water intake with any injections in the aPVT [F(2,16) = 1.00, NS] or pPVT [F(2,16) = 0.76, NS], or on food intake with any injections in the aPVT [F(2,16) = 2.56, NS] or pPVT [F(2,18) = 0.61, NS] (data not shown). These results indicate that hypothalamic OX acts through local SP in the aPVT to alter ethanol drinking.
Figure 6.
Injection of a neurokinin 1 receptor antagonist blocks orexin-induced ethanol drinking. Pretreatment with a sub-threshold dose of the neurokinin 1 receptor antagonist L-733,060 (0.5 nmol in 0.3 μl) compared to saline vehicle prevents the increase in intermittent-access 20% ethanol drinking induced by injection of orexin-B (OX-B, 1 nmol) into the anterior paraventricular thalamus (aPVT) as compared to saline vehicle. Neither L-733,060 at this dose nor OX-B alters ethanol drinking with injection into the posterior paraventricular thalamus (pPVT) (Experiment 7; N = 18, n = 9/group). Data are mean ± S.E.D.; *p < 0.05, #p = 0.06 vs. vehicle.
DISCUSSION
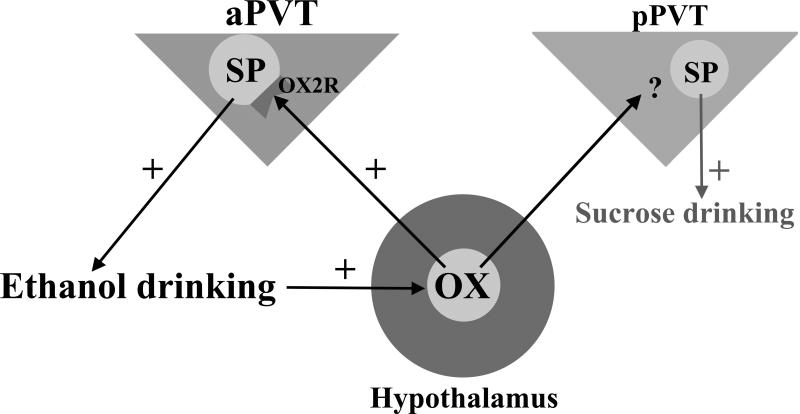
This study identifies a new function for SP in subregions of the PVT. The results demonstrate that this neuropeptide, similar to OX, endogenously promotes intermittent-access ethanol drinking through actions specifically in the aPVT, whereas it promotes in the pPVT intake of the food sucrose rather than the drug ethanol. Moreover, they show that SP in the aPVT is stimulated by OX, particularly OX-B, likely projecting from the hypothalamus and that SP plays a role in mediating the effects of OX in this brain region (see Figure 7). These results provide support for the new idea that SP in the PVT contributes to addictive behavior, much like OX, a well-established target for drug addiction treatment (Khoo and Brown, 2014).
Figure 7.
Potential pathways, nuclei, and neuropeptide systems involved in intermittent-access 20% ethanol drinking. Ethanol drinking is hypothesized to be promoted by orexin/hypocretin (OX) from the hypothalamus acting at the orexin 2 receptor (OX2R) on substance P (SP)-containing neurons in the anterior paraventricular thalamus (aPVT). Ethanol drinking can also increase levels of OX to further excite this aPVT pathway. In contrast, SP in the posterior paraventricular thalamus (pPVT) promotes sucrose intake.
Our finding that SP injection in the aPVT stimulates ethanol consumption is the first evidence that SP itself can enhance ingestive behavior. While earlier studies have reported that moderate-to-high doses of SP in other brain regions suppress ethanol drinking (Nikolaev et al., 2002; Yang et al., 2009), the present study reveals a stimulatory effect of SP at low-to-moderate doses in the aPVT. This increase in ethanol drinking is consistent with studies showing ethanol self-administration and consumption to be reduced by systemic NK1R antagonists in rats and NK1R knockout in mice (George et al., 2008; Schank et al., 2013; Steensland et al., 2010). The distinct effects of different doses of SP, with low levels promoting and high levels inhibiting ethanol drinking, are similar to findings with other neuropeptides, such as corticotropin releasing factor, and with stress which interacts with corticotropin releasing factor (Le et al., 2002; Uhart and Wand, 2009), and they may explain the present finding of later drinking onset after the moderate compared to low dose of SP. Our additional findings, that two different NK1R antagonists in the aPVT suppress ethanol drinking, provide strong support for the idea that endogenous SP is responsible this behavior. These results were obtained with RP 67580, which has high affinity for rodent NK1R (Seabrook et al., 1996) and shows high selectivity for this receptor (Beaujouan et al., 1993), and also with L-33,060, which has a stronger affinity for human NK1R (Seabrook et al., 1996). The additional finding here, that SP in the pPVT stimulates sucrose drinking, underscores the behavioral and anatomical specificity of this peptide's action, with SP in the aPVT stimulating ethanol intake and SP in the pPVT stimulating palatable food intake. This effect in the pPVT of SP on sucrose drinking is consistent with evidence that a systemic NK1 antagonist reduces this behavior (Steensland et al., 2010), lending further support for the idea that central SP promotes ingestive behavior.
Our evidence showing SP in the PVT to have similar behavioral effects as OX is an important step toward establishing a link between these neuropeptides. Specifically, like OX (Barson et al., 2015), we show that SP stimulates ethanol drinking when injected into the aPVT and sucrose drinking when injected into the pPVT. Our additional finding, that SP and OX injected into the adjacent third ventricle similarly suppress food and water intake, not only confirms that the effects of these neuropeptides in the PVT are not due to leakage into the ventricle but also demonstrates that these peptides can have similar effects in multiple brain regions. This idea is supported by other studies, showing both SP and OX to reduce food intake when injected into the ventricle (Dib, 1999; Yamanaka et al., 1999), produce anxiety in an elevated plus maze when injected into the amygdala (Avolio et al., 2011; Bassi et al., 2014), and induce antinociception when injected into the periaqueductal gray (Azhdari Zarmehri et al., 2011; Bassi et al., 2007).
The similar effects of SP and OX in the PVT subregions may reflect a physical interaction between these neuropeptides, at least in the aPVT. The finding that OX increases gene expression and peptide levels of SP in this subregion is the first direct evidence for a close relationship between these neuropeptides. This is consistent with indirect evidence, showing that a mutation of OX2R decreases gene expression of tachykinin precursor 1 (Lindberg et al., 2007) and that OX1R exists on SP-containing dorsal root ganglion neurons (Colas et al., 2014). The smaller increase in SP peptide relative to mRNA levels may reflect the difference in the timing of their measurement, with levels possibly higher shortly after OX injection. The additional result, that OX stimulates SP expression in isolated thalamic neurons, confirms that the increase of SP in the aPVT is due to postsynaptic actions of OX on local neurons. In addition to affecting SP-containing neurons in the PVT, it is possible that OX affects the release into the PVT of SP peptide from afferents from other brain regions, such as the Edinger-Westphal nucleus, mesopontine tegmentum, or raphe nuclei (Otake, 2005). Although an OX2R antagonist is needed to show definitively that this OX-induced increase in SP is physiologically relevant, the OX dose used here was relatively low, and OX does not bind to NK1R (Gerashchenko et al., 2001; Hokfelt et al., 2001). In contrast to the aPVT, SP in the pPVT may not interact directly with OX. This is suggested by our evidence that SP peptide levels in this subregion were unaffected and mRNA levels were decreased by local OX. These effects in the pPVT may reflect actions of OX on presynaptic terminals (Kukkonen and Leonard, 2014) or its interaction with other local neurochemicals such as glutamate (Huang et al., 2006). Together, this evidence focuses attention on SP in the aPVT as being directly and selectively stimulated by OX.
Our results indicate that the OX-induced release of SP into the aPVT has behavioral consequences. Specifically, the finding that OX-B-induced ethanol drinking is blocked by aPVT injection of an NK1R antagonist at a subthreshold dose suggests that the local release of SP is involved in mediating OX's ability in the aPVT to affect ingestive behavior. Levels of OX in the hypothalamus are elevated both during drug seeking (Dayas et al., 2008; Harris et al., 2005) and after ethanol intake (Barson et al., 2015; Lawrence et al., 2006; Morganstern et al., 2010). Notably, antagonists of the OX and SP systems both reduce stress-induced ethanol seeking (Richards et al., 2008; Schank et al., 2014). Since the aPVT is activated by an incentive stimulus (Flagel et al., 2011), SP in the aPVT may endogenously promote ethanol intake in response to the desire for ethanol induced by OX. Further research is needed to determine whether SP in the aPVT is actually involved in the process of drug seeking and if this SP-induced seeking is prevented by blockade of endogenous OX signaling.
In light of evidence that OX-B preferentially targets OX2R and OX-A targets both OX2R and OX1R (Sakurai et al., 1998), our findings here implicate OX2R as the receptor responsible for many of the effects described. In the aPVT, OX-B was found to induce larger effects than OX-A, on ethanol drinking as well as SP expression. This is consistent with electrophysiological studies, showing OX-B to be more potent than OX-A in depolarizing and exciting neurons in the PVT (Huang et al., 2006; Ishibashi et al., 2005). Specifically, while a large majority of neurons in the PVT respond to one or both OX isoforms, their increase in firing rate is larger for OX-B than for OX-A at the same concentration (Ishibashi et al., 2005; Kolaj et al., 2014). This indicates that it is binding of OX2R that is primarily responsible for OX-induced neuronal activation in the PVT. This is substantiated by our recent finding that ethanol drinking is decreased by an antagonist of OX2R but not OX1R in the aPVT (Barson et al., 2015). The present results thus support a role specifically for OX2R in the aPVT in promoting ethanol intake and suggest that OX2R may also be the primary OX receptor involved in the aPVT-induced increase in SP expression.
Together, our findings support the participation of the PVT in the addiction neurocircuitry and demonstrate that certain neuropeptides in this nucleus have subregion-specific effects on substance intake, with the aPVT rather than the pPVT being the subregion involved in ethanol drinking. Most importantly, these results point to SP as a potential target for the treatment of addictive behavior.
Supplementary Material
Acknowledgements
This research was supported by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Numbers R01AA12882 (S.F.L.), K99AA021782/R00AA021782 (J.R.B.), and F32DK100058 (K.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We extend gratitude to The Rockefeller University's Genomics Resource Center and the Translational Technology Core Laboratory, which is supported by Grant Number UL1TR0043 from the National Center for Advancing Translational Sciences, NIH Clinical and Translational Science Award program.
Footnotes
Authors Contribution
J.R.B, K.P., and S.F.L. were responsible for the study concepts and design. J.R.B., K.P., H.T.H., M.A., and L.S. collected and analyzed the data. J.R.B. drafted the manuscript and prepared the figures. J.R.B and S.F.L. edited and revised the manuscript. All authors critically reviewed the content and approved the final version for publication.
REFERENCES
- Avolio E, Alo R, Carelli A, Canonaco M. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav Brain Res. 2011;218:288–295. doi: 10.1016/j.bbr.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Azhdari Zarmehri H, Semnanian S, Fathollahi Y, Erami E, Khakpay R, Azizi H, Rohampour K. Intra-periaqueductal gray matter microinjection of orexin-A decreases formalin-induced nociceptive behaviors in adult male rats. J Pain. 2011;12:280–287. doi: 10.1016/j.jpain.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi GS, de Carvalho MC, Brandao ML. Effects of substance P and Sar-Met-SP, a NK1 agonist, in distinct amygdaloid nuclei on anxiety-like behavior in rats. Neurosci Lett. 2014;569:121–125. doi: 10.1016/j.neulet.2014.03.065. [DOI] [PubMed] [Google Scholar]
- Bassi GS, Nobre MJ, de Araujo JE, Brandao ML. Anxiogenic effects of activation of NK-1 receptors of the dorsal periaqueductal gray as assessed by the elevated plus-maze, ultrasound vocalizations and tail-flick tests. Neuropeptides. 2007;41:365–374. doi: 10.1016/j.npep.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Spreafico R, Rustioni A. Substance P innervation of the rat and cat thalamus. I. Distribution and relation to ascending spinal pathways. J Comp Neurol. 1992;315:457–472. doi: 10.1002/cne.903150408. [DOI] [PubMed] [Google Scholar]
- Beaujouan JC, Heuillet E, Petitet F, Saffroy M, Torrens Y, Glowinski J. Higher potency of RP 67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br J Pharmacol. 1993;108:793–800. doi: 10.1111/j.1476-5381.1993.tb12880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai B, Guo W, Wei F, Dubner R, Ren K. Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury. Mol Pain. 2012;8:78. doi: 10.1186/1744-8069-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas D, Manca A, Delcroix JD, Mourrain P. Orexin A and orexin receptor 1 axonal traffic in dorsal roots at the CNS/PNS interface. Front Neurosci. 2014;8:20. doi: 10.3389/fnins.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Dib B. Food and water intake suppression by intracerebroventricular administration of substance P in food- and water-deprived rats. Brain Res. 1999;830:38–42. doi: 10.1016/s0006-8993(99)01379-7. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J Intern Med. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–481. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28:713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Hermes ML, Renaud LP. Intrinsic properties and neuropharmacology of midline paraventricular thalamic nucleus neurons. Front Behav Neurosci. 2014;8:132. doi: 10.3389/fnbeh.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Sui N, Kirouac GJ. Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav. 2009;93:506–514. doi: 10.1016/j.pbb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212:251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Lindberg J, Saetre P, Nishino S, Mignot E, Jazin E. Reduced expression of TAC1, PENK and SOCS2 in Hcrtr-2 mutated narcoleptic dog brain. BMC Neurosci. 2007;8:34. doi: 10.1186/1471-2202-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP, Maggio JE. Substance P receptors: localization by light microscopic autoradiography in rat brain using [3H]SP as the radioligand. Brain Res. 1984;307:147–165. doi: 10.1016/0006-8993(84)90470-0. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front Behav Neurosci. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Skirboll LR, Pert CB. Comparison of substance P and enkephalin distribution in rat brain: an overview using radioimmunocytochemistry. Neuroscience. 1985;14:837–852. doi: 10.1016/0306-4522(85)90147-2. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev SV, Bychkov ER, Lebedev AA, Dambinova SA. [The influence of substance P central administration on ethanol intake in rats chronically exposed to alcohol]. Ross Fiziol Zh Im I M Sechenova. 2002;88:907–913. [PubMed] [Google Scholar]
- Otake K. Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res. 2005;51:383–394. doi: 10.1016/j.neures.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th Edition Elsevier Academic Press; San Diego, CA: 2005. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, Weinshenker D, Schroeder JP. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39:1093–1101. doi: 10.1038/npp.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Tapocik JD, Barbier E, Damadzic R, Eskay RL, Sun H, Rowe KE, King CE, Yao M, Flanigan ME, Solomon MG, Karlsson C, Cheng K, Rice KC, Heilig M. Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol-preferring rats. Biol Psychiatry. 2013;73:774–781. doi: 10.1016/j.biopsych.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Shepheard SL, Williamson DJ, Tyrer P, Rigby M, Cascieri MA, Harrison T, Hargreaves RJ, Hill RG. L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]i mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yang AR, Yi HS, Mamczarz J, June HL, Jr., Hwang BH, June HL., Sr. Deficits in substance P mRNA levels in the CeA are inversely associated with alcohol-motivated responding. Synapse. 2009;63:972–981. doi: 10.1002/syn.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.