Abstract
Growing teratoma syndrome is a rare condition among patients with nonseminomatous germ cell tumors who present with enlarging metastatic masses during appropriate systemic chemotherapy and normalized serum markers. Retroperitoneal residual masses are a common finding after chemotherapy for the nonseminomatous tumors of the testis. These might contain mature teratoma, fibrotic tissue, or tumor. Mature teratoma, which is unresponsive to chemotherapy, might result from evolution of a malignant lesion during treatment or it might represent a metastasis from a focus of mature teratoma in the primary testicular tumor. This article reviews a case of a growing teratoma syndrome.
Keywords: Nonseminomatous germ cell tumors, Chemotherapy, Residual masses, Mature teratoma
Introduction
The efficiency of chemotherapy on nonseminomatous germ cell tumors (NSGCTs) is no longer to be demonstrated. The existence of a residual mass at the end of the treatment requires the excision of the former. That is, in fact, the only way to affirm the histologic nature conditioning the subsequent conduct of the treatment.1 The pathologic analysis of these residual masses might reveal either the persistence of malignant cells or the presence of a fibrosis, a necrosis, or finally, the existence of a mature teratoma.2 The latter situation has been encountered in our patient.
Case presentation
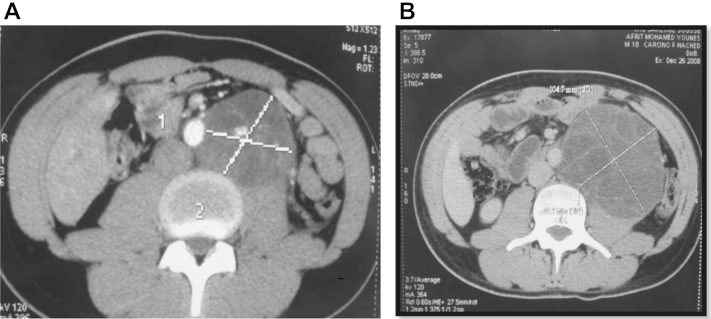
A 19-year-old patient consulted for a swelling of the left testicular. The clinical examination found a large, firm abdominal mass, attached to the deep plane, localized at the left flank. The examination of the external genital organs found an enormous mass at the left testicular of 15-cm long axis without associated inflammatory signs. An abdominal and pelvic computed tomography (CT) revealed a left retroperitoneal mass measuring 8 × 6 cm displacing the aorta to the right and compressing the left ureter (Fig. 1A) with bilateral hilar lymph nodes (maximum diameter 28 mm). It also showed a left testicular mass measuring 10 × 10 cm.
Figure 1.
(A) Retroperitoneal mass measuring 8 × 6 cm displacing the aorta to the right and compressing the left ureter. (B) Growing retroperitoneal mass measuring 12 × 12 cm.
Serum tumor markers were twice as high as the normal. Our patient had an orchiectomy followed by 3 cycles of chemotherapy (bleomycin, cisplatin, and etoposide) for a stage IIC mixed NSGCT containing a teratomatous component and an embryonal carcinoma. Serum tumor markers were normalized after the first cycle of chemotherapy.
At initial staging, hilar lymph nodes have regressed on CT data, instead the retroperitoneal mass has increased (maximum diameter 12 × 12 cm; Fig. 1B).
Our patient had a second – line chemotherapy (ifosfamide plus etoposide and cisplatin). Two months later, a comparative abdominal scanner has shown that the retroperitoneal mass continued to increase (maximum diameter was 12 × 15 cm) and was responsible of a hydronephrosis. Clinically, the patient complained of an abdominal discomfort.
Given the negative tumor marker and the imaging features, growing teratoma syndrome (GTS) was hypothesized. The patient underwent surgery that consisted of a complete resection of the mass.
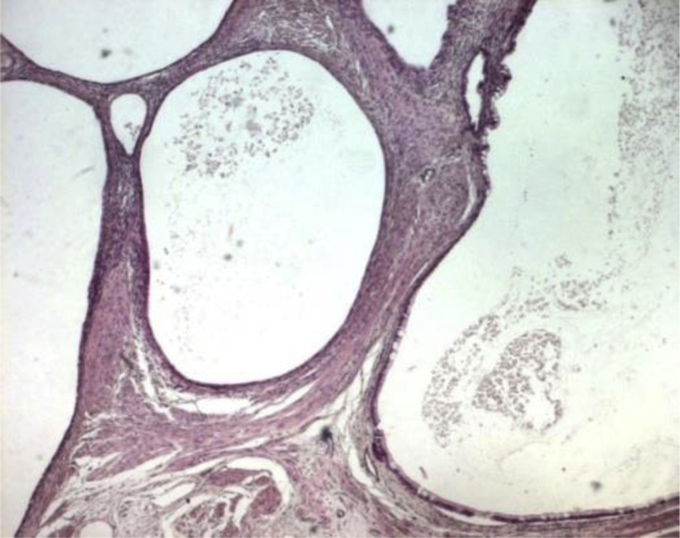
Pathologic examination of the resected lesion confirmed the diagnosis of mature teratoma in his multicystic form (Fig. 2) without viable tumor.
Figure 2.
Teratoma in his multicystic form.
Eighteen months later, our patient is in good health without any local or distant recurrence.
Discussion
The term GTS was coined by Logothetis et al, in 1982, to describe a rare entity among patients with NSGCTs, characterized by enlarging metastatic masses despite appropriate systemic chemotherapy and normalized serum markers. The diagnosis is confirmed by the presence of mature teratoma and the absence of any malignant germ cells on final surgical pathology.
The prevalence of GTS in metastatic NSGCT is between 1.9% and 7.6%.3 GTS is most commonly observed in the retroperitoneum but has also been described in the lung, mediastinum, supra clavicular lymph nodes, inguinal lymph nodes, forearm, mesentery, and liver. Our patient presented a retroperitoneal localization.
The etiology of GTS is unclear. The 2 most-quoted theories are that chemotherapy destroys only the immature malignant cells, leaving the mature benign teratomatous elements, and4 chemotherapy alters the cell kinetics toward transformation from a totipotent malignant germ cell toward a benign mature teratoma.
A third hypothesis offered by Hong et al5 proposes an inherent and spontaneous differentiation of malignant cells into benign tissues, as suggested by the experimental murine teratocarcinoma mouse model. In our case, the probable assumption is the transformation of the nonseminomatous tumors into a mature teratoma because the mass existed at the beginning of treatment.
GTS poses a diagnostic challenge for both medical oncologists and urologists because of its rarity and unusual presentation.
A growing mature teratoma is characterized by enlarging metastatic masses, despite appropriate systemic chemotherapy and normalized serum markers. The preferred treatment is complete surgical resection because teratoma was resistant to chemotherapy and radiation therapy.6 The chemotherapy used before establishing a diagnosis of GTS includes a variety of single agents, such as actinomycin D or cyclophosphamide, or various combinations of adriamycin, bleomycin, etoposide, vinblastine, cyclophosphamide, chlorambucil, methotrexate, nitrogen mustard, and cisplatin.6
In our case, we have administrated a second line of chemotherapy (ifosfamide plus etoposide and cisplatin), but the retroperitoneal mass continues to increase, and the surgical treatment was indicated only when patient presented an uretero-pyelocalicial expansion.
Finally, growing mature teratoma is unresponsive to systemic chemotherapy and requires surgical excision to avoid malignant transformation or complications such as compression of adjacent structures such as an ureterohydronephrosis, subocclusive syndromes, venous, and lymphatic stasis.7
Although GTS has an excellent prognosis, regular follow-up is critical, as very late malignant masses do occur in some patients. In fact, in an effort to avoid late diagnosis of GTS, Spiess et al8 recommend regular imaging in patients undergoing chemotherapy, possibly after 2 cycles of chemotherapy, to ensure careful monitoring of subtle changes in tumor size and appearance. Imaging findings and tumor markers can suggest the presence of mature lesions, but surgical excision or sampling of the lesions is mandatory to exclude recurrent malignancy.4
Conclusion
A growing mature teratoma is a progressive form of NSGCT characterized by a negative tumor marker and a specific CT scan features. It is unresponsive to chemotherapy testicular tumors. The only treatment is surgical excision to avoid its complications.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Available online 25 December 2013
References
- 1.Houlgatte A., Auberget J.L., Berlizot P., et al. Les tératomes matures d'origine testiculaire Réflexions à propos de douze cas. Progrès en Urologie. 1991:1–6. [PubMed] [Google Scholar]
- 2.Jeffery G.M., Theaker J.M., Lee A.H., et al. The growing teratoma syndrome. Br J Urol. 1991;67:195–202. doi: 10.1111/j.1464-410x.1991.tb15109.x. [DOI] [PubMed] [Google Scholar]
- 3.Gorbatiy Vladislav, Spiess Philippe E., Pisters Louis L. The growing teratoma syndrome: current review of the literature. Indian J Urol. 2009;25:186–189. doi: 10.4103/0970-1591.52910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimkin Katherine, Gupta Punita, Mccauley Roy, et al. The growing teratoma syndrome. Pediatr Radiol. 2004;34:259–262. doi: 10.1007/s00247-003-1045-z. [DOI] [PubMed] [Google Scholar]
- 5.Hong W.K., Wittes R.E., Hajdu S.T., et al. The evolution of mature teratoma from malignant testicular tumors. Cancer. 1977;40:2987–2992. doi: 10.1002/1097-0142(197712)40:6<2987::aid-cncr2820400634>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Gorbatiy Vladislav, Spiess Philippe E., 1st, Pisters Louis L. The growing teratoma syndrome: current review of the literature. Indian J Urol. 2009 Apr-Jun;25:186–189. doi: 10.4103/0970-1591.52910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aide N., Comoz F., Savin E. Enlarging residual mass after treatment of a nonseminomatous germ cell tumor: growing teratoma syndrome or cancer recurrence? J Clin Oncol. 2007;25:4494–4496. doi: 10.1200/JCO.2007.12.7530. PubMed: 17906212. [DOI] [PubMed] [Google Scholar]
- 8.Spiess P.E., Kassouf W., Brown G.A., et al. Surgical management of growing teratoma syndrome: the M. D. Anderson Cancer Center. Experience J Urol. 2007;177:1330–1334. doi: 10.1016/j.juro.2006.11.086. [DOI] [PubMed] [Google Scholar]