Abstract
Recent studies have shown that ozone (O3) is endogenously generated in living tissues, where it makes both positive and negative physiological contributions. A pathway for the formation of both O3 and hydrogen peroxide (H2O2) was previously proposed, beginning with the antibody or amino acid-catalyzed oxidation of water by singlet oxygen (1O2) to form hydrogen trioxide (H2O3) as a key intermediate. A key pillar of this hypothesis is that some of the H2O2 molecules incorporate water-derived oxygen atoms. However, H2O3 decomposes extremely readily in water to form 1O2 and water, rather than O3 and H2O2. This article highlights key literature indicating that the oxidation of organic molecules such as the amino acids methionine, tryptophan, histidine, and cysteine by 1O2 is involved in ozone formation. Based on this, an alternative hypothesis for ozone formation is developed involving a further reaction of singlet oxygen with various oxidized organic intermediates. H2O2 having water-derived oxygen atoms is subsequently formed during ozone decomposition in water by known reactions.
Keywords: Ozone, Singlet oxygen, Hydrogen peroxide, Amino acid oxidation, Baeyer-Villiger oxidation
Introduction
Ozone gas (O3,δ+ O = O–O δ−) is an important component of the stratosphere where it protects organisms on earth from the most damaging wavelengths of solar radiation [1]. On the other hand, tropospheric ozone may be harmful to the respiratory system [1]. Nevertheless, ozone gas finds some application in alternative medicine [2] as well as in water and wastewater treatment [3].
Over a decade ago, Wentworth et al. [4] reported that antibodies catalyze ozone formation in the presence of water and singlet oxygen (1O2), partly based on the observed occurrence, under such conditions, of reactions that were thought to be unique to oxidation by ozone. These reactions included the conversion of indigo carmine to isatin sulfonic acid and the conversion of cholesterol to 3β-hydroxy-5-oxo-5,6-sechocholestan-6-al (secosterol aldehdye A) and 3β-hydroxy, 5β-hydroxy- B-norcholestane-6β-carboxaldehyde (secosterol aldehyde B). On the positive side, ozone generation in this manner contributes to bacterial killing by neutrophils [4]. Moreover, one of the best photodynamic therapy (PDT) strategies for cancer treatment involves the use of antibodies conjugated to photosensitizers [5], and the anticancer effect of this type of PDT may partly be due to antibody-catalyzed ozone generation upon irradiation of the photosensitizers and formation of 1O2. On the other hand, the secosterol aldehydes produced by cholesterol ozonolysis potentially contribute to the pathogenesis of disorders such as atherosclerosis and Alzheimer’s disease [6,7].
The concept of ozone generation in biological systems generated reasonable skepticism, and experimental results were obtained proving that conversion of indigo carmine to isatin sulfonic acid, or the formation of cholesterol secosterol aldehydes could also be mediated by oxidants other than ozone [8,9]. For example, it was demonstrated that cholesterol 5-hydroperoxide, obtained by the reaction of cholesterol with singlet oxygen, undergoes Hock cleavage to form the secosterol aldehydes [9]. However, the singlet oxygen-Hock cleavage pathway predominantly generates secosterol aldehyde B [7,9], while the ozonolysis pathway predominantly generates secosterol aldehyde A [7]. The fact that the latter is the major secosterol aldehyde in atherosclerotic tissues [7] and is also produced in vitro by neutrophils [10] supports the occurrence of ozone-mediated cholesterol oxidation in vivo [11]. More recently, the formation of endogenous ozone by plant leaves was proved directly by GC-MS-SIM [1]. It was also reported that the antibiotic activity of a number of compounds including trans-resveratrol, salicylic acid, and cinnamic acid involves the endogenous formation of formaldehyde and ozone [2,12,13].
A pathway involving the oxidation of water by 1O2 was proposed for the antibody-catalyzed ozone formation [4], mainly based on the fact that some hydrogen peroxide molecules (H2O2) formed under the same conditions incorporate oxygen atoms from water molecules [14]. However, this proposal, which assumes that the O3 and H2O2 are products of the same pathway, has not been unequivocally proved.
Here, I reinterpret available literature and develop an alternative hypothesis for O3 formation involving the oxidation and deoxidation of specific types of organic compounds. Pathways of methionine-, tryptophan-, and histidine-catalyzed ozone formation as well as a pathway of ozone formation during formaldehyde oxidation are proposed as examples of O3 generation in this manner. A common feature of all the ozone-forming steps is that 1O2 reacts as an electrophile with a nucleophilic oxygen atom in the immediate precursor molecule, whose structure is such that loss of an O3 molecule results in co-formation of a non-charged product. I also propose that the decomposition of O3 in water may be largely responsible for the production of H2O2 containing water-derived oxygen atoms.
The antibody/amino acid-catalyzed water oxidation pathway and the need for alternative pathways of O3 formation
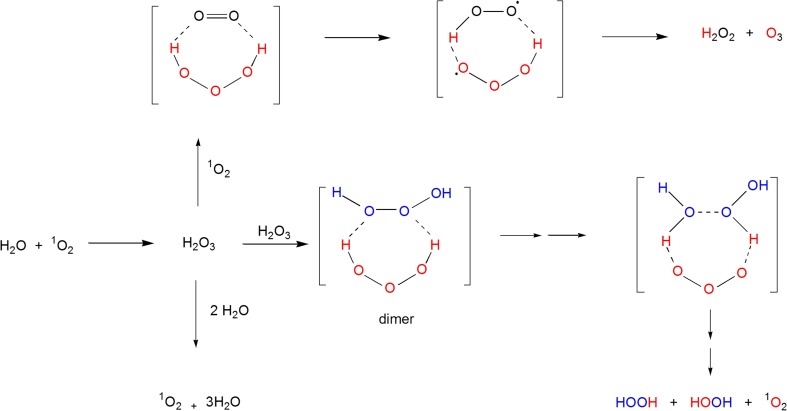
The antibody-catalyzed ozone formation occurs under conditions where H2O2 is also generated in a high yield of >500 molecules per antibody molecule [4,14]. Thus, O3 and H2O2 were postulated to be formed by a common pathway, the antibody-catalyzed water oxidation pathway which begins with the reaction of 1O2 with water to generate hydrogen trioxide (H2O3) as a key intermediate (Fig. 1) [4]. Two possible routes for the conversion of H2O3 to H2O2 and O3 have been proposed and shown to be feasible based on theoretical calculations [15,16]. The first of these routes involves a reaction of H2O3 with 1O2 while the other involves a reaction of the former with another molecule of H2O3 (Fig. 1). Notably, however, a third route for H2O3 decomposition involving its water catalyzed conversion to 1O2 and water (Fig. 1) is well established both theoretically and experimentally [16]. In fact, H2O3 is extremely unstable (with a half-life of less than a second) under aqueous conditions, where it basically decomposes to H2O and 1O2 [16]. The antibody-catalyzed formation of O3 and H2O2 was suggested to occur in a hydrophobic site where the H2O3 is shielded from water [14]. However, even in organic solvents, all attempts to unambiguously detect H2O2 and O3 as products of the decomposition of H2O3 have failed [16].
Fig. 1.
The water oxidation pathway involving the initial formation of H2O3 followed by decomposition of the latter by (i) reaction with singlet oxygen to form H2O2 and O3, (ii) reaction with another H2O3 molecule to form 2H2O2 and 1O2 with the intermediacy of ozone, or (iii) the water catalyzed decomposition to 1O2 and H2O [4,15,16]. The latter reaction is extremely facile under aqueous conditions [16]
The possible role of amino acid oxidation in the antibody-catalyzed formation of H2O2 as an alternative to the water oxidation pathway was discounted because of the consideration that, even if every photooxidizable amino acid residue (cysteine, methionine, histidine, tryptophan, and tyrosine) in an antibody molecule were consumed, this could not account for the >500 mole equivalents of H2O2 generated [14]. Curiously, however, Yamashita et al. [17] found that out of 19 amino acids tested (excluding tyrosine), the photooxidizable amino acids methionine, cysteine, histidine, and tryptophan catalyzed the formation of ozone in the presence of singlet oxygen, comparably to antibodies, and that peptides lacking these amino acids did not generate ozone. They postulated that the amino acids catalyzed ozone formation through the water oxidation pathway but did not propose how the amino acids performed the catalysis. However, a major role for H2O3 in ozone formation under these conditions is doubtful, even from the consideration that hydrophobic sites for its stabilization in amino acid solutions are unlikely to be present.
Hence, there is a need to explore alternative pathways for O3 and H2O2 formation that do not involve H2O3 as the key intermediate.
Evidence that the oxidation of amino acids and other organic molecules may be important for ozone generation
The common feature of the four amino acids that catalyze ozone formation [17] is that they are photooxidizable [14], and numerous studies have documented their oxidation by singlet oxygen under physiologically relevant conditions, both in the free form and as components of proteins [18–32].
As components of proteins, the reactivity of these amino acids with 1O2 greatly depends on their positions in the proteins, with residues exposed to solvent being oxidizable, while the less accessible residues remain unoxidized [20,21]. While only few amino acid residues in antibodies seem to get oxidized in the presence of singlet oxygen [22], such few residues might be sufficient for O3 and H2O2 formation. For example, just one exposed tryptophan residue was found to contribute 50 % of H2O2 production by a monoclonal antibody [23]. To date, the oxidative modification of specific tryptophan, methionine, and histidine residues in antibodies and monoclonal antibodies by singlet oxygen has been reported [23–26]. Of particular interest is the recent discovery that 1O2 mediates the formation of cross-linked histidine-histidine dimers [27] and that two identical conserved histidine residues in the highly flexible and solvent-accessible hinge region of an immunoglobulin G1 (IgG1) antibody undergo photooxidation to form such dimers [26].
Exposure of plant leaves to ozone causes damage to the leaves’ photosystem 2 (PS II) [33]. PS II damage was also found to occur during light-induced oxidative stress in a process that involves 1O2-mediated oxidation of specific tryptophan residues [29]. PS II damage under the latter conditions is consistent with endogenous ozone production by the leaves [1] through a process involving tryptophan oxidation. In a related study, exogenous histidine was found to promote oxygen uptake and damage to PS II of the cyanobacterium Synechocystis PCC 6803, and the increased oxygen uptake was attributed to ‘chemical trapping’ of singlet oxygen by histidine [30].
Tyihak et al. [12,13] more recently reported that compounds such as formaldehyde, cinnamic acid, and resveratrol were precursors of ozone. They postulated a formaldehyde/O3 pathway in which formaldehyde reacts with H2O2 to generate activated formaldehyde and 1O2, followed by participation of the latter in the water oxidation pathway to generate ozone [12,13]. However, in atmospheric chemistry, formaldehyde is known as an important ozone precursor by a mechanism involving its oxidation [34] and not merely the production of 1O2.
Thus, it is worthwhile to consider pathways of 1O2-mediated amino acid and formaldehyde oxidation, with the aim of identifying potential steps that could be involved in O3 formation.
Suggested pathways of ozone formation via reactions of singlet oxygen with amino acids or formaldehyde
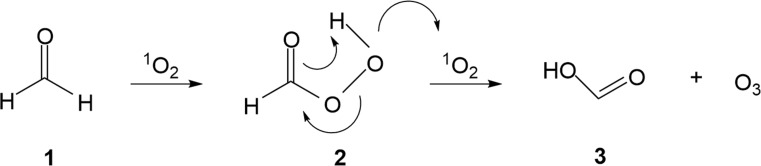
It was recently reported that various aliphatic and aromatic aldehydes react with 1O2 to form the corresponding organic acids [35]. The initial reaction of the aldehyde with 1O2 involves a hydride transfer and generates a peroxyacid, which undergoes a Baeyer-Villiger oxidation with a second aldehyde molecule to form two molecules of acid [35]. In the Baeyer-Villiger oxidation, the peroxyacid acts as a nucleophile while the aldehyde acts as an electrophile. Since 1O2 is a good electrophile, it may plausibly compete with the aldehyde in the Baeyer-Villiger oxidation step. Hence, formaldehyde 1 may be converted via peroxyformic acid 2 to formic acid 3 and ozone (Fig. 2). Thus, the formaldehyde/O3 pathway may involve formaldehyde reacting with H2O2 to produce 1O2 [12,13], followed by reactions depicted in Fig. 2.
Fig. 2.
Proposed mechanism of formation of ozone through reactions of formaldehyde (1) and singlet oxygen (1O2)
Tomono et al. [36] found that decomposition of the pure 1O2 generator, 1-methylnaphthalene-4-endoperoxide (MNPE) at 37 °C and pH 7.4 in the presence of cholesterol resulted in the formation of secosterol aldehyde B and a small amount of secosterol aldehyde A, and that addition of IgG resulted in a decrease in the former aldehyde and an increase in the latter. Increased formation of secosterol A in the presence of IgG is easily explained by the antibody-catalyzed ozone formation from 1O2. On the other hand, formation of secosterol A even in the absence of IgG might be partly explained by secosterol aldehyde B reacting with singlet oxygen analogously to the ozone-producing reactions of formaldehyde with singlet oxygen.
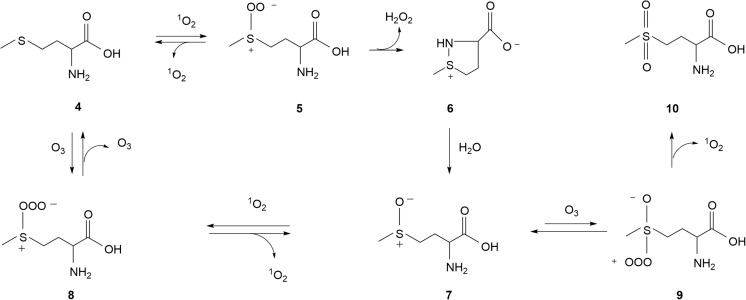
Between pH 6 and 10, the reaction of methionine 4 with 1O2 in water proceeds via persulfoxide 5 and the cyclic dehydromethionine 6 to form methionine sulfoxide 7 [36] (Fig. 3). Interestingly, under similar conditions, methionine 4 also reacts efficiently with O3 to generate sulfoxide 7 and 1O2 [37,38]. Since the reaction between H2S and O3 proceeds through an intermediate adduct H2S-O3 [39], the reaction of 4 with O3 to form 7 may also proceed through a trioxysulfoxide adduct, 8 (Fig. 3). Although methionine sulfoxide 7 may be further oxidized irreversibly to form methionine sulfone, this reaction is usually unfavorable [40]. A possible mechanism for the latter reaction might begin with a nucleophilic attack of O3 on the S atom of sulfoxide 7 to generate intermediate 9 whose decomposition affords the sulfone 10 (Fig. 3). Such ability of O3 to react as a nucleophile has been previously reported [3]. On the other hand, it is conceivable that the reaction of 1O2 at the nucleophilic oxygen of sulfoxide 7 can produce trisulfoxide adduct 8 which decomposes to O3 and methionine 4. This proposed reversibility of the reaction of methionine 4 and O3 to form sulfoxide 7 and 1O2 is indirectly supported by the fact that methionine sulfoxide reductases readily convert the sulfoxide 7 back to methionine [40,41]. The yield of O3 during the proposed reaction of 7 and 1O2 is expected to be good because ozone’s co-product, 4, will react with 1O2 to regenerate reactant 7. Likewise, the reported high yield of 1O2 and methionine sulfoxide 7 from O3 and methionine 4 should be due to the very short lifetime of 1O2 (less than a second as compared to 4.8 min half-life of ozone in water) [37,38].
Fig. 3.
Proposed pathway of O3 formation via the successive reactions of methionine (4) and methionine sulfoxide (7) with singlet oxygen (1O2). The competing conversion of 7 to sulfone 10 normally occurs to a limited extent
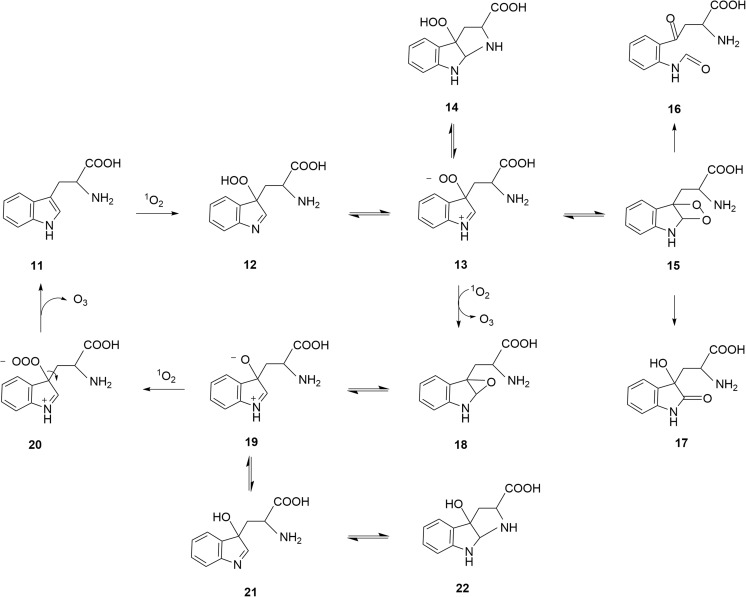
Figure 4 illustrates a suggested pathway for tryptophan-catalyzed ozone formation. First, tryptophan 11 reacts with singlet oxygen to form hydroperoxide 12 [18,28,32], which can exist in equilibrium with zwitterionic peroxide 13 and tricylic hydroperoxide 14. The latter is a well-known tryptophan photooxidation product [31,32]. Zwitterionic peroxide 13 may cyclize to form dioxetane 15 whose decomposition affords N-formylkynurenine 16 [28,32] or dioxindolylalanine 17 [32]. Alternatively, I propose that peroxide 13 may react with 1O2 to afford O3 and epoxide 18 in a concerted reaction like the above proposed reaction of peroxyacid 2 with 1O2 (Fig. 2). However, a stepwise reaction of 1O2 and 13 via a zwitterionic tetroxide is not ruled out. Epoxide 18 can exist in equilibrium with zwitterionic oxide 19, which may also react with 1O2 to form ozone and regenerate tryptophan 11 via trioxyanion 20. A concerted reaction between 19 and 1O2, bypassing 20 is also a possibility. Compounds 18 and 19 can exist in equilibrium with isomeric alcohols 21 and 22, which are known products [28,31,32].
Fig. 4.
Proposed pathways of O3 formation via reactions of tryptophan 11 and its oxidation products with singlet oxygen (1O2)
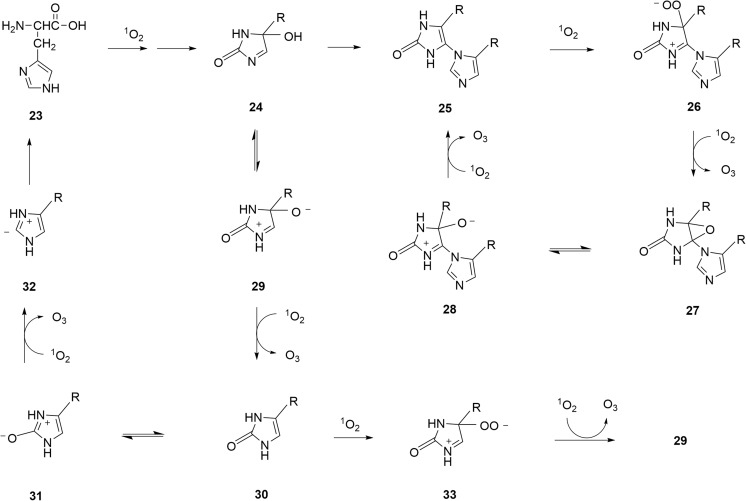
Figure 5 illustrates some of the potential pathways involved in the histidine-catalyzed ozone formation. First, histidine 23 is converted to hydrated imidazolone 24 [18,27,28], which is a precursor of the histidine-histidine cross-linked dimer 25 [26] that has been detected in the hinge region of an IgG 1 [26]. A cyclic oxidation-deoxidation pathway of this dimer will lead to O3 production via intermediates 26–28. Ozone formation may also occur during the conversion of compound 24 via zwitterionic oxide 29 to 2-oxohistidine 30, which is a known product [26]. The latter may be converted back to histidine 23 via zwitterionic oxide 31 and vinylic anion 32. Alternatively, 30 may be converted back to 29, with O3 formation, via zwitterionic peroxide 33.
Fig. 5.
Proposed pathways of O3 formation via reactions of histidine 23 and its oxidation products with singlet oxygen (1O2)
The pathways suggested in Figs. 4 and 5 for tryptophan and histidine-catalyzed ozone formation are not exhaustive, and other oxidized intermediates such as dioxindolylalanine 17 are also potential O3 precursors.
Ozone decomposition in water may play a key role in the formation of H2O2 containing water-derived oxygen
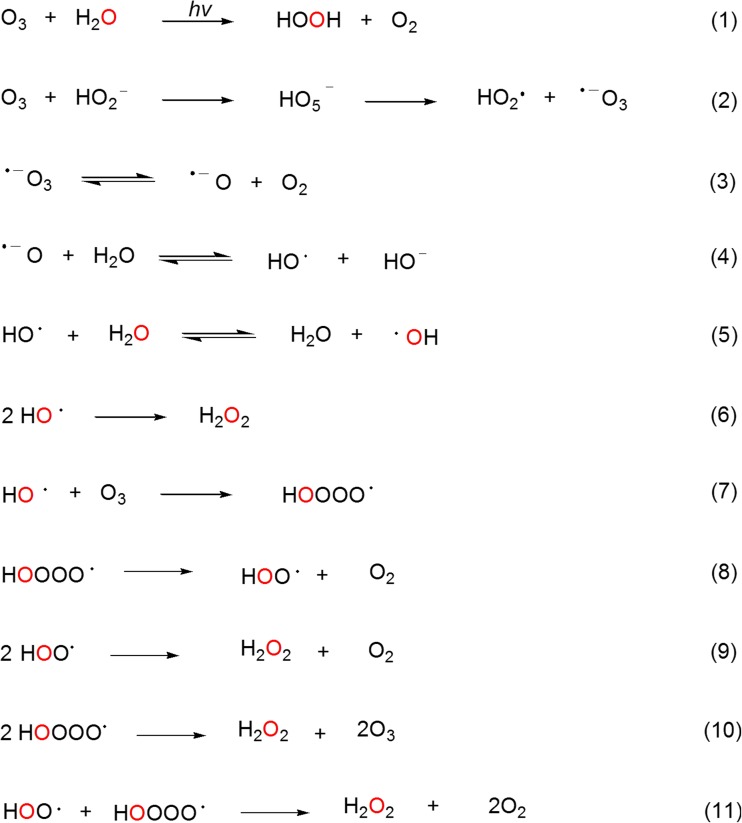
The incorporation of oxygen atoms from water into H2O2 molecules in the presence of 1O2 was considered as key evidence that water reacts with 1O2 to form H2O3 according the water-oxidation pathway [14]. However, H2O2 containing water-derived oxygen may potentially be formed during the decomposition of O3 by known reactions as depicted in Fig. 6. For example, when O3 formation occurs during irradiation of antibodies, equation 1 is likely to be a major contributor of such H2O2 [42]. H2O2 that does not contain water-derived oxygen can be generated during the oxidation of methionine (Fig. 3) or by ozonolysis of tryptophan or histidine via α-hydroxyhydroperoxide intermediates [43]. HO2 anions derived from such H2O2 molecules can initiate O3 decomposition and formation of hydroxyl radicals (∙ OH) according to equations 2–4 [44]. Hydroxyl radicals undergo rapid hydrogen exchange with water molecules [45]. Thus, even if some hydroxyl radicals formed according to equation 4 have ozone-derived oxygen atoms, their hydrogen exchange with water molecules will generate hydroxyl radicals having water-derived oxygen according to equation 5. There is reasonable chance that such hydroxyl radicals will meet and react to form H2O2 (equation 6) [46]. Other reactions that would lead to formation of H2O2 with water-derived oxygen include equations 7–11 [47]. By such pathways therefore, the oxidation of water to form H2O2 may occur as a consequence of O3 formation and decomposition, contrary to the previously proposed ozone formation as a consequence of water oxidation.
Fig. 6.
Proposed formation of H2O2 containing water-derived oxygen atoms as a consequence of the decomposition of O3
Concluding remarks
In the present article, potential pathways have been proposed for (i) the oxidation of aldehydes by 1O2 to form acids and O3 and (ii) the amino acid-catalyzed O3 formation via various oxidation and deoxidation reactions. Theoretical and experimental efforts to confirm these pathways will be possible because potential key intermediates have been identified.
Antibodies may play an important role in ozone production by neutrophils [4]. While efforts to understand the finer details of the antibody-catalyzed O3 formation have in the past focused on potential hydrophobic active sites for formation of H2O3 [14,22], more attention should now shift to mapping solvent accessible sites where methionine, histidine, tryptophan, cysteine, methionine, and disulfide bridges undergo oxidation, and how chemical modifications to these sites affect O3 generation.
Based on the types of O3-generating reactions suggested here, it will be possible to predict the potential of various other organic compounds to participate in or catalyze ozone formation. It will also be possible to synthesize new ozone-generating molecules, which may help to advance the use of ozone in various fields. For example, such molecules may serve as alternatives to the antibiotics currently used in crop protection.
Compounds such as 1-butylnaphthalene-4-propionate endoperoxide or 1-methylnaphthalene-4-propionate endoperoxide generate 1O2 under physiological conditions in the dark [48,49]. The potential application of such compounds to generate ozone for treatment of various diseases remains to be explored. For example, the malaria parasite, Plasmodium falciparum, which easily develops drug resistance, has been shown to be inhibited by ozone [50] and, interestingly, it abundantly produces a histidine-rich protein [51] that might be a good catalyst of ozone formation in the presence of singlet oxygen.
References
- 1.Balla J, Tyihak E. Direct measurement of emission of endogenous ozone from plants by GC-MS-SIM. Chromatographia Supp. 2010;71:S87–S91. doi: 10.1365/s10337-010-1594-x. [DOI] [Google Scholar]
- 2.Tyihak E, Moricz AM, Ott PG. BioArena studies: unique function of endogenous formaldehyde and ozone in the antibiotic effect—a review. Med Chem. 2012;8:75–84. doi: 10.2174/157340612799278388. [DOI] [PubMed] [Google Scholar]
- 3.Beltran FJ (2004) Ozone reaction kinetics for water and wastewater systems. CRC Press, pp 13–14
- 4.Wentworth P, Jr, McDunn JE, Wentworth AD, Takeuchi C, Nieva J, Jones T, Bautista C, Ruedi JM, Gutierrez A, Janda KD, Babior BM, Eschenmoser A, Lerner RA. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 5.Pereira PMR. Antibodies armed with photosensitizers: from chemical synthesis to photobiological applications. Org Biomol Chem. 2015 doi: 10.1039/c4ob02334j. [DOI] [PubMed] [Google Scholar]
- 6.Wentworth P, Jr, Nieva J, Takeuchi C, Galve R, Wentworth AD, Dilley RB, DeLaria GA, Saven A, Babior BM, Janda KD, Eschenmoser A, Lerner RA. Evidence for ozone formation in human atherosclerotic arteries. Science. 2003;302:1053–1056. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- 7.Wentworth AD, Song B-D, Nieva J, Shafton A, Sangeetha T, Wentworth P Jr (2009) The ratio of cholesterol 5,6 secosterols formed from ozone and singlet oxygen offers insight into the oxidation of cholesterol in vivo. Chem Comm (Camb) 3098-3100 [DOI] [PMC free article] [PubMed]
- 8.Kettle AJ, Clark BM, Winterbourn CC. Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone. J Biol Chem. 2004;279:18521–18525. doi: 10.1074/jbc.M400334200. [DOI] [PubMed] [Google Scholar]
- 9.Brinkhorst J, Nara SJ, Pratt DA. Hock cleavage of cholesterol-5α-hydroperoxide: an ozone-free pathway to the cholesterol ozonolysis products identified in arterial plague and brain tissue. J Am Chem Soc. 2008;130:12224–12225. doi: 10.1021/ja804162d. [DOI] [PubMed] [Google Scholar]
- 10.Tomono S, Miyoshi N, Shiokawa H, Iwabuchi T, Aratani Y, Higashi T, Nukaya H, Ohshima H. Formation of cholesterol ozonolysis products in vitro and in vivo through a myeloperoxidase-dependent pathway. J Lipid Res. 2011;52:87–97. doi: 10.1194/jlr.M006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyoshi N, Iuliano L, Tomono S, Ohshima H. Implications of cholesterol autoxidation products in the pathogenesis of inflammatory diseases. Biochem Biophys Res Comm. 2014;446:702–708. doi: 10.1016/j.bbrc.2013.12.107. [DOI] [PubMed] [Google Scholar]
- 12.Tyihak E, Moricz AM, Ott PG, Katay G, Mincsovics E. Biological characterization of ingredients in OPLC-BioArena-greenhouse-system: unique reactions of endogenous HCHO and O3 in in vitro and in vivo conditions. Chromatographia. 2012;75:983–990. doi: 10.1007/s10337-012-2254-0. [DOI] [Google Scholar]
- 13.Tyihak E, Moricz AM, Ott PG, Kiraly-Veghely Z, Katay G. BioArena system for knowing and understanding the biological world: a review with new experimental results. J AOAC Int. 2013;96:1189–1199. doi: 10.5740/jaoacint.SGETyihak. [DOI] [PubMed] [Google Scholar]
- 14.Wentworth P, Jr, Jones LH, Wentworth AD, Zhu X, Larsen NA, Wilson IA, Xu X, Goddard WA, III, Janda KD, Eschenmoser A, Lerner RA. Antibody catalysis of the oxidation of water. Science. 2001;293:1806–1811. doi: 10.1126/science.1062722. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Muller RP, Goddard WA., III The gas phase reaction of singlet dioxygen with water: a water-catalyzed mechanism. Proc Natl Acad Sci U S A. 2002;99:3376–3381. doi: 10.1073/pnas.052710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerkovnic J, Plesnicar B. Recent advances in the chemistry of hydrogen trioxide (HOOOH) Chem Rev. 2013;113:7930–7951. doi: 10.1021/cr300512s. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita K, Miyoshi T, Arai T, Endo N, Itoh H, Makino K, Mizugishi K, Uchiyama T, Masataka S. Ozone production by amino acids contributes to killing of bacteria. Proc Natl Acad Sci U S A. 2008;105:16912–16917. doi: 10.1073/pnas.0807952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei C, Song B, Yuan J, Feng Z, Jia G, Li C. Luminescence and Raman spectroscopic studies on the damage of tryptophan, histidine and carnosine by singlet oxygen. J Photochem Photobiol A Chem. 2007;189:39–45. doi: 10.1016/j.jphotochem.2007.01.005. [DOI] [Google Scholar]
- 19.Pattison DI, Rahmanto AS, Davies MJ. Photo-oxidation of proteins. Photochem Photobiol Sci. 2012;11:38–53. doi: 10.1039/C1PP05164D. [DOI] [PubMed] [Google Scholar]
- 20.Jensen RL, Arnbjerg J, Ogilby R. Reaction of singlet oxygen with tryptophan in proteins: a pronounced effect of the local environment on the reaction rate. J Am Chem Soc. 2012;134:9820–9826. doi: 10.1021/ja303710m. [DOI] [PubMed] [Google Scholar]
- 21.Lundeen RA, McNeill K. Reactivity differences of combined and free amino acids: quantifying the relationship between three-dimensional protein structure and singlet oxygen reaction rates. Environ Sci Technol. 2013;47:14215–14223. doi: 10.1021/es404236c. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Wentworth P, Jr, Wentworth AD, Eschenmoser A, Lerner RA, Wilson IA. Probing the antibody-catalyzed water-oxidation pathway at atomic resolution. Proc Natl Acad Sci U S A. 2004;101:2247–2252. doi: 10.1073/pnas.0307311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreethara A, Lau K, Hosken B, Macchi F, Zhan D, Shen A, Steinmann D, Schoneich C, Lentz Y. Role of surface exposed tryptophan as substrate generators for the antibody catalyzed water oxidation pathway. Mol Pharm. 2013;10:278–288. doi: 10.1021/mp300418r. [DOI] [PubMed] [Google Scholar]
- 24.Khor HK, Jacoby ME, Squler TC, Chu GC, Chelius D. Identification of methionine sulfoxide diastereomers in immunoglobin gamma antibodies using methionine sulfoxide reductase enzymes. mAbs. 2010;2:299–308. doi: 10.4161/mabs.2.3.11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano M, Kobayashi N, Yabuta M, Uchiyama S, Fukui K. Detection of histidine oxidation in a monoclonal immunoglobulin gamma (IgG) 1 antibody. Anal Chem. 2014;86:7536–7543. doi: 10.1021/ac501300m. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Zhang Z, Cheetham J, Ren D, Zhou ZS. Discovery and characterization of a photo-oxidative histidine-histidine cross-link in IgG1 antibody utilizing 18O-labeling and mass spectrometry. Anal Chem. 2014;86:4940–4948. doi: 10.1021/ac500334k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Lu W, Fang Y, Liu J. Evolution of oxidation dynamics of histidine: nonreactivity in the gas phase, peroxides in hydrated clusters, and pH dependence in solution. Phys Chem Chem Phys. 2014;16:22179–22191. doi: 10.1039/C4CP03550J. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Rodriquez ME, Guo M, Kenney ME, Oleinic NL, Anderson VE. Oxidative modification of cytochrome c by singlet oxygen. Free Radic Biol Med. 2008;44:1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasson TMD, Rexroth S, Barry BA. Light-induced oxidative stress, N-formylkynurenine, and oxygenic photosynthesis. PLoS ONE. 2012;7:e42220. doi: 10.1371/journal.pone.0042220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehman AU, Cser K, Sass L, Vass I. Characterization of singlet oxygen production and its involvement in photochemical damage of photosystem II in the cyanobacterium Cynechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim Biophys Acta. 2013;1827:689–698. doi: 10.1016/j.bbabio.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Ronsein GE, de Oliveira MCB, de Medeiros MHG, Mascio PD. Characterization of 1O2-derived oxidation products of tryptophan: a combination of tandem mass spectrometry analyses and isotopic labeling studies. J Am Soc Mass Spectrom. 2009;20:188–197. doi: 10.1016/j.jasms.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Ronsein GE, de Oliveira MCB, de Medeiros MHG, Mascio PD. Mechanism of dioxindolylalanine formation by singlet molecular oxygen-mediated oxidation of tryptophan residues. Photochem Photobiol Sci. 2011;10:1727–1730. doi: 10.1039/c1pp05181d. [DOI] [PubMed] [Google Scholar]
- 33.Kobayakawa H, Imai K. Effects of the interaction between ozone and carbon dioxide on gas exchange, photosystem II and antioxidants in rice leaves. Photosynthetica. 2011;49:227–238. doi: 10.1007/s11099-011-0024-0. [DOI] [Google Scholar]
- 34.Liu L, Flatoy F, Ordonez C, Braathen GO, Hak C, Junkermann W, Andreani-Aksoyoglu S, Mellqvist J, Galle B, Prevot ASH, Isaksen ISA. Photochemical modelling in the Po basin with focus on formaldehyde and ozone. Atmos Chem Phys. 2007;7:121–137. doi: 10.5194/acp-7-121-2007. [DOI] [Google Scholar]
- 35.Hajimohammadi M, Safari N, Mofakham H, Shaabani A. A new and efficient aerobic oxidation of aldehydes to carboxylic acids with singlet oxygen in the presence of porphyrin sensitizers and visible light. Tetrahedron Lett. 2010;51:4061–4065. doi: 10.1016/j.tetlet.2010.05.124. [DOI] [Google Scholar]
- 36.Fang Y, Liu F, Bennet A, Ara S, Liu J. Experimental and trajectory study on the reaction of protonated methionine with electronically excited singlet molecular oxygen (a1Δg): reaction dynamics and collision energy effects. Phys Chem B. 2011;115:2671–2682. doi: 10.1021/jp112237y. [DOI] [PubMed] [Google Scholar]
- 37.Kanofsky JR, Sima P. Singlet oxygen production from the reactions of ozone with biological molecules. J Biol Chem. 1991;266:9039–9042. [PubMed] [Google Scholar]
- 38.Munoz F, Mvula E, Braslavsky SE, von Sonntag C. Singlet dioxygen formation in ozone reactions in aqueous solution. J Chem Soc Perkin Trans. 2001;2:1109–1116. doi: 10.1039/b101230o. [DOI] [Google Scholar]
- 39.Vahedpour M, Baghary R, Khalili F (2013) Prediction of mechanism and thermochemical properties of O3 + H2S atmospheric reaction. J. Chem. Article ID 65968 http://dx.doi.org/10.1155/2013/659682
- 40.Ghesquere B, Jonckheere V, Colaert N, van Durme J, Timmerman E, Goethals M, Schymkowitz J, Rousseau F, Vanderkerckhove J, Gevaert K. Redox proteomics of protein-bound methionine oxidation. Mol Cell Proteomics. 2011;10:M110.006866. doi: 10.1074/mcp.M110.006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JC, You Z, Kim G, Levine RL. Methionine sulfoxide reductase A is a stereospecific methionine oxidase. Proc Natl Acad Sci U S A. 2011;108:10472–10477. doi: 10.1073/pnas.1101275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaze WH, Kang J-W, Chapin DH. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng. 1987;9:335–352. doi: 10.1080/01919518708552148. [DOI] [Google Scholar]
- 43.Sharma VK, Graham NJD. Oxidation of amino acids, peptides and proteins by ozone: a review. Ozone Sci Eng. 2010;32:81–90. doi: 10.1080/01919510903510507. [DOI] [Google Scholar]
- 44.Merenyi G, Lind J, Sonntag C. Reaction of ozone with hydrogen peroxide (peroxone process): a revision of current mechanistic concepts based on thermokinetic and quantum-chemical considerations. Environ Sci Technol. 2010;44:3505–3507. doi: 10.1021/es100277d. [DOI] [PubMed] [Google Scholar]
- 45.Codorniu-Hernandez E, Kusalik PG. Mobility mechanism of hydroxyl radicals in aqueous solution via hydrogen transfer. J Am Chem Soc. 2012;134:532–538. doi: 10.1021/ja208874t. [DOI] [PubMed] [Google Scholar]
- 46.Codorniu-Hernandez E, Hall KW, Ziemianowicz D, Carpendale S, Kusalik PG. Aqueous production of oxygen atoms from hydroxyl radicals. Phys Chem Chem Phys. 2014;16:26094–26102. doi: 10.1039/C4CP02959C. [DOI] [PubMed] [Google Scholar]
- 47.Staehelin J, Buhler RE, Hoigne J. Ozone decomposition in water studied by pulse radiolysis. 2. HO and HO4 as chain intermediates. J Phys Chem. 1984;88:5999–6004. doi: 10.1021/j150668a051. [DOI] [Google Scholar]
- 48.Tomono S, Miyoshi N, Sato K, Ohba Y, Ohshima H. Formation of cholesterol ozonolysis products through an ozone-free mechanism mediated by myeloperoxidase-H2O-chloride system. Biochem Biophys Res Comm. 2009;383:222–227. doi: 10.1016/j.bbrc.2009.03.155. [DOI] [PubMed] [Google Scholar]
- 49.Otsu K, Sato K, Sato M, Ono H, Ohba Y, Katagata Y. Impaired activation of caspase cascade during cell death induced by newly synthesized singlet oxygen generator, 1-buthylnaphthalene-4-propionate-endoperoxide. Cell Biol Int. 2008;32:1380–1387. doi: 10.1016/j.cellbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Lell B, Viebahn R, Kremsner PG. The activity of ozone against Plasmodium falciparum. Ozone Sci Eng. 2001;23:89–93. doi: 10.1080/01919510108961991. [DOI] [Google Scholar]
- 51.Verma P, Biswas S, Mohan T, Ali S, Rao DN. Detection of histidine-rich protein and lactate dehydrogenase of Plasmodium falciparum in malaria patients by sandwich ELISA using in-house reagents. Indian J Med Res. 2013;138:977–987. [PMC free article] [PubMed] [Google Scholar]