Abstract
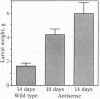
The growth rates of Manduca sexta (tobacco hornworm) larvae feeding on tomato plants constitutively expressing a prosystemin antisense gene were approximately 3 times higher than growth rates of larvae feeding on nontransformed control plants. The levels of proteinase inhibitor I and inhibitor II proteins in leaves of tomato plants expressing the antisense prosystemin gene remained at undetectable levels until the sixth day of larval feeding and then increased throughout the plants to 100-125 microg/g of leaf tissue after 14 days. In control plants, levels of proteinase inhibitor I and II proteins increased rapidly from the second day of larval feeding and by the eighth day contained levels of 225 microg/g of leaf tissue and 275 microg/g of leaf tissue, respectively, and then increased slowly thereafter. Prosystemin mRNA levels in antisense and control plants after 6 days and 12 days of larval feeding correlated with levels of inhibitor I and II protein levels. These experiments demonstrate that resistance of plants toward an insect pest can be modulated by genetically engineering a gene encoding a component of the inducible systemic signaling system regulating a plant defensive response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985 Oct;79(2):568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Gordon M. P., Smit B. A. Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2393–2396. doi: 10.1073/pnas.88.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Johnson R., Narvaez J., An G., Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B., Pearce G., Orozco-Cardenas M., Ryan C. A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992 Mar 20;255(5051):1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- McGurl B., Ryan C. A. The organization of the prosystemin gene. Plant Mol Biol. 1992 Nov;20(3):405–409. doi: 10.1007/BF00040600. [DOI] [PubMed] [Google Scholar]
- Narváez-Vásquez J., Orozco-Cárdenas M. L., Ryan C. A. Differential expression of a chimeric CaMV-tomato proteinase Inhibitor I gene in leaves of transformed nightshade, tobacco and alfalfa plants. Plant Mol Biol. 1992 Dec;20(6):1149–1157. doi: 10.1007/BF00028901. [DOI] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Trautman R., Cowan K. M., Wagner G. G. Data processing for radial immunodiffusion. Immunochemistry. 1971 Oct;8(10):901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]