Abstract
Gonadal steroids regulate the pattern of GnRH secretion. Arcuate kisspeptin (kisspeptin, neurokinin B, and dynorphin [KNDy]) neurons may convey steroid feedback to GnRH neurons. KNDy neurons increase action potential firing upon the activation of neurokinin B receptors (neurokinin-3 receptor [NK3R]) and decrease firing upon the activation of dynorphin receptors (κ-opioid receptor [KOR]). In KNDy neurons from intact vs castrated male mice, NK3R-mediated stimulation is attenuated and KOR-mediated inhibition enhanced, suggesting gonadal secretions are involved. Estradiol suppresses spontaneous GnRH neuron firing in male mice, but the mediators of the effects on firing in KNDy neurons are unknown. We hypothesized the same gonadal steroids affecting GnRH firing pattern would regulate KNDy neuron response to NK3R and KOR agonists. To test this possibility, extracellular recordings were made from KNDy neurons in brain slices from intact, untreated castrated or castrated adult male mice treated in vivo with steroid receptor agonists. As observed previously, the stimulation of KNDy neurons by the NK3R agonist senktide was attenuated in intact vs castrated mice and suppression by dynorphin was enhanced. In contrast to observations of steroid effects on the GnRH neuron firing pattern, both estradiol and DHT suppressed senktide-induced KNDy neuron firing and enhanced the inhibition caused by dynorphin. An estrogen receptor-α agonist but not an estrogen receptor-β agonist mimicked the effects of estradiol on NK3R activation. These observations suggest the steroid modulation of responses to activation of NK3R and KOR as mechanisms for negative feedback in KNDy neurons and support the contribution of these neurons to steroid-sensitive elements of a GnRH pulse generator.
GnRH neurons form the final common output from the central nervous system to control reproduction. These neurons release GnRH in a pulsatile pattern (1–4) to stimulate pituitary release of LH and FSH, which is essential for reproduction (5). Gonadal steroid hormones regulate GnRH pulse patterns (6), but only the β-isoform of estrogen receptor (ER), which is not critical for fertility, has been consistently detected in these cells (7–10). Steroid-sensitive afferent neurons thus likely convey feedback signals to GnRH neurons.
Several observations support a steroid-sensitive “GnRH pulse generator” in the hypothalamic arcuate nucleus (11–15). A likely contributor to this pulse generator is the arcuate KNDy neuron population, which is named for its colocalization of the neuropeptides kisspeptin, neurokinin B, and dynorphin (16). KNDy neurons project to GnRH neurons (16), and all three KNDy neuropeptides are important for reproductive function. Mutations in the genes encoding kisspeptin receptor, neurokinin B, or the high-affinity receptor for neurokinin B (neurokinin-3 receptor [NK3R]) have all been found in human patients with hypogonadotropic hypogonadism (17–19). Consistent with these observations, kisspeptin and NK3R agonists increase GnRH neuron activity and/or GnRH/LH release (20–24). In contrast, antagonism of the high-affinity receptor for dynorphin (κ-opioid receptor [KOR]) increases LH release (25).
The neuropeptides made by KNDy neurons also regulate activity of these neurons. Activation of NK3R and KOR modifies frequency of action potential firing in KNDy neurons (26, 27), and these modifications are sensitive to gonadal hormones (27). Specifically, NK3R-induced stimulation of action potentials is attenuated in KNDy neurons from intact vs castrate males, whereas KOR-mediated inhibition of action potential firing is enhanced in KNDy neurons from intact compared with castrate males. Together these data suggest potential mechanisms through which gonadal secretions have a negative feedback effect on KNDy neurons and thus the GnRH pulse generator. However, the identity of the gonadal secretions involved remains unclear.
Estrogens and androgens are both candidate mediators of the feedback effects on NK3R and KOR activation in KNDy neurons. KNDy neurons express both androgen receptor (AR) and ERs (28) and may also be regulated by steroid-sensitive afferents (29). The brain expresses aromatase, which converts testosterone to estradiol (30, 31). Both androgens and estrogens inhibit arcuate kisspeptin mRNA expression (28, 32), but steroidal modulation of mRNA expression may not necessarily reflect changes in kisspeptin protein levels (33), let alone KNDy neuron action potential firing rate and/or downstream GnRH neuron function. In this regard, some studies have shown that estradiol is a more potent suppressor of GnRH/LH release in males (34–37).
To better understand the role of gonadal steroids in the neuropeptide-mediated stimulation and inhibition of KNDy neurons, the present study tested the hypothesis that estradiol is the main steroid modifying action potential firing of individual KNDy neurons in male mice. Our approach was to compare response to NK3R and KOR agonists among intact, untreated castrate, and castrate male mice treated in vivo with physiological levels of steroid hormones or with specific steroid receptor agonists.
Materials and Methods
Animals
Tac2-enhanced green fluorescent protein (GFP) bacterial artificial chromosome transgenic mice (015495-UCD/STOCK Tg [Tac2-EGFP]381Gsat) were obtained from the Mouse Mutant Regional Resource Center (http://www.mmrrc.org/) and propagated in our colony to be used in both electrophysiological and single-cell PCR experiments. These mice express GFP driven by the promoter for Tac2, the mouse gene for neurokinin B. Within the arcuate nucleus, our previous work has shown that fluorescently identified cells in this mouse also express kisspeptin and/or dynorphin at high percentages (27) in support of their identity as KNDy neurons. Mice were maintained under a 14-hour light, 10-hour dark photoperiod (lights on 3:00 am EST) with 2916 chow (Harlan) and water available ad libitum. Male mice aged 79–123 days were used either intact or after bilateral castration under isofluorane anesthesia (VetOne); bupivacaine (0.25%, 8 μL per site; Hospira) was provided as a postoperative analgesic. SILASTIC brand implants (Dow Corning) containing steroids (34) were placed sc in the scapular region at the time of surgery. Mice were used 4–7 days after castration. All procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Experimental design
Reagents were purchased from Sigma Chemical Co unless otherwise noted. Intact and castrated (4–7 d after surgery) male mice were used. Treatments were administered beginning on the day of surgery. Although T is the main sex steroid in the circulation in males, it can be metabolized to either estradiol or DHT in the target tissues. Estradiol and DHT were chosen as treatments to target specifically estrogen or androgen receptors, respectively. Mice were placed in one of seven treatment groups: 1) intact, no further treatment; 2) castrated, no further treatment; 3) castrated plus an implant containing 0.625 μg estradiol suspended in sesame oil; 4) castrated plus two implants containing 400 μg DHT each in sesame oil (800 μg total); 5) castrated plus daily sc injections of 1 mg/kg of the ERα agonist 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT; Tocris Bioscience); 6) castrated plus daily sc injections of 1 mg/kg of the R enantiomer of the ERβ agonist (R)-2,3-bis(4-hydroxyphenyl)-propionitrile (DPN; Tocris Bioscience); or 7) castrated plus daily sc injections of 2 mg/kg DPN. DPN and PPT were suspended in a vehicle of 5% dimethylsulfoxide (DMSO), 95% sesame oil, administered between 9:30 am and 12:00 pm EST, and terminated the day before recording. All steroid and steroid receptor agonist treatments were administered in vivo and were not present in recording solutions.
Seminal vesicle mass
To confirm endocrine status, the ratio of the androgen-dependent seminal vesicles to body mass was determined at the time of the brain slice preparation. Compared with intact mice, castration reduced this ratio (intact, 6.2 ± 0.3 mg/g, n = 9; castrated, 2.3 ± 0.2 mg/g, n = 20, P < .05). Only DHT treatment (8.0 ± 0.4 mg/g, n = 12 mice) restored the ratio to that of intact mice, consistent with the production physiological circulating androgen levels by these implants (34). The remaining groups were similar to the castrated mice (estradiol, 2.3 ± 0.2 mg/g, n = 12 mice; PPT, 3.0 ± 0.5 mg/g, n = 5 mice; 1 mg/kg DPN, 2.8 ± 0.3 mg/g, n = 4 mice; 2 mg/kg DPN, 4.0 ± 0.4 mg/g, n = 4 mice).
Determination of ER agonist dose
Groups of castrated or intact adult male mice were treated daily with vehicle, PPT, or DPN for 4–6 days. PPT was administered at 1 mg/kg (38). DPN was administered 1, 2, or 4 mg/kg. Injections began the day of surgery and continued until the day before tissue harvest. Agonist concentration was devised to give a 50- to 100-μL injection per 25 g mouse and volume adjusted by body mass. At the time of euthanasia, mice were anesthetized under isoflurane and decapitated. Pituitaries were collected and RNA extracted via RNeasy (QIAGEN). Approximately 250 ng pituitary RNA was reverse transcribed (20 μL/reaction) as described (39) along with a standard curve of mouse pituitary RNA (5, 0.5, 0.05, 0.005 ng/μL final concentration). PrimeTime quantitative PCR (qPCR) assays for mRNAs of prolactin (Prl), and the housekeeping genes Ppia and Rps29 were purchased from Integrative DNA Technologies (Table 1). qPCR was performed using 10 ng cDNA per reaction in duplicate. Relative expression of Prl was normalized to the average relative expression of Ppia and Rps29 via the ΔΔcycle threshold method. Amplicon size was confirmed by agarose gel electrophoresis. All assay primers span an intron to minimize amplification of genomic DNA. PCR efficiencies were calculated from the slope of the standard curve.
Table 1.
qPCR Assays for PCRa
| Gene | IDT Assay Identification | Accession Number | Exons | Amplicon, bp | Amplicon Location, bp |
|---|---|---|---|---|---|
| Prl | Mm.PT.58.21865636 | NM_011164 | 3–4 | 142 | 266–407 |
| Ppia | Mm.PT.39a.2.gs | NM_008907 | 4–5 | 85 | 355–439 |
| Rps29 | Custom | NM_009093 | 1–2 | 127 | 119–245 |
| Ar | Mm.PT.47.17416675 | NM_013476 | 4–5 | 125 | 2065–2189 |
| Esr1 | Mm.PT.47.16003033 | NM_007956 | 6–7 | 100 | 1456–1555 |
| Esr2 | Mm.PT.47.17681375 | NM_207707 | 4–5 | 115 | 1036–1150 |
Abbreviation: IDT, Integrated DNA Technologies.
PrimeTime qPCR assays were from Integrated DNA Technologies (www.idtdna.com/pages/products/gene-expression/primetime-qpcr-assays-and-primers).
DPN has only approximately 70-fold selectivity for ERβ over ERα, compared with the 400-fold selectivity of PPT for ERα (40). We were unable to identify a reliable positive control for efficacy of DPN on a putative ERβ-specific target (41–44). This was despite using the pure active R-enantiomer DPN at a molar concentration greater than 3 times higher than that of PPT. Our strategy was thus to use doses of DPN that did not increase pituitary prolactin mRNA levels, an ERα-specific target, compared with the castrated-only group (38). Castration reduced pituitary Prl expression and PPT treatment restored expression to the same level as that observed in pituitaries from intact animals (Supplemental Figure 1). Pituitary Prl expression was lower in all DPN-treated mice compared with intact animals, but when compared with castrates, the highest DPN dose tested (4 mg/kg) increased (P < .05) Prl expression; 1 mg/kg had no effect and 2 mg/kg DPN was not different from either 1 mg/kg or 4 mg/kg DPN (Supplemental Figure 1). Based on these findings, 1 and 2 mg/kg DPN doses were tested in electrophysiological experiments.
Cell harvest for cDNA synthesis and single-cell PCR for transcripts of gonadal steroid receptors
Cells used for single-cell PCR were harvested from intact or castrated adult male mice as described (27). Briefly, the whole-cell, patch-clamp configuration was achieved, cytoplasm aspirated into the pipette, and the pipette withdrawn to generate an outside-out patch, sealing the cell contents within the pipette with that cell's own membrane. Harvests in which an outside-out patch could not be generated could be contaminated with material from other cells and were discarded. False harvests in which a pipette was lowered into the tissue but no aspiration of cell contents occurred were used to estimate background contamination (45). Additionally, a standard curve of mouse hypothalamic RNA (5, 0.5, 0.05, 0.005 ng/μL final concentration) was reverse transcribed. Single-cell cDNA, controls, and the standard curve were preamplified using TaqMan PreAmp Master Mix (Invitrogen) as described (27). TaqMan PrimeTime qPCR assays for mRNAs of Ar, Esr1, and Esr2 were purchased from Integrative DNA Technologies (Table 1). Quantitative PCR was performed using 2–5 μL of diluted preamplified DNA per reaction, in duplicate, for 40–50 cycles (TaqMan Gene Expression Master Mix; Invitrogen). Single cells were considered positive for a transcript if their threshold was a minimum of four cycles earlier than the preamplification blank, which consisted of reverse transcription and preamplification of the solution used to fill the pipettes for harvest.
Brain slice preparation
Solutions were bubbled with 95% O2–5% CO2 throughout the experiments and for 15 minutes or longer before use. Brain slices were prepared with modifications (46) as described (47). Brains were rapidly removed and placed in ice-cold high-sucrose saline solution containing (in millimoles/liter) 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal slices (300 μm) were cut with a VT1200 S (Leica Biosystems). Slices were incubated 30 minutes at room temperature in 50% high-sucrose saline and 50% artificial cerebrospinal fluid (ACSF) containing (in millimoles/liter) 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 2.5 CaCl2 (pH 7.4) and then transferred to 100% ACSF solution at room temperature for 1–6 hours before recording.
Electrophysiological recordings
Targeted single-unit extracellular recordings were used because this configuration has the least impact on the intrinsic properties of the recorded cell (48, 49). Recording pipettes (2–4.5 MΩ) were pulled from borosilicate glass (Schott 8250; World Precision Instruments) with a P-97 puller (Sutter Instruments). Pipettes were filled with HEPES-buffered solution containing (in millimoles) 150 NaCl, 10 HEPES, 10 glucose, 2.5 CaCl2, 1.3 MgCl2, and 3.5 KCl, and low-resistance (<25 MΩ) seals were formed between the pipette and neuron. Recordings were made in a voltage clamp with a 0-mV pipette holding potential and 2-mV/pA gain, and signals were filtered at 5 kHz using an EPC8 amplifier and PatchMaster software (version 2 × 42; HEKA Instruments, Inc).
Slices were transferred to a recording chamber on an Olympus BX51wI upright fluorescent microscope with constant perfusion of ACSF at 29ºC–33ºC and stabilized for 5 minutes or longer before recording from a cell. Brief illumination at 470 nm was used to identify GFP-positive cells. All acute treatments were diluted in ACSF and administered by bath. Vehicles were determined to have no effect on firing rate (27). After pipette seal formation and another stabilization period of 5 minutes or longer, neuron activity was recorded for a 5-minute untreated control period, followed by 4–5 minutes of treatment. At the end of each experiment, inactive cells were treated with high-potassium ACSF (20 mM K+). Cells that exhibited action currents in response were verified to be alive, and all data, including quiescence, were used. For cells not responding to K+, data analysis was truncated at the last action current. Firing characteristics were further evaluated for changes that might suggest poor health of a cell, such as extremely rapid firing and markedly reduced action current amplitude over a short duration. All data from such cells were excluded.
To test the effect of the in vivo treatments on NK3R-mediated responses in quiescent KNDy neurons, the NK3R agonist senktide (10 nM, in ≤0.1% DMSO; Phoenix Pharmaceuticals, Inc) was bath applied for 4–5 minutes to brain slices from each in vivo treatment group. A cell was considered responsive if it exhibited greater than 0.1 Hz frequency of action potential firing during senktide treatment.
To examine the effect of steroid hormones on KOR-mediated responses in KNDy neurons, dynorphin A (dynorphin; 1 μM; Tocris Bioscience) was bath applied for 5 minutes to brain slices from mice treated in vivo with either estradiol or DHT. Spontaneously active neurons were used because we hypothesized dynorphin would inhibit KNDy neurons. Because extracellular recordings monitor firing activity, inhibition of quiescent cells cannot be observed with this method. Percentage change of firing frequency was used to compare dynorphin responses among hormonal treatment groups because of the range of pretreatment spontaneous activity with different hormonal conditions.
Analyses
Results were unaffected by time of day, time from brain slice preparation, mouse age, duration of in vivo treatments, and location of the cell within the arcuate nucleus. Targeted extracellular recordings detect action currents, which are the currents underlying action potentials. Their frequency thus reflects the action potential firing rate. Action currents were identified using custom software written in IgorPro (WaveMetrics, Inc). Control action current frequency was averaged for the last 2 minutes before treatment. The first 3 minutes of treatment were not included in the analysis to allow time for solution exchange and drug penetration of the slice. Treatment action current firing frequency was determined during the next 2 minutes. Cells with basal firing frequency of 0.1 Hz or less were considered quiescent. Cells that remained quiescent during treatment were considered nonresponsive if they subsequently generated action currents in response to elevated K+. Spontaneously active cells with a change in firing frequency of greater than 20% were considered responsive; quiescent cells that generated action currents at a frequency greater than 0.1 Hz during treatment were also considered responsive. The percentage of responsive cells is reported, but for statistical rigor, both responsive and nonresponsive cells were included in statistical analyses. No more than two cells from a given animal and no fewer than four animals were included in the same experiment, and n indicates number of cells.
A subset of cells was not treated because they did not have the firing required for an intended experiment (eg, quiescent vs active). In these cases, the final 1–2 minutes recorded from cells that had stable recordings for 5 minutes or longer and were verified to be alive were included in an analysis of spontaneously firing vs quiescent cells from different hormonal treatment groups.
Data are reported as mean ± SEM. Nonparametric or parametric comparisons were used as appropriate for data distribution. Responses within groups were analyzed by a two-tailed Wilcoxon matched-pairs signed rank test. Comparisons among groups were analyzed by a Friedman two-way, repeated-measures ANOVA with Bonferroni's multiple comparisons test (senktide), Kruskal-Wallis test with Dunn's multiple comparisons test (seminal vesicle mass, spontaneous firing, percentage change with dynorphin), or a one-way ANOVA with uncorrected Fisher's least significant difference test (Prl expression). Significance was set at P < .05.
Results
Spontaneous activity of KNDy neurons differs with in vivo treatment
Firing activity in KNDy neurons in brain slices without further treatment is an indicator of the in vivo influences of treatments through various mechanisms. These data include the control periods of the cells presented in the experiments below as well as stable recordings from cells that were not further studied because they did not have the firing rate required for the intended experiment. Spontaneous action potential firing (>0.1 Hz firing frequency) was observed in 44% of cells (12 of 27) from intact, 49% of cells (18 of 37) from castrated, 54% of cells (13 of 24) from DHT-treated, and 38% of cells (9 of 24) from estradiol-treated mice. Spontaneous firing frequency was increased by castration vs that in cells from intact mice (intact, 0.8 ± 0.2 Hz, n = 12 cells; castrated, 4.9 ± 0.9, n = 18 cells, P < .05). Firing rate was restored to that of intact mice by treatment of castrated males with implants of either estradiol (1.1 ± 0.3 Hz, n = 9 cells) or DHT (2.6 ± 0.8 Hz, n = 13 cells). The number of spontaneously firing cells from the other treatment groups was too low for statistical comparison.
Both estradiol and DHT attenuate the NK3R-mediated increase in action potential firing frequency in KNDy neurons
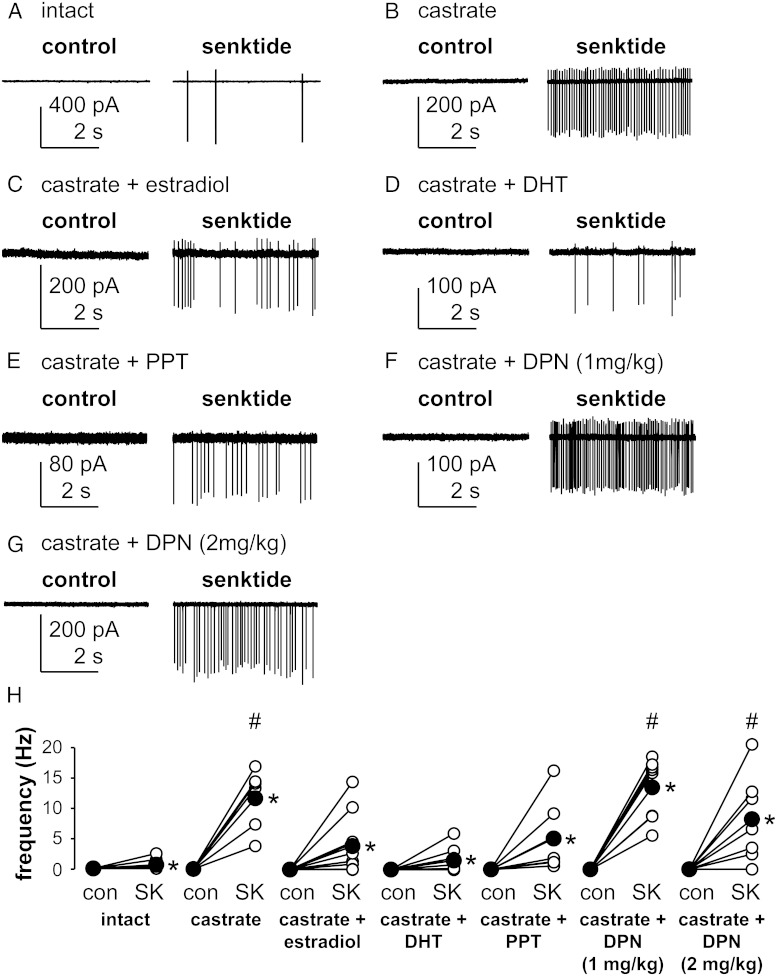
To test the hypothesis that estradiol is the main steroid modifying the response of KNDy neurons to activation of NK3R, we compared the response in intact, untreated castrated, or castrated males treated with estradiol or DHT implants. The NK3R agonist senktide (10 nM) was bath applied to brain slices during targeted extracellular recordings of quiescent (≤0.1 Hz firing frequency) KNDy neurons. Representative traces from these recordings are shown in Figure 1, A–D. Consistent with previous reports (27), senktide increased firing in only 62% of cells from intact mice (five of eight) compared with 100% of cells recorded from castrate mice (six of six). Senktide increased firing in 70% of cells from estradiol-treated (7 of 10) and 75% of cells from DHT-treated mice (six of eight), consistent with the percentage of responsive cells in intact mice. Both responding and nonresponding cells were included for statistical analyses of firing rate. Senktide increased firing rate (P < .05 vs presenktide control of 0.0 ± 0.0 Hz for all groups) in intact (0.6 ± 0.3 Hz, n = 8 cells), castrated (11.7 ± 2.0 Hz, n = 6 cells), and steroid-treated mice (estradiol, 3.9 ± 1.5 Hz, n = 10 cells; DHT, 1.5 ± 0.7 Hz, n = 8 cells; Figure 1H). Contrary to our hypothesis, this increase in firing frequency was attenuated by either estradiol or DHT treatment (both P < .05 vs castrated only).
Figure 1.
Estradiol (via ERα) and DHT attenuate the NK3R-induced increase in KNDy neuron firing frequency. A–G, Representative raw current traces from KNDy neurons before (left panels) and during (right panels) acute treatment with the NK3R agonist senktide (10 nM) in the various groups as labeled. Variable current amplitudes reflect small changes in pipette position during the recording and do not provide information about changes in cell function. H, Firing frequency of individual KNDy neurons during control period (con) and senktide (SK) treatment. Open circles show data from individual cells, filled circles show means, and lines between open circles connect data from the same cell; * marks differences within groups (P < .05), Wilcoxon matched-pairs signed rank test; # marks differences among groups (P < .05), Friedman two-way repeated-measures ANOVA with Bonferroni's multiple comparisons test.
Estradiol acts via ERα but not ERβ to inhibit the NK3R-mediated response in KNDy neurons
We next tested whether ERα or ERβ mediates the suppression of senktide-induced firing rate. Because mice with kisspeptin-targeted knockout of ERα exhibit altered steroid feedback (50), we hypothesized that ERα, not ERβ, mediates the estrogen attenuation of the KNDy neuron response to activation of NK3R. Senktide was bath applied to brain slices from castrated adult male mice that had been treated with daily injections of either the ERα agonist PPT (1 mg/kg) or the ERβ agonist DPN (1 or 2 mg/kg). Representative traces are shown in Figure 1, E–G. A response to senktide was observed in 100% of KNDy neurons from the mice treated with PPT (seven of seven) and 1 mg/kg DPN (eight of eight) and in 86% of cells (six of seven) from animals treated with 2 mg/kg DPN. Senktide increased firing frequency (P < .05 compared with presenktide control of 0.0 ± 0.0 Hz) in cells from all ER agonist groups (Figure 1H). PPT attenuated the senktide-induced increase in the mean firing rate of KNDy neurons (5.1 ± 2.2 Hz, n = 7 cells, P < .05 compared with castrates). In contrast, neither dose of DPN altered senktide-induced firing compared with that in castrates (1 mg/kg, 13.5 ± 1.8 Hz, n = 8 cells; 2 mg/kg, 8.2 ± 2.7 Hz, n = 7 cells, both P > .5 compared with castrated males). Together these data support the hypothesis that ERα mediates estradiol inhibition of the NK3R-mediated increase in action potential firing in KNDy neurons.
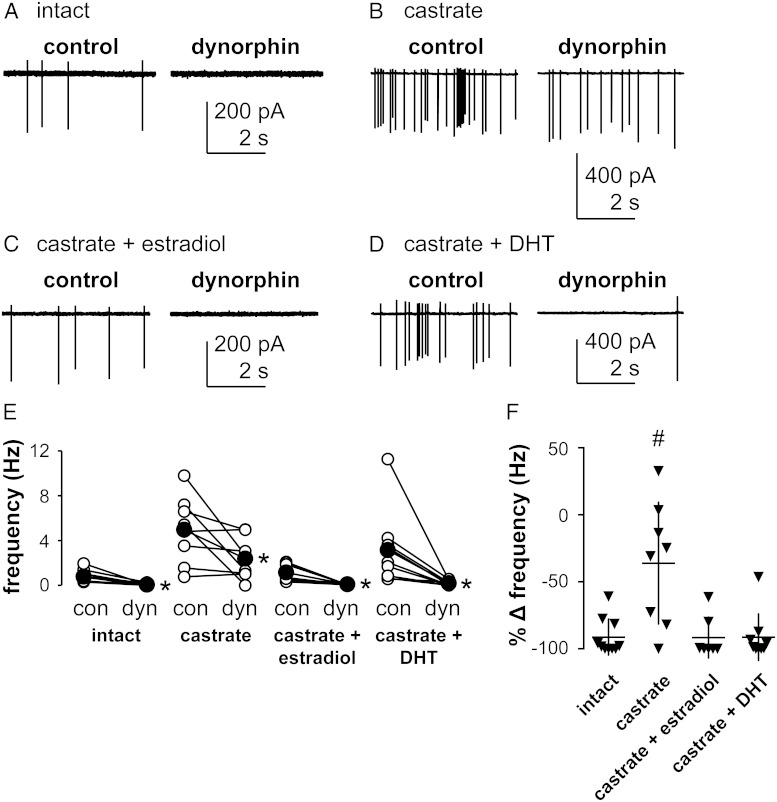
Both estradiol and DHT enhance the KOR-mediated decrease in action potential firing frequency in KNDy neurons
Dynorphin inhibits KNDy neuron firing activity in a gonad-dependent manner, with inhibition being greater in intact than castrated males (27). The effect of dynorphin on the firing rate of spontaneously active KNDy neurons from intact male mice or castrated male mice treated with estradiol or DHT was examined to test the hypothesis that estradiol enhances the dynorphin-mediated inhibition of KNDy neuron firing rate. Quiescent neurons were excluded from this experiment because inhibition of firing could not be observed. Representative traces from these recordings are shown in Figure 2, A–D. Dynorphin decreased firing (>20% decrease in frequency compared with pretreatment spontaneous activity) in 63% of KNDy neurons from the castrate-only group (five of eight) and in 100% of cells from intact animals (10 of 10), similar to our previous findings (27). Dynorphin also decreased firing in 100% of KNDy neurons studied from estradiol- (nine of nine) and DHT (seven of seven)-treated castrated males. Within each in vivo treatment group, dynorphin decreased action potential firing (intact: control, 0.8 ± 0.2 Hz, dynorphin, 0.1 ± 0.0 Hz, n = 10 cells; castrated: control, 5.0 ± 1.1 Hz, dynorphin, 2.4 ± 0.7 Hz, n = 8 cells; castrated + estradiol: control, 1.1 ± 0.3 Hz, dynorphin, 0.0 ± 0.0 Hz, n = 7 cells; castrated + DHT: control, 3.1 ± 1.1 Hz, dynorphin, 0.2 ± 0.1 Hz, n = 9 cells; all P < .05 vs predynorphin control, Figure 2E). Because of the range of spontaneous firing frequency among groups, percentage change of frequency before and during treatment was used to normalize the data for comparison among groups (Figure 2F). Compared with cells from intact males (91% ± 4% decrease), the percentage decrease in the firing rate of cells from castrated males was lower (36% ± 16% decrease, P < .05). Both estradiol (92% ± 6% decrease) and DHT (91% ± 6% decrease) treatment restored the percentage decrease in firing frequency during dynorphin treatment to that observed in cells from intact mice.
Figure 2.
Estradiol and DHT enhance the KOR-induced inhibition of KNDy neuron firing frequency. A-D, Representative raw current traces from KNDy neurons before (left panels) and during (right panels) acute treatment with dynorphin (1 μM) from the various groups as labeled. E, Firing frequency of individual KNDy neurons during the control period (con) and dynorphin (dyn) treatment. Open circles show data from individual cells, filled circles show means, and lines between open circles connect data from the same cell. *, Differences within groups (P < .05, Wilcoxon matched-pairs signed rank test). F, Percentage change (%Δ, dynorphin-control) in firing frequency of the cells plotted in panel E; horizontal lines, means; vertical lines, SEM. #, Differences among groups (P < .05, Kruskal-Wallis test with Dunn's multiple comparisons test).
KNDy neurons from both castrated and intact male mice express AR and/or ERα but not ERβ message
ERβ mRNA (Esr2) expression in KNDy neurons had not been examined in male mice. Single-cell PCR was performed on cell contents from fluorescently labeled arcuate neurons from intact or castrate Tac2-GFP adult male mice to determine the expression of Esr2 as well as AR (Ar) and ERα (Esr1) mRNA. GFP reliably identifies KNDy neurons in these animals (27). Two cells did not amplify for glyceraldehyde-3-phosphate dehydrogenase and were thus eliminated from further analysis, leaving 17 cells from intact and 22 cells from castrated mice for analysis (Table 2). The expression of Esr1 and Ar detected by single-cell PCR was consistent with that reported using in situ hybridization (28). In contrast, Esr2 was not detected in the cells studied from either intact or castrated mice. Of interest, there was considerable overlap of Ar and Esr1 expression. Of the cells that expressed Ar, 10 cells from intact (59%) and 12 cells from castrated (55%) animals also expressed Esr1.
Table 2.
Single-Cell PCR Resultsa
| Transcript | Intact Cells Expressing Transcript, (%, n = 17) | Castrated Cells Expressing Transcript, (%, n = 22) |
|---|---|---|
| Ar | 11 (65) | 15 (68) |
| Esr1 | 15 (88) | 16 (73) |
| Esr2 | 0 (0) | 0 (0) |
| Ar + Esr1 | 10 (59) | 12 (55) |
Results are shown as the number of cells expressing transcript (percentage of GFP identified cells).
Discussion
Pulsatile GnRH release is controlled by gonadal steroid feedback, but GnRH neurons typically lack most receptors critical for this feedback. Here we examined aspects of steroid feedback on arcuate kisspeptin (KNDy) neurons, which may convey these feedback effects to GnRH neurons. Our previous work demonstrated that gonadal feedback attenuated the stimulatory effects of senktide on the firing rate of KNDy neurons while increasing the inhibitory effects of dynorphin. Because only intact and castrated males were compared, this left open the question of the gonadal secretion(s) that mediated these feedback effects. We hypothesized that, as with GnRH neurons in male mice (34), estradiol would be the primary negative feedback hormone for KNDy neurons. Contrary to our hypothesis, the present results demonstrate that agonists of both ERα and AR modify response of KNDy neurons to activation of either NK3R or KOR in castrated adult male mice.
In addition to the influence of gonadal steroids on NK3R- and KOR-mediated responses, both estradiol and DHT decreased the firing frequency of spontaneously active neurons to levels observed in intact mice. Previous studies observed no change in spontaneous firing frequency of KNDy neurons between castrated and intact male or female mice (27, 51, 52), although arcuate NKB neurons exhibited reduced excitability in estradiol-treated females compared with ovariectomized females (52). It should be noted that these past and the present comparisons were made from short-duration recordings that may not reflect long-term patterns of activity of these cells. The present data may be biased toward spontaneously active neurons because some cells with no spontaneous firing activity were excluded from these analyses because verification of viability (high K+) was not done to permit subsequent studies on other cells in that slice. Despite these possible limitations, the present findings suggest that androgens and/or estrogens may influence spontaneous activity of KNDy neurons monitored over a short duration in addition to their effects on the responsiveness of these cells to their own peptide products. Both of these actions are consistent with steroid-negative feedback changing the firing rate of KNDy neurons to reduce overall reproductive neuroendocrine output.
Although most KNDy neurons in all treatment groups responded to either senktide or dynorphin, there were a few neurons in which the firing rate did not change enough to meet our criteria for response. Many populations of neurons from which recordings are obtained are heterogeneous by one measure or another (53, 54). The existence of subgroups of KNDy neurons is indicated by studies using a variety of methods. These include the percentage of cells coexpressing the mRNAs of the three KNDy peptides (27) or expressing ERα, ERβ and AR, the functional response of the cells to various tachykinins (26), and the percentage of cells that project to GnRH neurons. With regard to the latter, reports vary with sex and method. In females, the connectivity between KNDy neurons and GnRH neurons varies from none at the dendrites and soma of GnRH neurons (leaving open the possibility of interactions at the terminals) (55) to approximately one-quarter of KNDy neurons projecting to GnRH neurons (56). In males, 60% of KNDy neurons project to GnRH neurons by postnatal day 2 (57). The present results in which at minimum approximately two-thirds of the cells respond to the in vitro treatments are consistent with a typical level of heterogeneity observed in this system.
The action of estradiol on the response of KNDy neurons to activation of NK3R in males appears to be mediated by ERα. Estradiol-mediated inhibition of the NK3R-induced increase in KNDy neuron firing was mimicked by specific agonism of ERα but not ERβ. Single-cell PCR identified only ERα, suggesting KNDy neurons from male mice may lack ERβ in contrast to these cells in females (32). The apparent absence of ERβ in KNDy neurons in males agrees with previous work that showed little to no expression of ERβ in the arcuate nucleus of males (58–60). It is important to point out that in vivo steroid-induced changes in synaptic connectivity or cellular function anywhere within the brain slice could alter KNDy neuron activity because we intentionally left all communication within the slice unblocked. Expression of the steroid receptor within these cells is thus not a prerequisite for response. The present observations are, however, consistent with previous studies using global knockouts, which indicated that ERα but not ERβ is necessary for fertility in both males and females (61–63). Likewise, estradiol-induced down-regulation of kisspeptin and neurokinin B mRNA expression in the female mouse arcuate nucleus is mediated via ERα (32, 64). Furthermore, kisspeptin-specific ERα knockout advanced pubertal onset, disrupted cyclicity, and diminished the ovariectomy-induced increase in LH secretion in female mice (50), although more recent studies using this model suggest kisspeptin neurons may not be involved in negative feedback in adult females (65). Resolution of this question will require studies in which ERα is deleted from specific kisspeptin populations in adulthood to remove developmental effects of long-term knockouts.
One caveat to studies with the ERβ agonist is that we were unable to generate a positive control for efficacy of DPN on a putative ERβ-specific target despite attempting to repeat several published observations using quantitative RT-PCR (41–44). Our studies of effects via ERβ must thus be interpreted with caution but are still of interest with regard to the receptor mediating the response to DHT. Whereas DHT cannot be aromatized into estradiol, it can be metabolized into 5α-androstane-3β,17β-diol, which can act on some isoforms of ERβ (66). ERβ-specific agonism did not mimic the effects of DHT, indicating DHT action via AR rather than its metabolite's action on ERβ expressed in other neurons that transsynaptically alter KNDy neuron response to senktide.
There are several mechanisms through which estradiol and DHT may influence spontaneous KNDy neuron firing activity and/or the response to senktide or dynorphin. First, firing activity could be altered by steroid-mediated changes in expression or modulation of ion channels and other molecules contributing to action potential firing in KNDy neurons. Second, inputs other than KNDy neuropeptides, such as fast synaptic transmission, may mediate steroid-mediated inhibition of KNDy neuron activity (29, 67). Third, estradiol and DHT may change expression levels of NK3R or KOR. Previous work using single-cell PCR found that half as many KNDy neurons from castrated mice expressed KOR mRNA and twice as many expressed NK3R mRNA than KNDy neurons from intact males (27). This differs from another study in male mice using in situ hybridization that found T did not affect the percentage of arcuate kisspeptin neurons expressing either receptor message (24). Because these studies focused on mRNA, tests of protein colocalization may ameliorate this apparent contradiction. Altered subcellular localization of either NK3R or KOR is another possible mechanism for steroid-induced changes in responsiveness. Finally, steroids may alter the expression of KNDy neuropeptides because both estradiol and DHT suppress arcuate expression of kisspeptin, neurokinin B, and/or dynorphin steady-state mRNA levels (24, 28, 32, 64). The present studies cannot distinguish among these and other possible mechanisms, leaving this area open for further investigation.
The present study focuses on KNDy neurons, which form an important direct or indirect input to GnRH neurons, but steroid-negative feedback likely regulates GnRH release at multiple levels in the central nervous system. In this regard, the inhibitory actions of both estradiol and androgen at the KNDy neuron are not necessarily reflected by changes in GnRH neuron function. Inhibition of NK3R-mediated stimulation and enhancement of KOR-mediated inhibition in KNDy neurons by both estradiol and DHT would presumably decrease the amount of kisspeptin released to stimulate GnRH neurons, which express kisspeptin receptor and are directly depolarized by kisspeptin (22, 68–71). In a study using the same experimental model used here, however, the increase in peaks of action potential firing observed in GnRH neurons after castration was inhibited by estradiol but DHT had no effect (34). Of note, androgens did appear to inhibit GnRH neuron action potential frequency in male mice at pharmacologically high doses that mimic steroid abuse (72). The contrasting effect of physiological levels of androgen provided by DHT in KNDy vs GnRH neurons in males supports past (73) and recent (15) evidence that additional central circuits beyond kisspeptin-expressing neurons alter GnRH output. Estradiol may also have a role in GnRH neurons via ERβ, the acute activation of which excites GnRH neurons from female mice (10, 74).
A candidate for nonkisspeptin relay of steroid feedback to GnRH neurons and for which some mechanistic evidence exists is γ-aminobutyric acid (GABA)ergic neurons. GABA excites GnRH neurons because of their intracellular chloride concentration (75–77). Estradiol inhibits GABAergic postsynaptic currents in GnRH neurons from males (78), consistent with its inhibitory effect on the action potential firing rate of these cells (34). One source of GABAergic input to GnRH neurons may be KNDy neurons because half of these cells coexpress mRNA for glutamic acid decarboxylase-67, an enzyme that synthesizes GABA (79). Inhibition of KNDy neuron activity by estradiol would thus likely reduce both kisspeptin and GABA release from these cells. The effects of DHT on GABAergic transmission have not been studied in males, but in females both DHT in combination with estradiol and a hyperandrogenemic state produced by prenatal androgen exposure increase GABAergic transmission to GnRH neurons as well as close appositions from arcuate kisspeptin neurons (80–82). Although DHT inhibited KNDy neurons and would thus likely reduce GABA from this source, it may have different effects on other GABAergic inputs to GnRH neurons (83–87) and thereby increase the excitatory drive to GnRH neurons independent of kisspeptin that could counteract the loss of this stimulation.
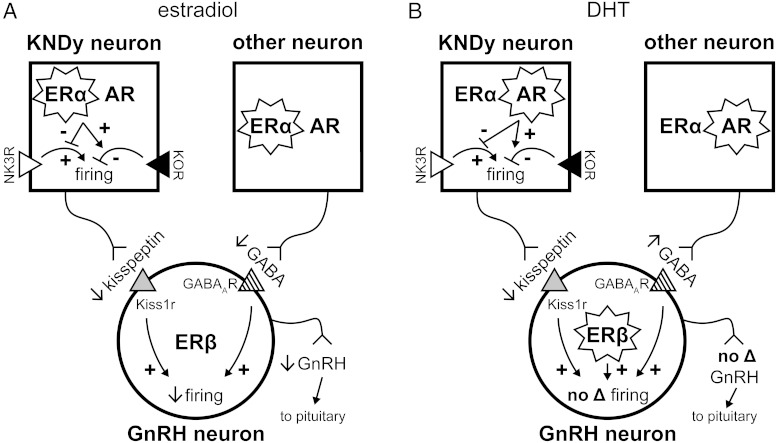
Based on the present observations and previous work highlighted here, we propose a model for gonadal steroid feedback on GnRH neurons via KNDy neurons and other inputs, using GABA as an example for the latter (Figure 3). In males, estradiol plays an overall inhibitory role, attenuating NK3R-mediated stimulation and enhancing KOR-mediated inhibition of KNDy neuron firing activity. This decreases the amount of stimulatory kisspeptin released to GnRH neurons. Estradiol also decreases GABAergic excitation of GnRH neurons, ultimately decreasing GnRH release. The effects of DHT on NK3R- and KOR-mediated responses in and kisspeptin release from KNDy neurons mirror those of estradiol. However, these effects are counteracted in the GnRH neuron by a DHT-mediated increase in GABAergic (or other) excitation as well as by a possible activation of ERβ by the diol metabolite of DHT, resulting in no net change in GnRH neuron activity in DHT-treated males. In the intact male with circulating T that may be metabolized to estradiol and/or DHT in the brain, any excitatory central effects of AR activation on GnRH neuron activity are counteracted by the inhibitory actions of estradiol and by androgen inhibition at the level of the pituitary (34, 88). This is supported by high levels of aromatase in the hypothalamus to convert T to estradiol (30) as well as the ultimate inhibition of LH release by both estradiol and androgen (24, 34).
Figure 3.
Proposed model for gonadal steroid feedback on KNDy, GnRH, and other neurons in male mice. Different pathways are activated by estradiol (A) and DHT (B) to differentially affect GnRH output (see text for details). We propose that in the brain of the intact male, estradiol-negative feedback prevails. A, The low levels of estradiol in males do not act through ERβ to affect GnRH output. B, ERβ may be activated by a diol metabolite of DHT. A star surrounding a steroid receptor indicates activation; +, stimulation; −, inhibition. GABAAR, GABAA receptor; Kiss1r, kisspeptin receptor.
The current studies demonstrate an inhibitory role of both estradiol and androgen on KNDy neurons via inhibition of action potential firing induced by NK3R activation and enhancement of inhibition of firing by KOR activation. These findings elucidate in part mechanisms of steroid feedback to a potential key component of the GnRH pulse generator. The present data also suggest that components beyond kisspeptin neurons are important for the overall regulation of GnRH release.
Acknowledgments
We thank Elizabeth Wagenmaker for her excellent technical assistance and editorial comments, and Dr R. Anthony DeFazio, Tova Berg, and Luhong Wang for their editorial comments.
This work was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01HD34860 (to S.M.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- AR
- androgen receptor
- DMSO
- dimethylsulfoxide
- DPN
- 2,3-bis(4-hydroxyphenyl)-propionitrile
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- KNDy
- arcuate neurons coexpressing neurokinin B, dynorphin and kisspeptin
- KOR
- κ-opioid receptor
- NK3R
- neurokinin-3 receptor
- PPT
- 4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol
- qPCR
- quantitative PCR.
References
- 1. Carmel PW, Araki S, Ferin M. Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology. 1976;99:243–248. [DOI] [PubMed] [Google Scholar]
- 2. Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. [DOI] [PubMed] [Google Scholar]
- 3. Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130:503–510. [DOI] [PubMed] [Google Scholar]
- 4. Levine JE, Duffy MT. Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology. 1988;122:2211–2221. [DOI] [PubMed] [Google Scholar]
- 5. Belchetz P, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. [DOI] [PubMed] [Google Scholar]
- 6. Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod. 1987;36:1207–1218. [DOI] [PubMed] [Google Scholar]
- 7. Hrabovszky E, Steinhauser A, Barabas K, et al. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. [DOI] [PubMed] [Google Scholar]
- 8. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. [DOI] [PubMed] [Google Scholar]
- 9. Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63:120–131. [DOI] [PubMed] [Google Scholar]
- 10. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–260. [DOI] [PubMed] [Google Scholar]
- 12. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315. [DOI] [PubMed] [Google Scholar]
- 13. Nishihara M, Hiruma H, Kimura F. Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology. 1991;54:321–326. [DOI] [PubMed] [Google Scholar]
- 14. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ezzat A, Pereira A, Clarke IJ. Kisspeptin is a component of the pulse generator for GnRH secretion in female sheep but not the pulse generator. Endocrinology. 2015;156:1828–1837. [DOI] [PubMed] [Google Scholar]
- 16. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 20. Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154:3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015;156:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–1012. [DOI] [PubMed] [Google Scholar]
- 24. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. [DOI] [PubMed] [Google Scholar]
- 26. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. [DOI] [PubMed] [Google Scholar]
- 27. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 29. DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci. 2014;34:16296–16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90:295–298. [DOI] [PubMed] [Google Scholar]
- 31. Ryan KJ, Naftolin F, Reddy V, Flores F, Petro Z. Estrogen formation in the brain. Am J Obstet Gynecol. 1972;114:454–460. [DOI] [PubMed] [Google Scholar]
- 32. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 33. Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154:2739–2749. [DOI] [PubMed] [Google Scholar]
- 34. Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74:931–937. [DOI] [PubMed] [Google Scholar]
- 35. Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology. 1997;138:3686–3694. [DOI] [PubMed] [Google Scholar]
- 36. McManus CJ, Goodman RL, Llanza NV, et al. Inhibition of luteinizing hormone secretion by localized administration of estrogen, but not dihydrotestosterone, is enhanced in the ventromedial hypothalamus during feed restriction in the young wether. Biol Reprod. 2005;73:781–789. [DOI] [PubMed] [Google Scholar]
- 37. Santen RJ. Is aromatization of testosterone to estradiol required for inhibition of luteinizing hormone secretion in men? J Clin Invest. 1975;56:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avtanski D, Novaira HJ, Wu S, et al. Both estrogen receptor α and β stimulate pituitary GH gene expression. Mol Endocrinol. 2014;28:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34:15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. [DOI] [PubMed] [Google Scholar]
- 41. Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94. [DOI] [PubMed] [Google Scholar]
- 42. Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor α and β specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muir JL, Pfister HP. Time course of the corticosterone and prolactin response following predictable and unpredictable novelty stress in Rattus norvegicus. Physiol Behav. 1987;40:103–107. [DOI] [PubMed] [Google Scholar]
- 44. Otsuki M, Gao H, Dahlman-Wright K, et al. Specific regulation of lipocalin-type prostaglandin D synthase in mouse heart by estrogen receptor β. Mol Endocrinol. 2003;17:1844–1855. [DOI] [PubMed] [Google Scholar]
- 45. Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145:728–735. [DOI] [PubMed] [Google Scholar]
- 46. Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci. 2005;25:5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. [DOI] [PubMed] [Google Scholar]
- 48. Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proc Online. 2003;5:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32:3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153:5384–5393. [DOI] [PubMed] [Google Scholar]
- 52. Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155:2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology. 2008;149:3130–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156:2582–2594. [DOI] [PubMed] [Google Scholar]
- 56. Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C, Boehm U. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology. 2015;156:32–38. [DOI] [PubMed] [Google Scholar]
- 57. Kumar D, Periasamy V, Freese M, Voigt A, Boehm U. In utero development of kisspeptin/GnRH neural circuitry in male mice. Endocrinology. 2015;156:3084–3090. [DOI] [PubMed] [Google Scholar]
- 58. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. [DOI] [PubMed] [Google Scholar]
- 59. Mitra SW, Hoskin E, Yudkovitz J, et al. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. [DOI] [PubMed] [Google Scholar]
- 60. Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. [DOI] [PubMed] [Google Scholar]
- 61. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. [DOI] [PubMed] [Google Scholar]
- 62. Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96:12887–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742. [DOI] [PubMed] [Google Scholar]
- 65. Dubois SL, Acosta-Martinez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365:43–47. [DOI] [PubMed] [Google Scholar]
- 67. Wang L, Moenter SM. Fast glutamatergic transmission to hypothalamic kisspeptin neurons is regulated in an estrous cycle-dependent manner. Presented at: Annual Meeting of the Society for Neuroscience; 2014; Washington, DC. [Google Scholar]
- 68. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. [DOI] [PubMed] [Google Scholar]
- 69. Han S-K, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Penatti CAA, Davis MC, Porter DM, Henderson LP. Altered GABAA receptor-mediated synaptic transmission disrupts the firing of gonadotropin-releasing hormone neurons in male mice under conditions that mimic steroid abuse. J Neurosci. 2010;30:6497–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gore AC. GnRH: The Master Molecule of Reproduction. Boston: Kluwer Academic Publishers; 2002. [Google Scholar]
- 74. Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: toward an emerging consensus. J Neuroendocrinol. 2011;23:557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu X, Porteous R, d'Anglemont de Tassigny X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31:2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891. [DOI] [PubMed] [Google Scholar]
- 78. Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;29:9809–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72:33–41. [DOI] [PubMed] [Google Scholar]
- 81. Sullivan SD, Moenter SM. Neurosteroids alter γ-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144:4366–4375. [DOI] [PubMed] [Google Scholar]
- 82. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the GnRH system of the ewe: changes in identified GnRH inputs and in glial association. Endocrinology. 2003;144:3663–3676. [DOI] [PubMed] [Google Scholar]
- 84. Pompolo S, Pereira A, Kaneko T, Clarke IJ. Seasonal changes in the inputs to gonadotropin-releasing hormone neurones in the ewe brain: an assessment by conventional fluorescence and confocal microscopy. J Neuroendocrinology. 2003;15:538–545. [DOI] [PubMed] [Google Scholar]
- 85. Sullivan SD, DeFazio RA, Moenter SM. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci. 2003;23:8578–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. [DOI] [PubMed] [Google Scholar]
- 87. Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144:4967–4974. [DOI] [PubMed] [Google Scholar]
- 88. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]