Abstract
CD36/FAT (fatty acid translocase) is associated with human and murine nonalcoholic fatty liver disease, but it has been unclear whether it is simply a marker or whether it directly contributes to disease pathogenesis. Mice with hepatocyte-specific deletion of Janus kinase 2 (JAK2L mice) have increased circulating free fatty acids (FAs), dramatically increased hepatic CD36 expression and profound fatty liver. To investigate the role of elevated CD36 in the development of fatty liver, we studied two models of hepatic steatosis, a genetic model (JAK2L mice) and a high-fat diet (HFD)-induced steatosis model. We deleted Cd36 specifically in hepatocytes of JAK2L mice to generate double knockouts and from wild-type mice to generate CD36L single-knockout mice. Hepatic Cd36 disruption in JAK2L livers significantly improved steatosis by lowering triglyceride, diacylglycerol, and cholesterol ester content. The largest differences in liver triglycerides were in species comprised of oleic acid (C18:1). Reduction in liver lipids correlated with an improvement in the inflammatory markers that were elevated in JAK2L mice, namely aspartate aminotransferase and alanine transaminase. Cd36 deletion in mice on HFD (CD36L-HFD) reduced liver lipid content and decreased hepatic 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-FA uptake as compared with CON-HFD. Additionally, CD36L-HFD mice had improved whole-body insulin sensitivity and reduced liver and serum inflammatory markers. Therefore, CD36 directly contributes to development of fatty liver under conditions of elevated free FAs by modulating the rate of FA uptake by hepatocytes. In HFD-fed animals, disruption of hepatic Cd36 protects against associated systemic inflammation and insulin resistance.
Fatty liver (hepatic steatosis) occurs in 10%–25% of people worldwide and is considered the hepatic manifestation of the metabolic syndrome (1). The liver has a high capacity for free fatty acid (FFA) uptake and plays an important role in lipid metabolism. Hepatic fatty acid uptake occurs mainly through the family of slc27a fatty acid transport proteins (FATP) and the scavenger receptor CD36 (fatty acid translocase). FATP2 and FATP5 are the predominant FATPs in the liver and in vivo knockdown of FATP5 prevents and reverses fatty liver in mice (2). This demonstrates that loss of specific transporters in hepatocytes can affect liver FA flux and total lipid content. After uptake, FFAs are esterified into neutral lipid and packaged for secretion or stored. However, if the rate of uptake is greater than the capacity for esterification, accumulation of FFAs can promote inflammation.
Several factors are associated with the development of fatty liver including a high-fat diet, type 2 diabetes, hormones, and genetic mutations. In humans, there is a strong association between GH deficiency and increased risk for, or the presence of, nonalcoholic fatty liver disease (NAFLD) (3–5). Several animal models have shown that hepatic steatosis develops when GH signaling (specifically in the liver) is disrupted due to the loss of Janus kinase 2 (Jak2), GH receptor, or signal transducer and activator of transcription 5 (6–8). In JAK2L mice, hepatic Jak2 deficiency also leads to increased GH-stimulated adipose lipolysis, elevated circulating FFAs, and a 16-fold increase in Cd36 expression.
CD36 is a member of the class B scavenger receptor family with the ability to bind oxidized low-density lipoprotein (9), long-chain fatty acids, phospholipids, and collagen (10). Its high expression on macrophages, adipocytes, cardiomyocytes, and muscle cells is important for fatty acid (FA) uptake and lipid metabolism in these cells, and overexpression in muscle causes muscle steatosis (11). CD36 expression is much lower in normal hepatocytes but is increased with lipid-rich diets, hepatic steatosis, and NAFLD (12–14). Furthermore, increased hepatic Cd36 expression can increase FA uptake, and triglyceride (TG) accumulation (15–17) in several mouse strains Cd36 was identified as the gene most correlated with fatty liver (18). Still, its role in regulating liver lipid metabolism is unclear because both loss and gain of Cd36 function increased liver TG content (16, 19, 20). We therefore set out to define the precise role of CD36 in the pathogenesis of fatty liver.
Building on the observations in JAK2L mice, we generated mice lacking both Jak2 and Cd36 in hepatocytes and assessed the effect on liver lipid content, composition, and physiology. Concomitant deletion of Cd36 and Jak2 in hepatocytes led to near normalization of liver lipid content and inflammatory markers observed in JAK2L mice. Next, to understand the role of CD36 in normal liver lipid metabolism, we generated hepatocyte-specific Cd36 single knockout mice (CD36L mice). Control (CON) and CD36L mice were fed a low-fat (13% calories from fat) or a 60% fat diet and analyzed for effects on metabolism. We found that loss of Cd36 in the liver significantly blunted the development of hepatic steatosis under conditions of elevated FA. Cd36 deletion also affected the FA composition of liver lipids and improved the serum markers of liver inflammation. Finally, hepatocyte-specific loss of Cd36 significantly improved whole-body insulin sensitivity in mice fed a high-fat diet (HFD). Together, these data demonstrated that CD36 is not just a marker of disease but also plays an active role in fatty acid uptake and has significant effects on liver lipid content and composition and on insulin sensitivity.
Materials and Methods
Animals
Male JAK2L, JAK2L/CD36L (DKO), liver-specific Cd36 knockout (CD36L) and all CONL mice were bred and maintained in a constant 12-hour light, 12-hour dark cycle and normal chow diet (22% caloric intake from fat) or low-fat diet (LFD) (13% caloric intake from fat) from Pico lab Diet (number 5058 and number 5053) and water ad libitum. For the HFD feeding experiments, mice were placed on 60% fat diet (Research Diets D12492) for 6 weeks starting at age 5–6 weeks. All animal studies were performed in compliance with the approved protocols of the University of California, San Francisco, Institutional Animal Care and Use Committee.
Measurement of serum metabolites
Glucose levels were measured with a Contour glucometer (Bayer) via tail vein blood. Blood was collected from the retroorbital sinus for final blood collection or via the tail vein for fasting and refed samples and spun at 15 000 × g for plasma collection or allowed to clot prior to centrifugation at 10 000 × g for 10 minutes at 4°C for serum collection. Plasma and serum were stored at −80°C until used for analyses. For serum insulin measurements, mice were fasted overnight (16 h) or fed ad libitum prior to blood collection at 10:00 am. Insulin levels were determined by an ELISA (ALPCO Diagnostics) according to the manufacturer's instructions. Nonesterified fatty acids and total cholesterol esters were measured using commercially available kits (Wako Chemicals). Plasma triglycerides were analyzed using an Infinity triglyceride reagent (Thermo Scientific). Serum aspartate aminotransferase (AST) and alanine transaminase (ALT) were analyzed by the University of California, Davis, Comparative Pathology Laboratory.
RNA isolation and real-time quantitative RT-PCR
Tissue was harvested and immediately snap frozen in liquid nitrogen prior to homogenization in TRIzol reagent (Invitrogen). Total RNA was extracted using an RNeasy minikit (QIAGEN Inc) as previously described (21). Gene expression primers and probe sequences were designed using Primer Express or purchased from Applied Biosystems (ThermoFisher Scientific) and are included in Supplemental Table 1. TaqMan real-time quantitative RT-PCR was performed with a Bioline SensiFAST Probe One-Step kit using total RNA (50 ng for tissue, 25–50 ng for hepatocytes) for each sample in duplicate. Expression levels were normalized to either RpS9 or 18S rRNA and relative levels compared with control samples using the 2-δδCt method (22).
Liver histology
Liver was fixed in 4% PFA and then dehydrated in 70% ethanol. Sectioning and hematoxylin and eosin staining was performed by the University of California, San Francisco (UCSF), Mouse Pathology Core. Images were taken on an Olympus BX51 microscope.
Western blotting
For detection of CD36 and actin, tissue was homogenized in radioimmunoprecipitation assay buffer (50 mM Tris; 150 mM NaCl; 1% Triton X-100; 1% deoxycholate; 0.1% sodium dodecyl sulfate; 1 mM EDTA, pH 7.4) and incubated on ice for 20 minutes. After centrifuging for 20 minutes at a maximum speed at 4°C, the protein concentration of the supernatant was determined using a bicinchoninic acid assay. Fifty micrograms of liver protein or 12.5 μg heart protein was used for Western blotting. Primary antibodies were used at 1:1000 dilution for CD36 (Novus Biologicals) and β-actin (Abcam), followed by fluorescently tagged secondary antibodies at 1:1000 dilution (Alexafluor-555 for CD36 and Alexafluor-647 for β-actin).
Liver lipidomics analysis
Liver tissue samples were homogenized in methanol-water (1:1 [vol/vol]) using the QIAGEN tissue lyser. After homogenization, lipids were extracted from the homogenate with dichloromethane-isopropanol-methanol (25:10:65 [vol/vol/vol]) containing the following internal standards at a concentration of 200 nM: glyceryl triheptadecanoate, 1,2-dinonadecanoin, cholesteryl heptadecanoate, 1,2-dilauroyl-sn-glycero-3-phosphocholine, 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine, and palmitoyl-L-carnitine-(N-methyl-d3) hydrochloride. Lipid extracts were then analyzed by ultraperformance liquid chromatography (UPLC)-tandem mass spectrometry using a Waters Acquity UPLC coupled to an AB Sciex QTRAP 5500 mass spectrometer. Lipid classes were separated by reversed-phase chromatography on a Waters Acquity UPLC BEH300 C4 column, 1.7 μm, 2.1 × 50 mm. Lipid species were analyzed on the mass spectrometer using positive ion electrospray ionization in the multiple reaction monitoring mode. Lipid chromatography chromatogram peak integration was performed with AB Sciex MultiQuant software. All data reduction was performed with Pfizer in-house software.
In vivo 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)-FA acid uptake
The BODIPY-FA uptake experiment was adopted from Khalifeh-Soltani et al (23). Briefly, BODIPY-C16 (Life Technologies) was resuspended in dimethylsulfoxide, and then a 0.1 μg/μL working stock was made in 1× PBS, 0.25% BSA (Sigma-Aldrich) (pH 7.4). Mice were fasted 4 hours and then injected ip with 0.5 μg BODIPY-C16 per gram of body weight. Controls were injected with respective volume of buffer without BODIPY-C16. Serum samples were collected prior to BODIPY injection, 1 and 3 hours after injection via the tail vein and then at 5 hours after injection via cardiac puncture. Tissues were collected at 5 hours and homogenized in radioimmunoprecipitation assay buffer. One hundred microliters of cleared tissue homogenate or 10 μL serum was analyzed using a fluorescent plate reader (Ex 485nm, Em 515nm, cutoff 495 nm). Fluorescence from saline-injected tissue was used as background and tissue fluorescence was normalized to tissue weight.
Triglyceride secretion
After a 4-hour fast, mice were injected with 500 mg/kg of Tyloxapol in sterile saline. Blood was collected via the tail vein at 0, 1, 2, 4, and 6 hours after injection in lithium-heparin-coated capillary tubes (Sarstedt Inc) and centrifuged at 2000 × g for 20 minutes at 4°C to collect plasma. Plasma TG was determined as described above.
Metabolic tests and fasting experiments
For insulin tolerance tests (ITT), mice were fasted for 4 hours and then given an ip bolus of 0.75 U insulin (Novolin; Novo Nordisk) per kilogram of body weight. For glucose tolerance tests (GTTs), mice were injected ip with 2 g of glucose (Fisher Scientific) per kilogram of body weight after a 16-hour fast. Glucose levels at indicated time points were measured from tail vein blood as described above.
Statistical analysis
Data are presented as means ± SEM. Statistical significance was determined by an unpaired two-tailed Student's t test or an ANOVA using Prism (Graph Pad). A value of P < .05 was considered statistically significant.
Results
Concomitant disruption of Cd36 in JAK2L mice reduces hepatic TG content
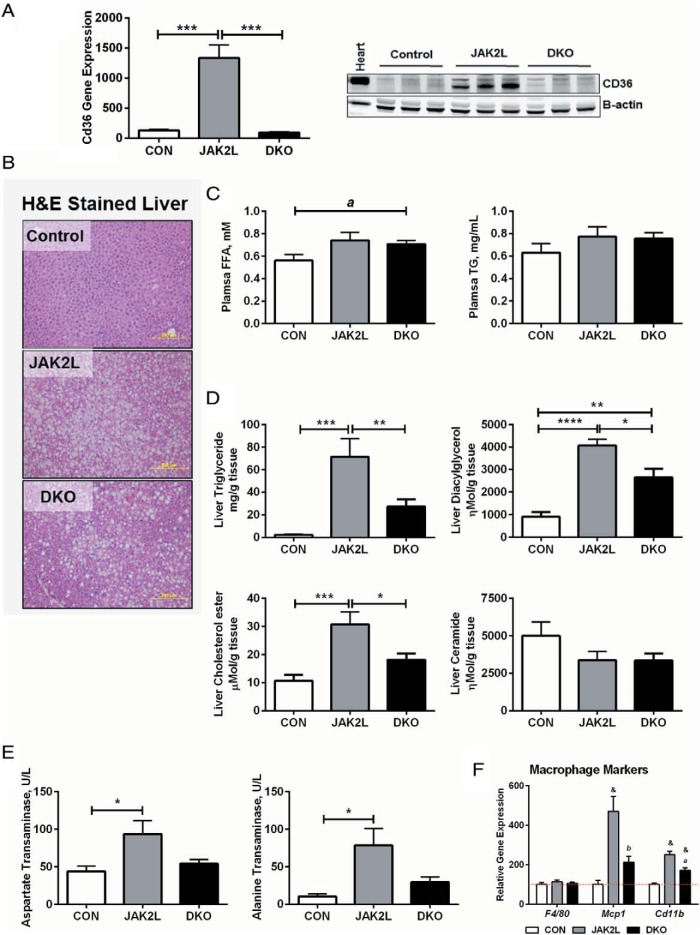
One of the most striking findings of JAK2L mice was the profound up-regulation of hepatic Cd36 expression associated with severe steatosis and inflammation (7) (Figure 1A). To address whether elevated hepatic CD36 protein in JAK2L mice was simply a marker or contributed to the development of steatosis, we generated mice with concomitant disruption of hepatocyte Jak2 and Cd36 (double knockout mice [DKO]) by crossing Jak2 floxed mice with Cd36 floxed mice (7, 24) and then to mice carrying the albumin promoter driven Cre recombinase to generate CON (Jak2 F/F, Cd36 F/F, AlbCre −/−), JAK2L (Jak2 F/F, Cd36 +/+, AlbCre +/− or Jak2 F/F, Cd36 F/+, AlbCre +/−) and DKO mice (Jak2 F/F, Cd36 F/F, AlbCre +/−). As previously observed (7), quantitative RT-PCR of whole liver showed that Cd36 expression was dramatically increased in JAK2L livers as compared with CON livers (Figure 1A). In DKO mice, Cd36 expression was reduced to levels lower than in CON mice. In addition, CD36 protein expression was also significantly reduced in DKO mice compared with JAK2L mice (Figure 1A, right panel). We confirmed that Jak2 and Igf-1 gene expressions were significantly reduced in both JAK2L and DKO mice as compared with the CON (Supplemental Figure 1). There were no differences in body weight between the JAK2L and DKO mice (Table 1).
Figure 1.
Liver lipid content is reduced in JAK2/CD36 DKO mice compared with JAK2L mice. CON, JAK2L, and JAK2/CD36 liver-specific KO mice (DKO) were fed a normal chow diet and samples collected at 9 weeks of age from ad libitum-fed mice. A, Hepatic Cd36 gene expression by quantitative RT-PCR and CD36 protein expression in liver by Western blot. B, Representative hematoxylin and eosin-stained liver sections from CON, JAK2L, and DKO mice at ×10 magnification. C, Plasma FFAs and TGs collected at 9 weeks (see also Table 1). D, Content of liver TG, DAG, CE, and ceramide content. E, Serum liver damage markers AST and ALT levels (n = 6–9/group). *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001. F, Expression of F4/80, Mcp1, and Cd11b in the liver of CON, JAK2L, and DKO mice (n = 7–10/group); &, P < .001 compared with CON mice; b, P < .01, a, P < .001 compared with JAK2L mice. All data are shown as mean ± SEM.
Table 1.
Basic Metabolic Parameters in CON, JAK2L, and DKO Mice
| CON | JAK2L | DKO | |
|---|---|---|---|
| Body weight, g | 29.39 ± 1.25 | 25.99 ± 1.11 | 26.55 ± 0.62 |
| Liver weight, g | 1.35 ± 0.06 | 2.01 ± 0.21a | 1.65 ± 0.08 |
| Liver weight, % | 4.60 ± 0.11 | 7.60 ± 0.45b | 6.21 ± 0.23a,c |
| Body fat, % | 18.38 ± 1.16 | 23.25 ± 2.20 | 19.55 ± 0.08 |
| Lean mass, % | 80.64 ± 1.25 | 77.13 ± 2.04 | 81.63 ± 0.56 |
| Plasma FFA, mM | 0.56 ± 0.05 | 0.63 ± 0.01 | 0.71 ± 0.03d |
| Plasma TG, mg/mL | 0.63 ± 0.08 | 0.74 ± 0.11 | 0.75 ± 0.05 |
| Glucose, mg/dL | |||
| Ad libitum fed | 169.9 ± 10.7 | 157.0 ± 13.9 | 176.3 ± 14.1 |
| Fasted | 67.3 ± 6.1 | 118.5 ± 10.3b | 98.9 ± 7.2d |
| Insulin, ng/mL | |||
| Ad libitum fed | 0.354 ± 0.059 | 0.454 ± 0.061 | 0.736 ± 0.154 |
| Fasted | 0.034 ± 0.016 | 0.056 ± 0.025 | 0.029 ± 0.005 |
| Liver glycogen, mg/g | 30.09 ± 4.25 | 24.56 ± 2.70 | 44.86 ± 4.02c,d |
| Bilirubin, mg/dL | 0.13 ± 0.02 | 0.08 ± 0.01 | 0.08 ± 0.01d |
Data represents mean ± SEM.
P < .01 compared with CON.
P < .001 compared with CON.
P < .01 compared with JAK2L
P < .05 compared with CON.
We observed significant steatosis in JAK2L mice (Figure 1B). However, concomitant loss of Cd36 reduced the abundance of lipid droplets in DKO livers as compared with JAK2L livers. The liver weight (total and percentage) of DKO mice was also reduced to near normal (Table 1). Plasma TG and FFA levels were elevated in the JAK2L and DKO mice as compared with CON but not different between the DKO and JAK2L mice (Figure 1C). As expected, there was a profound increase in liver TG content in the JAK2L mice as compared with the CON mice. However, despite the elevated plasma FFA levels, hepatocyte-specific deletion of Cd36 in JAK2L mice led to a 60% reduction in the liver TG in the DKO mice (Figure 1D). Total TG was 2.1, 71.3, and 27.3 mg/g tissue in CON, JAK2,L and DKO mice, respectively. There was no difference in the TG content of gastrocnemius and cardiac muscle between the JAK2L and DKO mice (Supplemental Figure 2, A and B). As expected, fat pad weights were significantly lower in the JAK2L and DKO mice compared with CONs but were unchanged between the JAK2L and DKO (Supplemental Figure 3).
In JAK2L mice, FA incorporation into diacylglycerol (DAG) was increased 4.5-fold compared with CON but reduced by 35% in the DKO vs JAK2L mice (Figure 1D, top right panel). We also measured liver cholesterol ester (CE) content (cholesterol bound to a FA) and observed it to be 3-fold higher CE in JAK2L mice compared with CON. CE content was decreased by 40% in DKO mice compared with JAK2L mice. Total ceramides (sphingosine bound to a FA) were lower in both JAK2L and DKO mice (Figure 1D, bottom right panel) as compared with CON. To determine whether the effects of Cd36 deletion were restricted to lipids, we also measured hepatic glycogen levels. Glycogen levels in JAK2L livers were not different from CON mice, but DKO livers had remarkably higher glycogen levels as compared with both JAK2L and CON mice (Supplemental Figure 2C).
Increased uptake of nonesterified FAs can lead to lipotoxicity and an increase in serum markers of hepatic inflammation such as AST and ALT. Both AST and ALT were elevated in JAK2L mice as compared with CON but were reduced in DKO mice compared with JAK2L mice (Figure 1E). Similarly, macrophage markers (monocyte chemoattractant protein-1 [Mcp1] and cluster of differentiation molecule 11b [Cd11b]) in whole liver were significantly elevated in JAK2L mice, but this increase was blunted in the DKO mice (Figure 1F). Taken together, the deletion of Cd36 in JAK2L livers led to significant reduction in hepatic lipid content and improved markers of liver inflammation, indicating that increased Cd36 expression in JAK2L mice was not simply a marker of steatosis but actively contributed to its severity.
CD36 regulates hepatic FA composition by increasing unsaturated FA content
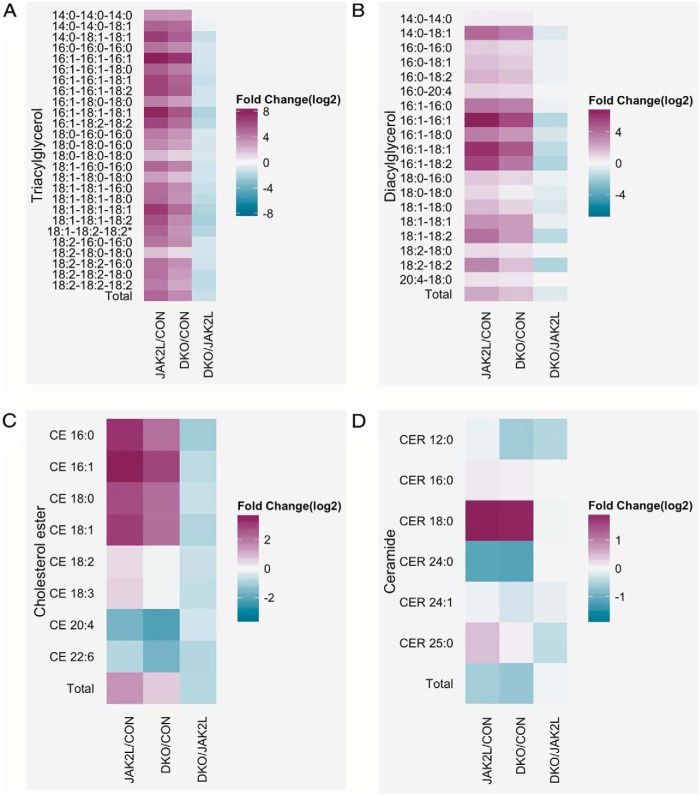
CD36 facilitates the transport of long-chain fatty acids in several cell types, and its overexpression is known to increase FA uptake (25, 26). Therefore, we sought to determine if differences in Cd36 expression would affect the fatty acid composition of specific liver lipids. Figure 2A shows the fold change in individual TG species in the JAK2L and DKO livers as compared with the CON liver and in DKO compared with JAK2L. The mass spectrometric analysis demonstrated that all TG species were elevated in JAK2L mice, with some species (TG-16:1–16:1–16:1 and TG-16:1–18:1–18:1) being greater than 200-fold increased (Supplemental Table 2). Similarly, all TG species were increased in DKO livers compared with CON but to a lesser extent than in JAK2L livers. As such, all TGs were less abundant in DKO livers as compared with JAK2L, especially for TGs comprised of two oleic acids (C18:1). TG-16:1–18:1–18:1, TG-18:1–18:1–18:1, and TG-18:1–18:1–18:2 were decreased 4-fold in DKO livers compared with JAK2L livers.
Figure 2.
High Cd36 expression alters FA composition of liver lipids. A–D, Mass spectrometric analysis of liver lipids from ad libitum-fed CON, JAK2L, and DKO was performed. The heat maps show the fold difference (log 2 transformed) between the groups for each lipid species for triacylglycerols (A), DAGs (B), CEs (C), and ceramides (CER) (D) (n = 6–8/group, data shown as mean ± SEM). *, P < .05, #, P < .01, &, P < .001 compared with CON mice; c, P < .05, b, P < .01, a, P < .001 compared with JAK2L mice.
DAG species were elevated in JAK2L and DKO livers compared with CON, but Cd36 loss led to reductions in DAGs in DKO mice compared with the JAK2L mice (Figure 2B). For example, as compared with the CON mice, DAG-16:1–16:1 was increased 120-fold in JAK2L liver but only 40-fold in DKO liver. Compared with JAK2L mice, the largest change in DAG levels for the DKO mice was observed for DAG-16:1–18:2 and DAG-18:2–18:2 (reduced 3.4-fold). FA conjugation to cholesterol was also increased in JAK2L livers but to a lesser extent in DKO livers (Figure 2C). Cholesterol conjugated to C20:4 and 22:6 was decreased in both the JAK2L and DKO mice as compared with CON, but essential FA esters (CE-18:2 and CE-18:3) were unchanged in DKO mice compared with CON.
Finally, although total ceramide levels were decreased in the JAK2L and DKO mice as compared with the CON, closer examination showed that ceramide CER-18:0 was elevated in JAK2L and DKO livers (Figure 2D), whereas CER-24:0 (the most abundant ceramide) was decreased 2-fold. All ceramide-FA conjugates were lower in DKO livers compared with JAK2L livers. Other ceramide derivatives such as glucosylceramide were also lower in the JAK2L and DKO livers, whereas lactosylceramide and ceramide-1-phosphate were elevated (Supplemental Figure 4, A–C). Structural phospholipids such as phosphatidylcholine and sphingomyelin were unchanged among the groups (Supplemental Figure 4, D and E). These data demonstrated that hepatocyte-CD36 modulated the FA composition of neutral lipids with high CD36 protein expression, shifting the balance toward an accumulation of unsaturated FAs.
Loss of Cd36 has modest effect on expression of lipid metabolism gene expression in the livers of JAK2L mice
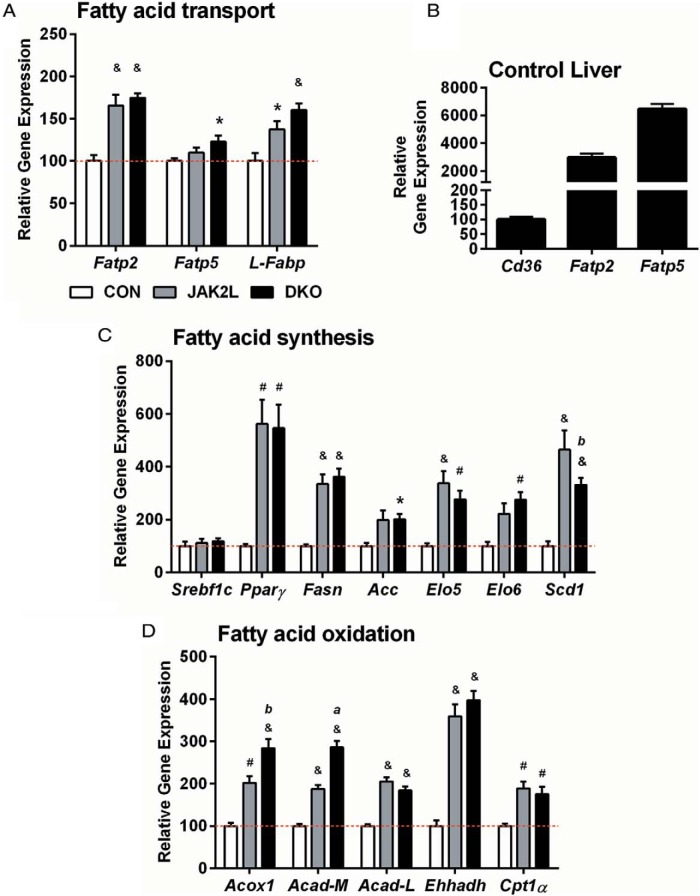
The steady-state pool of lipids in the liver and their FA composition depends on the balance of FA uptake/synthesis and FA use. To probe for mechanisms by which Cd36 deletion reduced liver lipid content, we examined the expression pattern of key genes that regulate these processes. Compared with CON livers, Fatp2 was increased 1.7-fold in the JAK2L and DKO livers (Figure 3A). Although modest, this increase may be biologically relevant as Fatp2 is normally 30-fold more abundant than Cd36 in the CON livers (Figure 3B).
Figure 3.
Scd1 gene expression is decreased in DKO mice compared with JAK2L mice. A, Hepatic gene expression patterns for FA transport genes normalized to CON expression. B, Expression of Fatp2 and Fatp5 in CON liver normalized to Cd36 expression (n = 4 per group). Hepatic gene expression patterns for FA synthesis (C) and β-oxidation genes (D) normalized to CON expression (n = 4–11/group, data shown as mean ± SEM). *, P < .05, #, P < .01, &, P < .001 compared with CON mice; c, P < .05, b, P < .01, a, P < .001 compared with JAK2L mice.
Next, we examined the expression of genes involved in de novo FA synthesis. Previously, stable isotope studies demonstrated that FA synthesis was not increased in the livers of JAK2L mice vs CON. Indeed, Srebp1, a transcription factor that regulates the lipogenesis program was not increased in JAK2L or DKO mice. Nonetheless, as shown previously, Pparγ, fatty acid synthase (Fasn), and acetyl-CoA carboxylase (Acc) were all increased in the JAK2L livers as compared with CON mice (Figure 3C), but the increased expression of these genes was not affected by the loss of Cd36 in DKO livers. Additionally, Elo5, Elo6, and stearyl-CoA desaturase 1 (Scd1) (genes involved in processing newly synthesized palmitate, C16:0) were also increased in both JAK2L and DKO mice compared with CON, suggesting that hepatocyte-specific deletion of Cd36 did not decrease the rate of FA synthesis in JAK2L livers. Of these genes, only Scd1 (desaturates 16:0 to 16:1 and 18:0 to 18:1) was reduced significantly in DKO compared with the JAK2L liver.
Lastly, we analyzed genes involved in FA oxidation and found that expression of Acox-1, acyl-CoA dehydrogenase (Acad), Ehhadh, and Cpt1α, all known to regulate β-oxidation, were increased in both JAK2L and DKO mice as compared with CON mice (Figure 3D). Notably, Acox1 (the first enzyme of FA oxidation) and Acad-M (necessary for the oxidation of medium chain FAs) were significantly elevated in DKO vs JAK2L mice.
Hepatocyte-specific disruption of Cd36 (CD36L) leads to reduced liver triglyceride on HFD
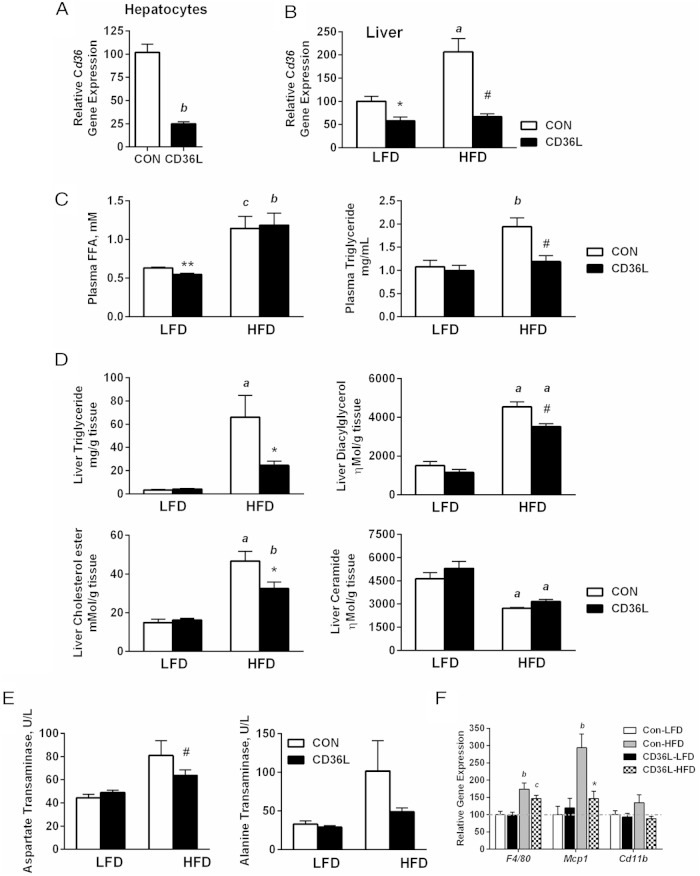
Hepatocyte-specific deletion of Cd36 in JAK2L mice demonstrated the importance of CD36 as a modulator and not just a marker of hepatic steatosis in a very specific genetic model. However, the extreme elevation in circulating GH levels in JAK2L mice make understanding the precise nature of the underlying mechanisms difficult, especially due to FA flux resulting from increased adipose lipolysis. We demonstrated that Cd36 expression correlated positively with liver fat, but prior results from others had shown that global Cd36 deletion (Cd36−/− mice) led to increased liver lipid content (7, 20, 27). Therefore, to determine what, if any, role CD36 played in normal liver lipid metabolism, we crossed the floxed Cd36 mice to mice carrying the albumin promoter-driven Cre recombinase to generate hepatocyte-specific Cd36 single knockout mice (CD36L). Cd36 expression was reduced 4-fold in CD36L hepatocytes derived from normal chow-fed mice (Cd36 F/F, AlbCre +/−) as compared with CON hepatocytes (Cd36 F/F, AlbCre −/) (Figure 4A). Cd36 expression in normal hepatocytes is low, and we reasoned that because CD36 is not the primary fatty acid transporter in the liver but is elevated with a HFD, and in NAFLD perhaps it is important in the liver under conditions with increased circulating FFA levels.
Figure 4.
Liver triglycerides are reduced in hepatocyte-specific Cd36 knockout mice on HFD. A–F, Hepatocyte-specific CD36L and CON mice were fed a LFD (13% fat) until 6 weeks of age and then either maintained on a LFD or switched to HFD (60% fat) for 6 more weeks prior to metabolic testing and tissue collection. A, Cd36 gene expression in primary hepatocytes isolated from mice on normal chow diet. B, Cd36 gene expression from whole liver of mice on LFD and HFD. C, Plasma FFA and TG levels of ad libitum-fed mice. D, Total hepatic TG, DAG, CE, and ceramide content. E, Serum liver damage markers AST and ALT levels (n = 6–8/group). F, Markers of macrophage infiltration, F4/80, Mcp1, and Cd11b, in the liver (n = 5–8/group). All data are shown as mean ± SEM. *, P < .05, #, P < .01 &, P < .001 compared with CON on same diet; c, P < .05, b, P < .01, a, P < .001 compared with same genotype.
To test this hypothesis, we placed mice on either a 13% fat diet (LFD) or a 60% fat diet (HFD) for 6 weeks. On the LFD, Cd36 gene expression in whole liver was 40% lower in CD36L mice as compared with CON mice (Figure 4B). The discrepancy in Cd36 expression between hepatocytes and whole liver may be explained by the expression of Cd36 in nonhepatocyte liver cell types such as Kupffer cells (28). In agreement with other data, HFD increased Cd36 expression 2-fold in CON but not in CD36L mice (Figure 4B [16]). The HFD also significantly increased circulating FFA and TG levels in CON-HFD mice (Figure 4C and Table 2), but whereas FFA levels were also increased in CD36L-HFD mice, plasma TG levels remained surprisingly low. Next, we analyzed the liver lipid content and found that on the LFD, there was no difference in liver TG content between CON and CD36L mice (Figure 4D, top left panel). The HFD induced a 20-fold increase in liver TG content in CON mice, but TG content was only 8-fold higher in CD36L-HFD mice as compared with CD36L-LFD mice. As compared with CON-HFD mice, TG content was 63% lower in CD36L-HFD mice. We also measured muscle and cardiac TG content and detected lower levels in CD36L mice on both diets, but the difference was only statistically significant on the HFD (Supplemental Figure 5, A and B). Liver DAG accumulation was reduced by 22% in CD36L vs CON mice on both the LFD and HFD diets, although the HFD diet increased DAG content as compared with the LFD-fed mice (Figure 4D, top right panel). There was no difference in liver CE content between CON and CD36L mice on the LFD but CE levels were elevated in CON-HFD mice vs CD36L-HFD. Ceramide content was not affected by genotype, but the HFD significantly reduced hepatic ceramide content in CON-HFD and CD36L-HFD mice. These data along with the ceramide content of JAK2L and DKO mice suggested that ceramide content is regulated by high FA flux.
Table 2.
Basic Metabolic Parameters in CON and CD36L Mice on LFD and HFD
| CON-LFD | CD36L-LFD | CON-HFD | CD36L-HFD | |
|---|---|---|---|---|
| Body weight, g | 27.01 ± 1.23 | 30.03 ± 1.71 | 37.63 ± 1.61a | 35.66 ± 1.32b |
| Liver weight, g | 1.45 ± 0.129 | 1.58 ± 0.12 | 1.99 ± 0.29 | 1.80 ± 0.10 |
| Liver weight, % | 5.35 ± 0.34 | 5.24 ± 0.21 | 5.17 ± 0.68 | 4.99 ± 0.20 |
| Body fat, % | 12.63 ± 0.72 | 10.83 ± 0.60 | 32.50 ± 2.10a | 29.05 ± 1.94a |
| Lean mass, % | 86.29 ± 0.68 | 87.56 ± 0.43 | 66.80 ± 1.99a | 70.22 ± 1.80a |
| Plasma FFAs, mM | 0.63 ± 0.01 | 0.55 ± 0.02c | 1.14 ± 0.16b | 1.19 ± 0.15d |
| Plasma TG, mg/mL | 1.08 ± 0.14 | 1.00 ± 0.11 | 1.94 ± 0.19d | 1.19 ± 0.13e |
| Glucose, mg/dL | ||||
| Ad libitum fed | 127.6 ± 7.9 | 116.3 ± 2.6 | 151.7 ± 13.1 | 133.0 ± 8.2 |
| Fasted | 59.9 ± 3.7 | 60.5 ± 3.6 | 87.3 ± 11.6 | 82.0 ± 10.3 |
| Insulin, ng/mL | ||||
| Ad libitum fed | 0.98 ± 0.27 | 0.88 ± 0.18 | 7.48 ± 2.12a | 4.42 ± 1.41 |
| Fasted | 0.46 ± 0.08 | 0.27 ± 0.06 | 0.49 ± 0.11 | 0.37 ± 0.10 |
| Liver glycogen, mg/g | 66.44 ± 4.90 | 38.48 ± 2.60f | 52.80 ± 2.18b | 66.81 ± 3.71a,e |
| Bilirubin, mg/dL | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 |
Data are shown as mean ± SEM.
P < .001 HFD compared with CON-LFD.
P < .05 HFD compared with CON-LFD.
P = .005 via t test.
P < .01 HFD compared with CON-LFD.
P < .01 compared with CON on same diet.
P < .001 compared with CON on same diet.
Finally, HFD feeding increased serum AST and ALT levels in CON-HFD mice, but the increase in these inflammatory markers was lower in CD36L-HFD mice (Figure 4E). Additionally, there was a reduction in the expression of macrophage markers (F4/80, Mcp1, and Cd11b) in CD36L-HFD liver as compared with CON-HFD (Figure 4F), demonstrating that loss of Cd36 also protects from inflammation associated with hepatic steatosis.
Fasting acutely increase circulating FA levels (29). To determine whether CD36 regulates liver TG content during fasting, mice were fasted for 24 hours or fed ad libitum. Fasting increased plasma FFAs 1.8-fold in CON mice compared with fed mice (Supplemental Figure 6A). Fed FFA levels were slightly elevated in CD36L mice, but fasted levels were similar to CON. Plasma TG levels were also increased in fasted mice (Supplemental Figure 6B). There was no difference in liver TG levels between CON and CD36L mice in the fasted or fed state (Supplemental Figure 6C). During a shorter fast (9 h), when FFA levels are thought to peak (29), we observed lower liver TGs in CD36L mice compared with CON, although the difference was not statistically significant (Supplemental Figure 6D). From these data we conclude that CD36 is crucial for regulating liver lipid content under conditions of high FFA flux. Furthermore, increased Cd36 expression in response to HFD may be a mechanism used by the liver to handle increased chronic FA load.
Deletion of Cd36 from liver alters content of unsaturated fatty acids
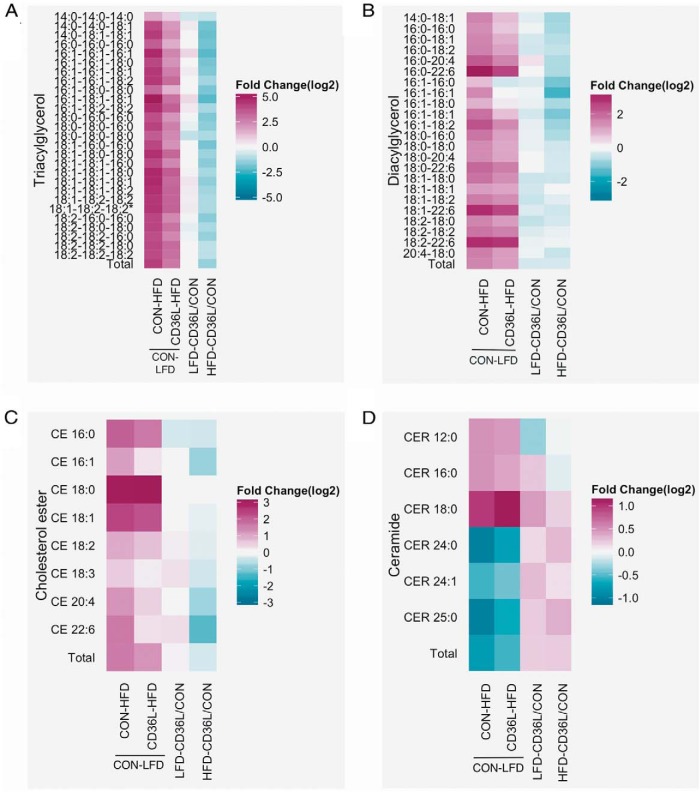
We then examined the specific composition of lipid species in CON and CD36L livers. Similar to JAK2L and DKO mice, higher plasma FFA levels in HFD fed mice lead to increased TG levels in both CON-HFD and CD36L-HFD mice compared with CON-LFD mice (Figure 5A and Supplemental Table 3). Significant differences were seen in TGs composed of unsaturated FAs. On the LFD, although there was no difference in total liver TG content between CD36L and CON mice, the lipidomics analysis revealed that some TG species were increased whereas others were decreased (Figure 5A). TG-16:1–16:1–16:1 and TG-16:1–18:1–18:1 were elevated 1.7-fold, whereas TG-18:0–18:0–18:0 was decreased 1.8-fold in CD36L-LFD as compared with CON-LFD (Figure 5A, column 3). On the HFD, loss of Cd36 significantly reduced all TG species in CD36L-HFD mice compared with the CON-HFD mice (Figure 5A, column 4). TG-16:1–16:1–16:1 and TG-16:1–18:1–18:1 were almost 5-fold lower in CD36L-HFD compared with CON-LFD.
Figure 5.
Lipidomics analysis showing the FA composition of various liver lipids in CON and CD36L mice A–D, Mass spectrometry analysis of liver lipids from CON and CD36L mice on LFD and HFD. The heat maps show the fold difference (log 2 transformed) between CON-HFD and CD3L-HFD relative to CON-LFD (columns 1 and 2), CD36L-LFD relative to CON-LFD (column 3), and CD36L-HFD relative to CON-HFD (column 4) for each lipid species for triacylglycerols (A), DAGs (B), CEs (C), and (D) ceramides (CER) (n = 7–8/group, data shown as mean ± SEM). *, P < .05, #, P < .01, &, P < .001 compared with CON on same diet; c, P < .05, b, P < .01, a, P < .001 compared with same genotype.
Unlike TGs, almost all DAG species were lower in CD36L-LFD mice as compared with CON-LFD. On average, DAGs containing C18:1 and the essential FA C18:2 were 25%–35% less abundant in CD36L-LFD livers (Figure 5B). HFD feeding also increased all DAG species in both genotypes, but individual DAGs were lower in CD36L-HFD livers compared with CON-HFD. Two DAGs, DAG-16:1–16:0 and DAG-16:1–16:1, were also modestly reduced in CD36L-HFD mice as compared with CON-LFD mice. On HFD, CE-18:0 was increased 9-fold in both CON-HFD and CD36L-HFD mice compared with the LFD mice. All other CEs were reduced in CD36L-HFD vs CON-HFD mice (CE-22:6 2.6-fold and CE-16:1 1.8-fold).
Lastly, there was no significant effect of genotype on ceramide FA composition. As seen with JAK2L and DKO mice, elevated FAs increased the amount of CER-18:0 and decreased CER-24:0 in the CON-HFD and CD36L-HFD mice compared with the LFD fed mice (Figure 5D). Ceramide conjugates glucosylceramide, lactosylceramide, and ceramide-1-phosphate were not affected by HFD feeding (Supplemental Figure 7, A–C). Phosphatidylcholine and sphingomyelin were also unaffected by genotype but were also reduced in mice on the HFD (Supplemental Figure 7, D and E).
FA uptake and desaturation is decreased in CD36L mice on HFD
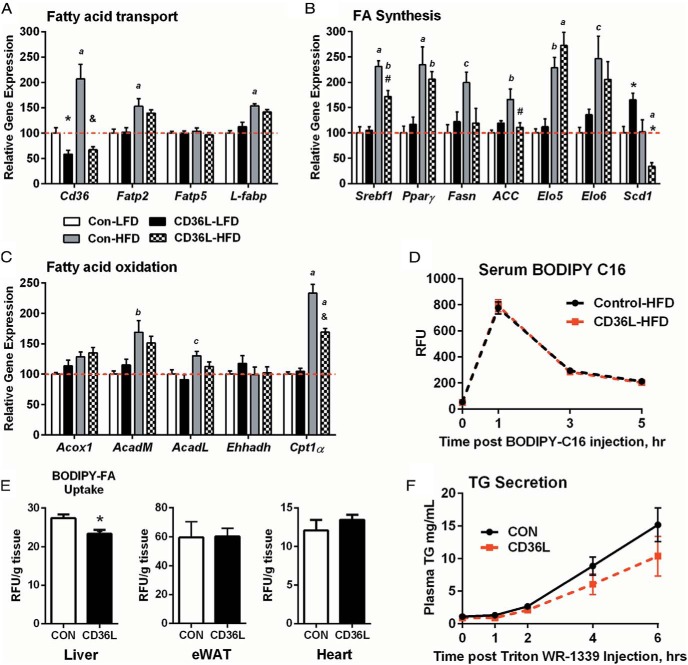
Next, we measured the expression of genes involved in lipid metabolism in the liver. There were no changes in the expression of FA transport genes (Figure 6A). However, HFD feeding did increase the expression of Fatp2 and L-Fabp, supporting our hypothesis that Fatp2 expression in JAK2L and DKO mice increased in response to elevated circulating FA. HFD also increased the expression of genes involved in de novo FA synthesis but Srebp1c, Fasn, Acc, and Scd1 expressions were lower in CD36L-HFD mice as compared with CON-HFD mice. Interestingly, Scd1 was significantly elevated in CD36L-LFD mice, consistent with the observation that some TG species comprising monounsaturated fatty acids were elevated in CD36L-LFD mice as compared with CON-LFD mice (Figure 5A). The difference in Scd1 expression between CD36L-LFD and CD36L-HFD mice suggested that the effect of CD36 on regulating Scd1 (and in turn lipid FA composition) is dependent on the composition and availability of FAs in the diet. Except for AcadM and Cpt1α, β-oxidation genes did not show any appreciable difference between groups (Figure 6C).
Figure 6.
Hepatic BODIPY-FA-C16 uptake is impaired in CD36L mice on a HFD. A–C, Hepatic gene expression patterns for FA transport (A), FA synthesis (B), and β-oxidation genes (C) normalized to CON-LFD expression (n = 5–9/group). *, P < .05, #, P < .01, &, P < .001 compared with CON on same diet; c, P < .05, b, P < .01, a, P < .001 compared with same genotype. D and E, In vivo BODIPY-FA-C16 uptake was performed in CON and CD36L mice on HFD for 6 weeks. D, Serum BODIPY-FA-C16 fluorescence at indicated time points. E, Liver, epididymal WAT (eWAT) and cardiac tissue BODIPY accumulation normalized to tissue weight (n = 6–7 per group). **, P < .01 compared with CON. F, TG secretion assay was performed in CON and CD36L mice on a 22% fat diet after a 4-hour fast (n = 10 per group). All data are shown as mean ± SEM.
To directly assess the effect of hepatic Cd36 deletion on FA uptake, in vivo, we measured accumulation of BODIPY-FL-C16 after bolus ip injection. We used HFD fed mice because baseline expression of Cd36 is very low on LFD. Figure 6D shows that the rate of BODIPY-C16 appearance and removal from circulation was the same in CON and CD36L mice. Uptake of BODIPY-FL-C16 into liver was reduced 15% in CD36L-HFD mice as compared with CON-HFD (Figure 6E). Although modest, this reduction in FA uptake would lead to significant differences in liver lipid content over time. As expected, there was no difference in adipose or cardiac tissue FA uptake. Therefore, we concluded that CD36 directly modulated hepatic FA uptake and ultimately neutral lipid content in response to high FA load. We also measured very low-density lipoprotein (VLDL)-TG secretion. Because the TG secretion rate is linked to liver TG content (30), we used mice on the normal chow diet to separate any effect of Cd36 deletion from differences in hepatic TG content between CON-HFD and CD36L-HFD mice. We found lower TG secretion in the CD36L mice as compared with CON (Figure 6F). Lower VLDL-TG secretion would, if anything, be expected to increase liver lipid content and may explain why liver TG levels were not decreased in the CD36L-LFD mice compared with the CON-LFD mice.
CD36L mice have improved insulin sensitivity on LFD and HFD
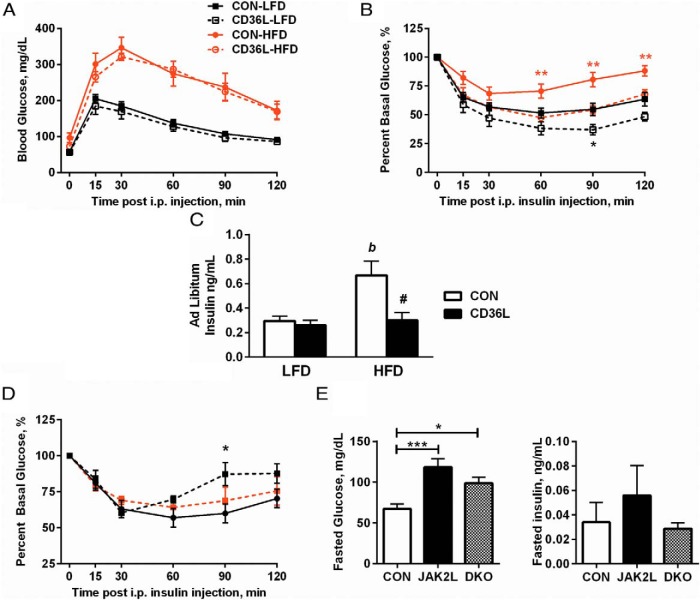
Hepatic steatosis promotes hepatic insulin resistance, but even modest changes in liver lipid content can have profound effects on whole-body glucose metabolism (31, 32). Despite high circulating TG and FFA levels and higher liver TG content, global Cd36 deletion mice (Cd36−/− mice) have improved insulin sensitivity (20, 27). Therefore, we sought to determine whether there was any effect of hepatic Cd36 deletion on glucose and insulin homeostasis. Fed and fasted glucose levels were higher in HFD fed mice vs LFD but were unchanged between CON and CD36L mice (Table 2). Next, we performed GTTs and observed no differences between genotypes, but glucose tolerance was severely impaired in HFD-fed mice (Figure 7A). Whole-body insulin sensitivity was then assessed by an ITT. Despite similar liver lipid content, there was improved insulin sensitivity in CD36L-LFD mice vs CON-LFD mice (Figure 7B). This suggested that hepatic CD36 can regulate insulin sensitivity independent of liver lipid content. As expected, HFD worsened insulin sensitivity in both groups, but whereas insulin sensitivity was markedly diminished in CON-HFD mice, CD36L-HFD mice were as insulin sensitive as CON-LFD mice. Remarkably, there was no increase in ad libitum insulin levels in CD36L-HFD mice, indicating protection from diet-induced hyperinsulinemia (Figure 7C). Next, we determined whether Cd36 deletion from JAK2L livers would also improve insulin sensitivity. Increased GH secretion on the JAK2L background would be expected to promote insulin resistance via the known antiinsulin effects of GH (33, 34). Insulin tolerance tests demonstrated worsened insulin sensitivity in JAK2L mice compared with CON, although the difference in percent basal glucose was statistically significant only at the 90-minute time point (Figure 7D). No significant effect on insulin sensitivity was observed for the DKO mice. Fasting glucose and insulin levels (Figure 7E) were elevated in JAK2L mice compared with CON. Fasting glucose levels were not different in DKO vs JAK2L mice, but fasting insulin levels were as low as in CON mice.
Figure 7.
Insulin sensitivity is improved in CD36L mice on LFD and HFD. A and B, GTT and ITT in 12-week-old mice after 6 weeks a LFD or HFD (n = 6–8 per group), *, P < .05, **, P < .01 compared with CON on same diet. C, Ad libitum insulin levels in CON and CD36L mice on day of tissue collection (n = 8 per group for LFD mice and n = 5 per group for HFD mice). #, P < .01 compared with CON on same diet; b, P < .01 compared with same genotype. D and E, Metabolic parameters in CON, JAK2L, and DKO mice. D, ITT (n = 5–10/group) in 9-week-old mice. E, Glucose and insulin levels after 16-hour fast (n = 5–11 per group). *, P < .05; ***, P < .001. All data are shown as mean ± SEM.
Together these data demonstrated that disruption of CD36 in the liver not only reduced liver fat content but also improved associated conditions such as inflammation and insulin resistance.
Discussion
The incidence of NAFLD continues to increase with significant consequences to human health including insulin resistance and advanced liver disease. Hepatic steatosis, HFD feeding, and NAFLD are associated with increased hepatic Cd36 expression, although Cd36 is not expressed at high levels in the normal liver. We addressed the role of CD36 in regulating liver lipid content by specifically deleting hepatocyte Cd36 in two steatosis models: 1) a genetic (JAK2L) and 2) a nutritional (HFD feeding) model. We confirmed that HFD increases hepatic Cd36 expression and have also observed a suppressive effect of GH signaling on Cd36 that was specific to the liver (data not shown). In both steatosis models, elevated Cd36 expression was associated with profound liver lipid accumulation, but we conclusively showed that hepatocyte-specific Cd36 disruption significantly reduced liver TG, DAG, and CE content. We also showed for the first time that CD36 significantly altered the FA composition of liver lipids, with high Cd36 expression promoting the accumulation of unsaturated FA. Furthermore, CD36 deletion from hepatocytes impaired FA uptake into the liver, conferred protection from steatosis-induced inflammation, and improved whole-body insulin sensitivity.
Previous studies have shown that in mice with global Cd36 deletion (CD36 null, Cd36−/− mice), liver TG was higher in fasted mice but unchanged in ad libitum-fed mice (11, 19, 20, 27). Severely impaired FA uptake into muscle, heart, and adipose tissue of Cd36−/− mice led to significant elevations in plasma FFAs and TGs (35). Increased FFA flux into the liver via intact hepatic FA transporters (Fatp2 and Fatp5) is expected to occur despite the loss of whatever FA uptake capacity is mediated by CD36 protein. Therefore, higher liver TG in Cd36−/− mice appears to be secondary to impaired FA uptake in peripheral tissue. Importantly, in our models, plasma FFAs were significantly increased without defects in peripheral FA uptake. Furthermore, we observed elevations in hepatic Fatp2, Fatp5, and L-Fabp expression that could compensate for the loss of Cd36. Despite this, hepatic Cd36 deletion dramatically reduced liver fat content. Indeed, the fact that FFA flux to the liver remained high in our models likely explains why steatosis was not entirely rescued in DKO and CD36L-HFD mice.
The proposed mechanisms by which CD36 regulates liver fat are varied and appear to be diet dependent. Cd36−/− mice have 2-fold higher liver TG when fed a high-fructose/low-fat chow diet in which increased lipogenesis drives steatosis but no change in liver TG when fed a HFD (20, 36). Additionally, reduced VLDL-TG secretion was proposed to compound the susceptibility of Cd36−/− to high-carbohydrate/prolipogenic steatosis (36). On the other hand, Cd36−/− mice on a high-carbohydrate liquid diet or alcohol diet had reduced TG accumulation due to a down-regulation of de novo lipogenesis (DNL) (37). In our hepatocyte-specific deletion model, the LFD, although not a high-carbohydrate lipogenic diet, does provide 62% of calories from carbohydrates. It is unclear whether the susceptibility of Cd36−/− mice to carbohydrate-induced steatosis is autonomous to the liver. Nevertheless, this susceptibility, coupled with decreased in VLDL-TG secretion, may explain why we did not see a decrease in liver TG in CD36L-LFD vs CON-LFD mice.
We previously measured hepatic DNL in JAK2L mice using stable isotopes. Despite a marked up-regulation of FA synthesis genes in JAK2L livers, the rate of lipogenesis was unchanged when compared with CON mice (7). With the exception of Scd1, there was no difference in the expression of genes regulating lipogenesis between JAK2L and DKO mice. Therefore, any subtle change in lipogenesis would be unlikely to account for the 60% reduction in liver TG between DKO and JAK2L mice. For CON and CD36L mice, the HFD increased the expression of FA synthesis genes in CON mice, but several of these genes (Srebp1c, Fasn, Acc, and Scd1) were reduced in CD36L-HFD mice. Whether these changes in gene expression could have contributed to the 63% reduction in liver TG content in CD36L-HFD mice is unclear. Nonetheless, both DKO and CD36L data suggest that Scd1 expression, at least, is regulated by CD36 function. Careful measurement of liver DNL under varying diet conditions is necessary to identify the specific contribution of CD36 to DNL. In the JAK2L and HFD models, elevated FFAs are a big driver of the steatosis, and our data support the observation that reducing Cd36 expression when FFAs are high improves liver fat (38). Therefore, we propose that the primary mechanism leading to reduced liver lipids in DKO and CD36L-HFD mice is impaired hepatic FA uptake due to an inability to up-regulate Cd36 expression.
An outstanding question is what happens to the FAs not taken up by CD36-deficient livers? Body weights do not suggest that DKO and CD36L-HFD mice are eating less food and there are no obvious increases in fat content in other tissues. Experiments to determine whether DKO and CD36L are metabolizing or excreting more fat than their respective controls should be undertaken in the future to address this question.
NAFLD is highly associated with insulin resistance, and hepatic CD36 is higher in NAFLD and nonalcoholic steatohepatitis patients with insulin resistance than those without (13). Cd36−/− mice have improved whole-body and muscle insulin sensitivity but worsened hepatic insulin sensitivity. Improved muscle glucose use coupled with increased FFA flux to the liver has been proposed as the underlying mechanism (20, 27). We found that hepatocyte-specific Cd36 deletion improved whole-body insulin sensitivity in both CD36L-LFD and CD36L-HFD mice without affecting glucose levels or tolerance. Without hyperinsulinemic-eugylcemic clamp data, we can only speculate as to which tissues mediate these insulin-sensitizing effects, but the likeliest explanation is that increased whole-body insulin sensitivity is primarily due to improved hepatic insulin sensitivity. Steneberg et al (39) recently demonstrated that increased Cd36 expression induced by hypersinsulinemia induces hepatic insulin resistance. For CD36L-HFD mice, the improvement in liver fat, changes in liver FA composition, and reduced muscle TG could also contribute to improved insulin sensitivity of these mice. However, the JAK2L and DKO ITT data suggest that Cd36 deletion may be insufficient to promote whole-body insulin sensitivity, depending on the underlying cause of insulin resistance (significantly elevated GH levels in the case of the JAK2L and DKO mice).
For CD36L-LFD mice, improved insulin response may be independent of hepatic lipid content because there was no significant difference in total liver TG, CE, or ceramide content compared with CON-LFD mice, though there were reductions in specific species of these lipids. Only DAG was similarly reduced in CD36L mice on both diets (23% lower compared with CON). This modest but consistent reduction should be investigated in the future because the role of DAG in inhibiting insulin signaling by activating protein kinase C-ϵ has been well established (40, 41).
Collectively these data demonstrate that CD36 is an important regulator of hepatic FA uptake under conditions of elevated FA. Hepatic Cd36 expression was highly regulated and impacted the severity of hepatic steatosis as well as improved whole-body insulin sensitivity. This work resolves a long-standing area of confusion over the role of CD36 in liver lipid metabolism and firmly demonstrates that CD36 is important functionally and is not just a marker of disease. Finally, this points toward the possibility of modulating the rate of hepatic FA uptake as a means of treating NAFLD or the associated metabolic diseases.
Acknowledgments
We thank Jennifer Bolen (University of California, San Francisco, Mouse Pathology core) for the preparation and staining of the liver slides, Lauren Sites for technical assistance in performing the mass spectrometric lipid analysis, and Dr Rahul Deo for assistance in analyzing the lipidomics data. We also thank Dr Kamran Atabai, Amin Khalifeh-Soltani, and Stephen Sakuma for assistance with the in vivo BODIPY-FA uptake assay.
This work was supported by National Institutes of Health Grant 1R01DK091276 (to E.J.W.) and American Heart Association postdoctoral fellowship Grant 12POST11910057 (to C.G.W.). We also acknowledge the support of the University of California, San Francisco (UCSF), Cardiovascular Research Institute, the UCSF Diabetes Center (Grant P30 DK063720), and the UCSF Liver Center (Grant P30 DK026743).
Disclosure Summary: D.M.E. and N.B.V. are employed at and receive compensation from Pfizer. The other authors have nothing to disclose.
Footnotes
- Acad
- acyl-CoA dehydrogenase
- Acc
- acetyl-CoA carboxylase
- ALT
- alanine transaminase
- AST
- aspartate aminotransferase
- BODIPY
- 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- Cd11b
- cluster of differentiation molecule 11b
- CD36/FAT
- fatty acid translocase
- CD36L
- hepatocyte-specific deletion of CD36
- CE
- cholesterol ester
- CON
- control mice
- DAG
- diacylglycerol
- DKO
- double-knockout mice
- DNL
- de novo lipogenesis
- FA
- fatty acid
- Fasn
- fatty acid synthase
- FATP
- fatty acid transport protein
- FFA
- free fatty acid
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- ITT
- insulin tolerance test
- Jak2
- Janus kinase 2
- JAK2L
- hepatocyte-specific deletion of JAK2
- LFD
- low-fat diet
- Mcp1
- monocyte chemoattractant protein-1
- NAFLD
- nonalcoholic fatty liver disease
- Scd1
- stearyl-CoA desaturase 1
- TG
- triglyceride
- UCSF
- University of California, San Francisco
- UPLC
- ultraperformance liquid chromatography
- VLDL
- very low-density lipoprotein.
References
- 1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. [DOI] [PubMed] [Google Scholar]
- 2. Doege H, Baillie RA, Ortegon AM, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130(4):1245–1258. [DOI] [PubMed] [Google Scholar]
- 3. Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39(4):909–914. [DOI] [PubMed] [Google Scholar]
- 4. Ichikawa T, Hamasaki K, Ishikawa H, Ejima E, Eguchi K, Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52(6):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Langendonk JG, Meinders AE, Burggraaf J, et al. Influence of obesity and body fat distribution on growth hormone kinetics in humans. Am J Physiol. 1999;277(5 Pt 1):E824–E829. [DOI] [PubMed] [Google Scholar]
- 6. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937–19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sos BC, Harris C, Nordstrom SM, et al. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest. 2011;121(4):1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui Y, Hosui A, Sun R, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46(2):504–513. [DOI] [PubMed] [Google Scholar]
- 9. Endemann G, Stanton LW, Madden KS, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268(16):11811–11816. [PubMed] [Google Scholar]
- 10. Febbraio M, Guy E, Coburn C, Knapp FF, Jr, Beets AL, Abumrad NA, et al. The impact of overexpression and deficiency of fatty acid translocase (FAT)/CD36. Mol Cell Biochem. 2002;239(1–2):193–197. [PubMed] [Google Scholar]
- 11. Ibrahimi A, Bonen A, Blinn WD, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274(38):26761–26766. [DOI] [PubMed] [Google Scholar]
- 12. Bechmann LP, Gieseler RK, Sowa JP, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30(6):850–859. [DOI] [PubMed] [Google Scholar]
- 13. Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. [DOI] [PubMed] [Google Scholar]
- 14. Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1281–G1287. [DOI] [PubMed] [Google Scholar]
- 15. Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20(2):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koonen DP, Jacobs RL, Febbraio M, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. [DOI] [PubMed] [Google Scholar]
- 17. Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPAR[gamma] in promoting steatosis. Gastroenterology. 2008;134(2):556–567.e551. [DOI] [PubMed] [Google Scholar]
- 18. Hui ST, Parks BW, Org E, et al. The genetic architecture of NAFLD among inbred strains of mice. Elife. 2015;4:e05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–32529. [DOI] [PubMed] [Google Scholar]
- 20. Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109(10):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson CG, Schupp M, Burkhardt BR, Wu J, Young RA, Wolf BA. Liver-specific overexpression of pancreatic-derived factor (PANDER) induces fasting hyperglycemia in mice. Endocrinology. 2010;151(11):5174–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δδC[T]) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 23. Khalifeh-Soltani A, McKleroy W, Sakuma S, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med. 2014;20(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagendran J, Pulinilkunnil T, Kienesberger PC, et al. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J Mol Cell Cardiol. 2013;63:180–188. [DOI] [PubMed] [Google Scholar]
- 25. Coburn C, Hajri T, Ibrahimi A, Abumrad N. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J Mol Neurosci. 2001;16(2):117–121. [DOI] [PubMed] [Google Scholar]
- 26. Krammer J, Digel M, Ehehalt F, Stremmel W, Füllekrug J, Ehehalt R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int J Med Sci. 2011;8(7):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goudriaan JR, Dahlmans VE, Teusink B, et al. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res. 2003;44(12):2270–2277. [DOI] [PubMed] [Google Scholar]
- 28. Bieghs V, Wouters K, van Gorp PJ, et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138(7):2477–2486, 2486.e2471–e2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steyn FJ, Leong JW, Huang L, et al. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology. 2012;153(1):273–282. [DOI] [PubMed] [Google Scholar]
- 30. Newberry EP, Xie Y, Kennedy S, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278(51):51664–51672. [DOI] [PubMed] [Google Scholar]
- 31. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Savage DB, Choi CS, Samuel VT, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116(3):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dominici FP, Cifone D, Bartke A, Turyn D. Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J Endocrinol. 1999;161(3):383–392. [DOI] [PubMed] [Google Scholar]
- 34. Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274(27):19055–19062. [DOI] [PubMed] [Google Scholar]
- 36. Nassir F, Adewole OL, Brunt EM, Abumrad NA. CD36 deletion reduces VLDL secretion, modulates liver prostaglandins, and exacerbates hepatic steatosis in ob/ob mice. J Lipid Res. 2013;54(11):2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clugston RD, Yuen JJ, Hu Y, et al. CD36-deficient mice are resistant to alcohol- and high-carbohydrate-induced hepatic steatosis. J Lipid Res. 2014;55(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ma Y, Huang Y, Yan L, Gao M, Liu D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm Res. 2013;30(5):1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Söderström I, Edlund H. Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem. 2015;290(31):19034–19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31):32345–32353. [DOI] [PubMed] [Google Scholar]
- 41. Samuel VT, Liu ZX, Wang A, et al. Inhibition of protein kinase Cϵ prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]