Abstract
A major challenge in assisted reproductive technology is to develop conditions for in vitro oocyte maturation yielding high-quality eggs. Efforts are underway to assess whether known hormonal and local factors play a role in oocyte developmental competence and to identify the molecular mechanism involved. Here we have tested the hypothesis that FSH improves oocyte developmental competence by regulating the translational program in the oocyte. Accumulation of oocyte proteins (targeting protein for the Xenopus kinesin xklp2 and IL-7) associated with improved oocyte quality is increased when cumulus-oocyte complexes are incubated with FSH. This increase is due to enhanced translation of the corresponding mRNAs, as indicated by microinjection of constructs in which the 3′ untranslated region of the Tpx2 or Il7 transcripts is fused to the luciferase reporter. A transient activation of the phosphatidyl-inositol 3-phosphate/AKT cascade in the oocyte preceded the increase in translation. When the epidermal growth factor (EGF) receptor is down-regulated in follicular cells, the FSH-induced rate of maternal mRNA translation and AKT activation were lost, demonstrating that the effects of FSH are indirect and require EGF receptor signaling in the somatic compartment. Using Ptenfl/fl:Zp3cre oocytes in which the AKT is constitutively activated, translation of reporters was increased and was no longer sensitive to FSH stimulation. More importantly, the oocytes lacking the phosphate and tensin homolog gene showed increased developmental competence, even when cultured in the absence of FSH or growth factors. Thus, we demonstrate that FSH intersects with the follicular EGF network to activate the phosphatidyl-inositol 3-phosphate/AKT cascade in the oocyte to control translation and developmental competence. These findings provide a molecular rationale for the use of FSH to improve egg quality.
After a period of quiescence that in humans last for decades, fully grown oocytes reenter the cell cycle just prior to ovulation and complete their maturation, yielding fertilizable eggs. Two distinct developmental processes are completed over a period of 12 hours in the mouse, or 36 hours in humans. These are often referred to as nuclear and cytoplasmic maturation. The ability to enter the cell cycle and correctly segregate chromosomes during the first meiotic division is termed meiotic competence, which, in mice, is established at the time of the follicle antrum formation (1–3). When meiotically competent oocytes are isolated from their follicles, they are able to reenter meiosis and reach metaphase II (MII) but usually fail to sustain embryo development (4). Additional metabolic and structural modifications are required to complete the oocyte differentiation program. These events take place during the periovulatory period and are essential to support embryo development, a property also defined as developmental competence (5).
In mammals, the acquisition of meiotic competence is followed by the establishment of a transcriptionally silent chromatin state (6–8). Therefore, in the last stages of oogenesis, gene expression is no longer regulated at the transcriptional level but relies on a well-orchestrated program of translation of stored maternal transcripts (as reviewed in references 9 and 10). Even though the molecular mechanisms responsible are still poorly understood, it is likely that developmental competence requires extensive translational regulations.
An unbiased survey of the transcripts recruited to the polysomes during oocyte maturation (from germinal vesicle [GV] to MII stage) indicated an enrichment in maternal mRNAs carrying well defined cis-acting elements on the 3′ untranslated region (UTR) (11). Through interactions with cognate RNA binding proteins, these elements regulate protein synthesis in the oocyte according to the temporal requirement of meiosis progression (11). The disruption of the regulatory circuits between cis elements and RNA binding proteins impairs the progression through meiosis I, underscoring the importance of the 3′ UTR in these regulations (11).
Further studies demonstrated that the translational program during oocyte maturation is not completely oocyte autonomous because it requires the presence of cumulus cells. Translation of a subset of transcripts is regulated by the activation of the follicular epidermal growth factor (EGF) network (12). Importantly the inactivation of this somatic-induced control of translation does not impair the ability of the oocyte to reach the MII stage (nuclear maturation) but compromises significantly their developmental competence (12). These findings indicate that the oocyte translational program during maturation consists of two components: a cell-autonomous component that controls cell cycle progression and a somatic-dependent component that is responsible, at least in part, for the developmental competence. Signals involved include the EGF-like growth factors amphiregulin (AREG) and epiregulin (EREG) secreted in the follicle in response to the LH surge (13). AREG, or EGF itself, promotes developmental competence when used during in vitro maturation (IVM) of the cumulus-enclosed oocytes (CEOs) in several mammalian species (14–17). A similar property has also been reported for FSH (17–23), opening the possibility that some of the FSH effects are mediated by EGF-like growth factors.
During the follicular phase of the ovarian cycle, FSH controls oocyte growth and follicle development (24–26) and induces the expression of LH and EGF receptors in mural granulosa cells (27–29). FSH concentration increases at the time of the midcycle LH surge (30–32), strongly indicating that FSH also functions during the periovulatory period. Although it is established that FSH improves egg quality, the mechanism of action is unknown. The aim of the present study was to test the hypothesis that FSH participates in the control of the oocyte translational program during the periovulatory period and that this regulation is responsible for the increase in the oocyte ability to sustain early embryo development.
Materials and Methods
Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco (protocol AN101432). Egfrδ/fl:Cyp19cre, Areg null, and Ptenfl/fl:Zp3cre were generated as previously described (36–38). Twenty-one to 24-day-old mice were injected ip with 5 IU pregnant mare serum gonadotropin (Calbiochem) to stimulate follicle development. After 45–47 hours, the animals were killed by cervical dislocation and the ovaries excised. Groups of mice were injected ip with an ovulatory dose of 5 IU human chorionic gonadotropin (hCG; Goldline Labs), and after 14 hours the CEOs were isolated from the ampullae.
Oocyte collection and culture
CEOs were isolated by puncturing the antral follicles and released in HEPES-buffered MEM medium supplemented with 3 mg/mL BSA, penicillin, streptomycin, and 2 μM milrinone. Only oocytes fully enclosed in three or more cumulus cells layers and with a light homogeneous cytoplasm were used. The basic culture medium was MEM-α (Gibco by Life Technologies) supplemented with pyruvate, penicillin, streptomycin, and 3 mg/mL BSA. The basic medium was supplemented with 2 μM milrinone, 100 nM AREG (R&D Systems Inc), 10 ng/mL ovine FSH (National Hormone and Peptide Program, Torrance, California) or not supplemented. CEOs were cultured for different times at 37°C in 5% CO2. Removal of cumulus cells was performed mechanically with a narrow borosilicate glass pipette or enzymatically with hyaluronidase.
Western blot
Oocytes and CEOs were extracted in Laemmli sample buffer (Bio-Rad Laboratories) supplemented with mercaptohetanol and a cocktail of phosphatase and protease inhibitors (Roche). The cellular extracts were boiled and separated on 8% polyacrylamide gel and transferred to a polyvinyl difluoride membrane. Targeting protein for the Xenopus kinesin xklp2 (TPX2), testis expressed gene 19.1 (TEX19.1), α-tubulin, phospho-AKT Ser473, total AKT, and total EGF receptor (EGFR) were immunolocalized by incubating the membrane with the respective antibodies (see Table 1).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Human TPX2 | A recombinant segment of the C-terminal domain of human TPX2 [UniProt number Q9ULW0] | TPX2 antibody | Novus Biological, NB500-179 | Rabbit whole antiserum | 1:500 |
| TEX19.1 | Detailed information on antibody preparation are given in Tarabay et al (57) | 7Tex-1F11 | Generous gift by Dr Stephan Viville | Mouse monoclonal | 1:500 |
| α-Tubulin | Monoclonal anti-α-tubulin antibody | Sigma, T6074 | Mouse monoclonal | 1:10 000 | |
| Phospho-AKT Ser473 | Synthetic phosphopeptide corresponding to residues around Ser473 of human Akt | Phospho-Akt (Ser473) (D9E) XP | Cell Signaling, 4060 | Rabbit monoclonal | 1:1000 |
| AKT | Synthetic peptide corresponding to residues in the carboxy-terminal sequence of mouse Akt | Akt (pan) (C67E7) rabbit mAb | Cell Signaling, 4691 | Rabbit polyclonal | 1:1000 |
| EGFR | Epitope mapping at the C-terminus of EGFR of human origin | EGFR Antibody (1005): sc-03 | Santa Cruz Biotechnology, 1005 | Rabbit monoclonal | 1:200 |
Abbreviation: mAb, monoclonal antibody.
Enzyme-linked immunosorbent assay
A commercially available ELISA kit was used to detect and quantify the level of IL-7 protein in the IVM spent media following the manufacturer's instructions (R&D Systems Inc). This assay has a sensitivity of 8.3 pg/mL. CEOs were cultured for 24 hours with or without FSH in groups of 30–50 at a density of 1 CEO/μL. The spent media were pulled to reach 100 μL. Serial dilutions 1:2 ranging from 500 pg/mL to 7.8125 pg/mL were used to produce a standard curve. The absorbance was read on a plate reader at 450 nm after blank subtraction. The intraassay variation was 2.8%–4.3%.
Luciferase assay
Renilla luciferase (RL) reporter plasmids were constructed from pRL-TK vector (Promega). Generation of the Tpx2, Il7 and Tex19.1 reporter were carried out as described previously (11). All reporters contain a T7 promoter enabling in vitro transcription to synthesize cRNAs (Ambion by Life Technologies). The Firefly luciferase (FL) cRNA was obtained from pcDNA3 plasmid and polyadenylated with a Poly(A) tailing kit (Ambion). CEOs were injected with reporter cRNA (12.5 ng/μl RL containing the indicated 3′ UTR) together with 12.5 ng/μL polyadenylated FL cRNA as a normalizing RNA. Injected CEOs were incubated for 3 hours in basic culture medium supplemented with 2 μM milrinone at 37°C 5% CO2 and then washed and cultured in the different culture treatments. After 16–17 hours, the CEOs were denuded, collected in lysis buffer, and snap frozen. Luciferase activities in the oocyte extracts were measured using a dual-luciferase reporter assay kit (Promega), and luminescence was detected with a SpectraMax L luminometer (Molecular Devices). Data are reported as ratios of RL and FL.
Real-time RT-PCR
Total RNA was extracted from denuded oocytes (RNeasy Micro plus; QIAGEN) and the cDNA prepared by random hexamers priming (Superscript III first strand synthesis system; Invitrogen by Life Technologies). Real-time PCR was performed on a Viaa 7 real-time PCR system (Applied Biosystems by Life Technologies) using cDNA equivalent to 0.8 oocytes as substrate, Kapa Sybr Fast Abi Prism mix (Kapa Biosystems), and 0.2 μM of each specific primer (Dppa3: forward, GACCCAATGAAGGACCCTGAA, reverse, GCTTGACACCGGGGTTTAG; Il7: forward, GCCTGTCACATCATCTGAG, reverse, AAAGTTTGGTTCATTATTCGGG; Rpl19: forward, CTGAAGGTCAAAGGGAATGTG, reverse, GGACAGAGTCTTGATGATCTC; Tpx2: forward, CATCAAAGATGAGGAAGAGGA, reverse, GGGTATCAAAGGAGAACGG). A three-step protocol (95°C for 30 sec, 60°C for 30 sec, 72°C for 20 sec) was repeated for 40 cycles, followed by acquisition of the melting curve. The expected amplicon sizes were as follows: 129 bp for Dppa3, 135 bp for Il7, 194 bp for Rpl19, and 129 bp for Tpx2. The data are expressed as 2−δδCT calculated as the difference between the cycle threshold (CT) of the gene of interest normalized for Dppa3 and Rpl19 (δCT treated) further normalized to GV (δCT untreated).
In vitro fertilization and embryo culture
The sperm were collected from the caudae epididymis and vasa deferentes of B6D2F1 adult males and capacitaded for 1–1.5 hours in Human Tubal Fluid medium (Millipore). Fifteen to 20 μL of sperm suspension were coincubated with CEOs for 5 hours at 37°C in 5% CO2, 5% O2, and 90% N2 in Human Tubal Fluid medium. At the end of the coincubation, the presumptive zygotes were mechanically stripped of the remaining cells and either cultured up to embryonic day (E) 5.0 in potassium supplemented optimized medium plus amino acids (Millipore) or fixed for 20 minutes in paraformaldehyde 2% and 0.2% Triton X-20, and mounted on slides in Vectashield containing 1 μg/mL 4,6-diamidino-2-phenylindole (Vector Laboratories Inc) or 20 mM TOPRO1 (Invitrogen by Life Technologies) to stain the DNA. Slides were analyzed on an inverted laser-scanning confocal microscope LSM5 Pascal (Zeiss) equipped with a UV lamp and a 633-nm laser. Only zygotes showing two pronuclei were considered fertilized.
Statistical analysis
Experiments were repeated at least three times. Data are expressed as the mean ± SEM. Normal distribution of the population was tested by the D'Agostino and Pearson omnibus normality test, the Shapiro-Wilk normality test, and the Kolmogorov-Smirnov normality test or only the Kolmogorov-Smirnov normality test when n = 5. When the population was normally distributed, statistical analysis was carried out using a one-way or two-way ANOVA (where appropriate), followed by a Tukey post hoc test for comparisons of multiple groups or a two-tailed paired or unpaired (where appropriate) t test for comparison of two groups. When the population was not normally distributed, statistical analysis was carried out using Kruskal-Wallis test followed by a Dunn's test for comparisons of multiple groups or Mann-Whitney test for comparison of two groups (*, P < .05; **, P < .01; ***, P < .001).
Results
FSH treatment of CEOs causes an increase in TPX2 and IL-7 protein accumulation and translation of their mRNA reporters in the oocyte
We have shown that oocyte quality is associated with translation of a subgroup of maternal mRNAs and corresponding changes in protein synthesis. If FSH effects on oocyte were also mediated by this mechanism, the levels of proteins associated with developmental competence should increase after exposure to this gonadotropin. To test this hypothesis, we investigated the accumulation of three oocyte proteins. TPX2 is a protein essential for spindle assembly and function (33), whereas IL-7 is a cytokine secreted by the oocyte and thought to be a survival signal for the cumulus cells (34). TEX19.1, expected not to change (12), was used as a control of the specificity of the FSH effect. CEOs were incubated with or without FSH and the accumulation of the three proteins in oocyte extracts or CEO spent media were measured. FSH induced a significant increase in TPX2 protein in the oocyte and IL-7 in the medium (Figure 1, A and B), without affecting the levels of total Tpx2 and Il7 mRNAs (Supplemental Figure 1A). Conversely, the protein levels of TEX19.1 in the oocyte were not affected by the FSH treatment (Figure 1A). These findings open the possibility that the FSH effects on oocyte quality are indeed mediated by increased translation of specific mRNAs.
Figure 1.
TPX2 and TEX19.1 accumulation and IL-7 secretion during oocyte maturation. A, CEOs were cultured for 17 hours in milrinone (GV) or in the absence (IVM) or presence of FSH (IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and extracts used for Western blot. Representative immunoblots of TPX2, TEX19.1, and α-tubulin (TUB) are reported. The bar graph represents the ratio TPX2 to α-tubulin or TEX19.1 to α-tubulin (mean ± SEM) in the different treatments (n ≥ 3). ***, Significant differences between IVM and IVM+FSH (paired t test, P < .001). B, CEOs were cultured for 24 hours in the absence (IVM) or presence of FSH (IVM+FSH). At the end of the incubation, spent media were collected and the IL7 content measured by an ELISA. The bar graph represents IL-7 concentration (mean ± SEM) in the two treatments (n ≥ 3). *, Significant differences (Mann-Whitney test, P < .05).
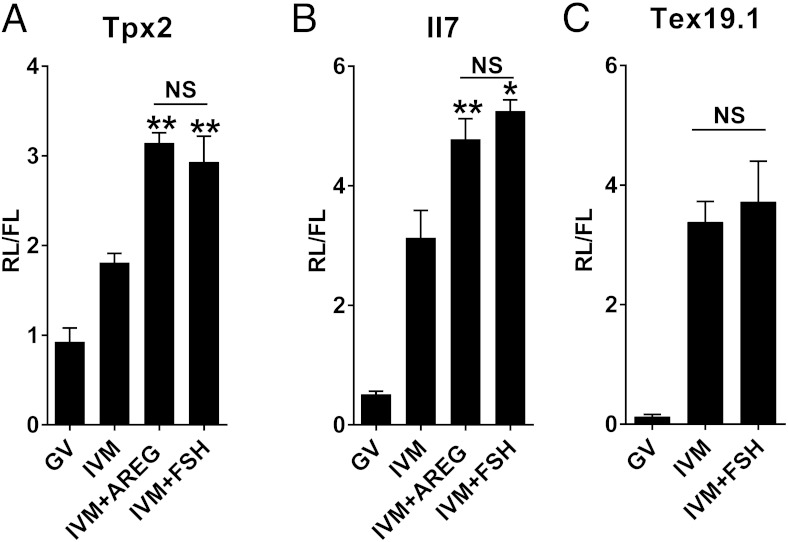
To investigate whether the FSH-dependent increase in protein is associated with an increased translation of the Tpx2 and Il7 mRNAs during oocyte maturation, reporters with the 3′ UTR of the two mRNA fused to luciferase were injected in oocytes still enclosed in cumulus cells. FSH treatment induced a significant increase in luciferase activity compared with control IVM treatment for both Tpx2 and Il7 reporters (Figure 2, A and B). Although the effect of FSH was quantitatively variable, an increased translation of the reporters was observed in all the experiments performed (Supplemental Figure 2). On average, FSH produces a 1.7-fold increase in the translation of the Tpx2 reporter and a 1.4-fold increase for Il7. This increase was additive to that associated with reentry into the cell cycle and was comparable with that triggered by AREG treatment. Conversely and in agreement with that reported for AREG and with the protein accumulation, FSH had no effect on the translation of a Tex19.1 reporter (Figure 2C). As an additional control, we monitored the translation of the FL reporter. The FL is not fused to any regulatory sequence and it is polyadenylated in vitro. The average luciferase activity for this reporter did not differ in any of the experimental groups (mean relative light units (RLUs) per oocyte ± SEM: GV 9094 ± 1347 RLU, IVM 9028 ± 1086 RLU, IVM+AREG 10 071 ± 1158 RLU, IVM+FSH 8677 ± 1642 RLU).
Figure 2.
FSH promotes an increase in translation of Tpx2 and Il7 but not Tex19.1 reporters. A–C, cRNA was injected in oocytes still enclosed in the cumulus cells and cultured for 16 hours in milrinone (GV) or different IVM treatments (IVM, IVM+AREG, IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and the luciferase activity measured in the oocyte extract. The bar graphs represent the ratio (mean ± SEM) between the renilla luciferase activity, translated under the control of the Tpx2 3′ UTR (n = 5), Il7 3′ UTR (n ≥ 3), or Tex19.1 3′ UTR (n = 3), and the FL activity used for injection normalization. *, **, Significant differences (P < .05 and P < .01, respectively) in the luciferase activity between IVM+AREG and IVM+FSH compared with IVM (one way ANOVA followed by Tukey's multiple comparison test). NS, not significant.
FSH requires the somatic EGF network to promote AKT activation and Tpx2 reporter translation
The AREG-induced increase in specific translation is preceded by and requires the transient activation of the phosphatidyl-inositol 3-phosphate (PI3K)/AKT pathway in the oocyte (12). Similarly, increased AKT phosphorylation at Ser473 was observed in oocytes cultured for 2.5 hours with FSH (Figure 3). These results indicate PI3K/AKT activation as a common downstream event triggered by both AREG and FSH.
Figure 3.
Oocyte AKT is phosphorylated in response to FSH treatment. CEOs were cultured for 2.5 hours in milrinone (GV) or different IVM treatments (IVM, IVM+AREG, IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and extracts used for Western blot. Representative immunoblots of phosphorylated (p-AKT) and total AKT (AKT) are reported. The bar graph represents the ratio p-AKT to AKT (mean ± SEM) in the different treatments (n = 6). *, Significant differences between treatments (Kruskal-Wallis test followed by Dunn's multiple comparison test, P < .05).
AREG signals through its receptor, EGFR, in follicular cells (35). To investigate whether the FSH signal intersects with the follicular EGF network, the oocyte AKT phosphorylation and Tpx2 reporter translation were assessed in CEOs from mice in which EGFR expression is selectively disrupted in cumulus cells (Figure 4A). To maximize the EGFR protein down-regulation, one Egfr allele was deleted globally, whereas the other was floxed and excised under the control of a Cyp19-driven cre recombinase (36, 37). The knockdown of the EGFR in the follicular cells prevented the FSH-induced AKT phosphorylation as much as the AREG-induced AKT activation (Figure 4B). Moreover, it prevented the AREG- and FSH-dependent potentiation of Tpx2 reporter translation (Figure 4C).
Figure 4.
The knockdown of the EGFR in the somatic compartment prevents both AREG- and FSH-induced AKT activation and Tpx2 reporter translation. A, CEOs were collected from Egfrfl/fl or Egfrδ/fl:Cyp19cre mice, homogenized, and extracts used for Western blots. Representative immunoblots for EGFR and α-tubulin (TUB) are reported. B, CEOs were collected from Egfrfl/+ or Egfrδ/fl:Cyp19cre mice and cultured for 2.5 hours in different IVM conditions (IVM, IVM+AREG, IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and extracts used for Western blots. Representative immunoblots of phosphorylated (p-AKT) and total AKT (AKT) are reported. The bar graph represents the ratio of p-AKT to AKT (mean ± SEM) in the different treatment/genotype (n = 6). ** and *, Significant differences (P < .01 and P < .05) between treatments in Egfrfl/+ mice, whereas no differences were observed in Egfrδ/fl:Cyp19cre mice (Kruskal-Wallis test followed by Dunn's multiple comparison test). C, CEOs were collected from Egfrfl/+ or Egfrδ/fl:Cyp19cre mice. Oocytes still enclosed in the cumulus cells were injected with Tpx2 reporter and cultured for 16 hours in milrinone (GV) or in in different IVM conditions (IVM, IVM+AREG, IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and the luciferase activity measured in the oocyte extract. The bar graph represents the ratio (mean ± SEM) between the RL activity, translated under the control of the Tpx2 3′ UTR and the FL activity used for injection normalization (n = 7). *, Significant differences (P < .05) in the luciferase activity between IVM+AREG and IVM+FSH compared with the IVM in Egfrfl/+ mice, whereas no differences were observed in Egfrδ/fl:Cyp19cre mice (two way ANOVA followed by Tukey's multiple comparison test).
Although these results indicate that FSH signals to the oocyte through the follicular EGF network, it is unclear whether Areg de novo synthesis mediates the FSH effects. The time course of oocyte AKT activation by FSH and AREG are comparable (Supplemental Figure 3), whereas one would expect faster AREG responses if it functioned distal to FSH. To further investigate whether the FSH effects are mediated by AREG, we followed the translation in Areg−/− mice. In this genetic background, FSH treatment has an effect on reporter translation similar to that observed in wild-type mice (Supplemental Figure 4A). In the same vein, when AKT phosphorylation after FSH is measured in Areg null oocytes, no significant changes could be observed (Supplemental Figure 4B).
Attempts to measure the EGFR phosphorylation state in cumulus cells treated with either FSH or AREG were not successful because of the limited amount of tissue available.
Constitutive oocyte AKT activation promotes Tpx2 and Il7 mRNA translation and developmental competence in the absence of FSH or growth factors
In the following experiments, a gain-of-function model was used to confirm the hypothesis that PI3K/AKT activation in the oocyte is required for the FSH action and to promote the developmental competence. The phosphate and tensin homolog gene (Pten), a phosphatase that degrades the PI3K product PI3P, was selectively deleted from the oocytes through a Zp3-driven cre recombinase (38).
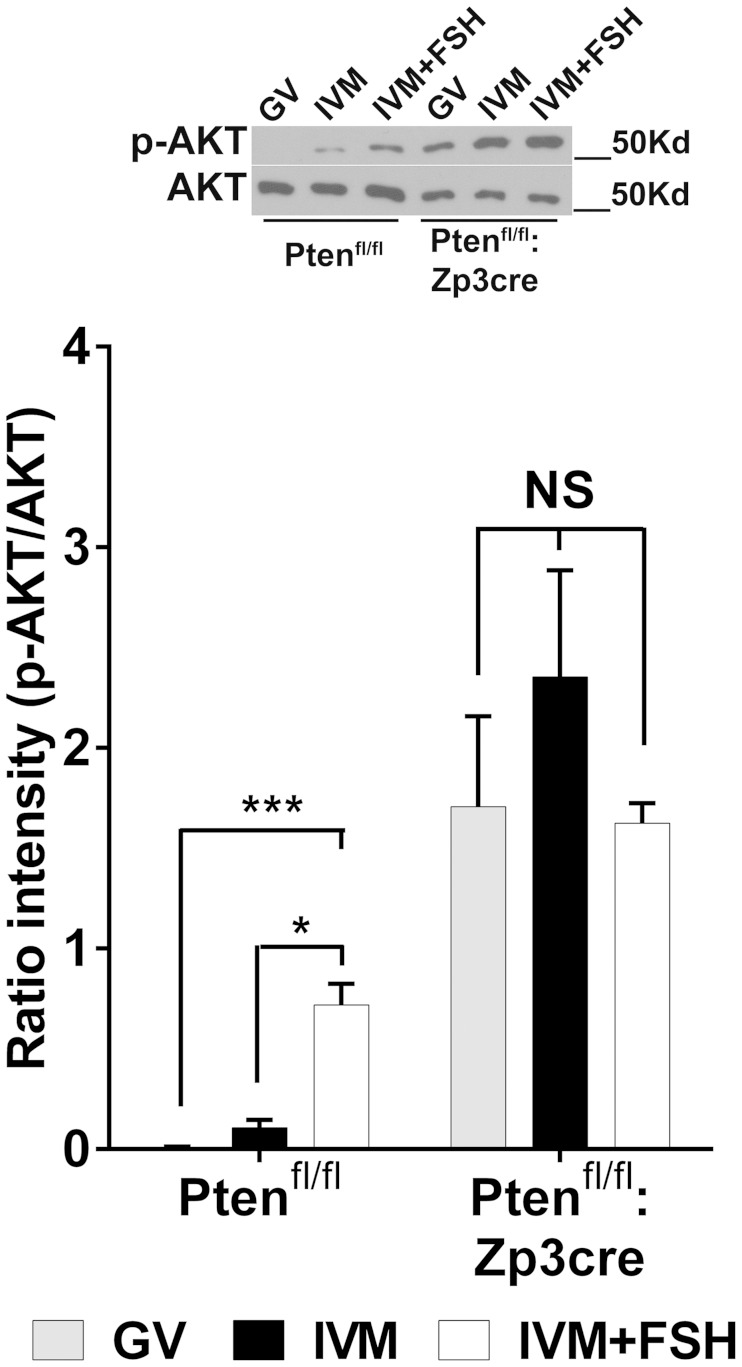
AKT phosphorylation was constitutively activated in oocytes from Ptenfl/fl:Zp3cre mice independently of the meiotic stage and the treatment (Figure 5). On the other hand and when used as a control, oocytes from Ptenfl/fl littermates showed AKT phosphorylation only when cultured with AREG or FSH.
Figure 5.
Oocyte-specific deletion of Pten constitutively activates AKT in the oocyte independent of the meiotic stage and culture treatment. CEOs were collected from Ptenfl/fl or Ptenfl/fl:Zp3cre mice and cultured for 2.5 hours in milrinone (GV) or in the absence (IVM) or presence of FSH (IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and extracts used for Western blots. Representative immunoblots of phosphorylated (p-AKT) and total AKT (AKT) are reported. The bar graph represents the ratio p-AKT to AKT (mean ± SEM) in the different treatment/genotype (Ptenfl/fl, n = 7, Ptenfl/fl:Zp3cre, n = 5). *** and *, Significant differences (P < .001 and P < .05, respectively) between IVM+FSH compared with GV and IVM in Ptenfl/fl mice, whereas no differences were observed in Ptenfl/fl:Zp3cre mice (Kruskal-Wallis test followed by Dunn's multiple comparison test).
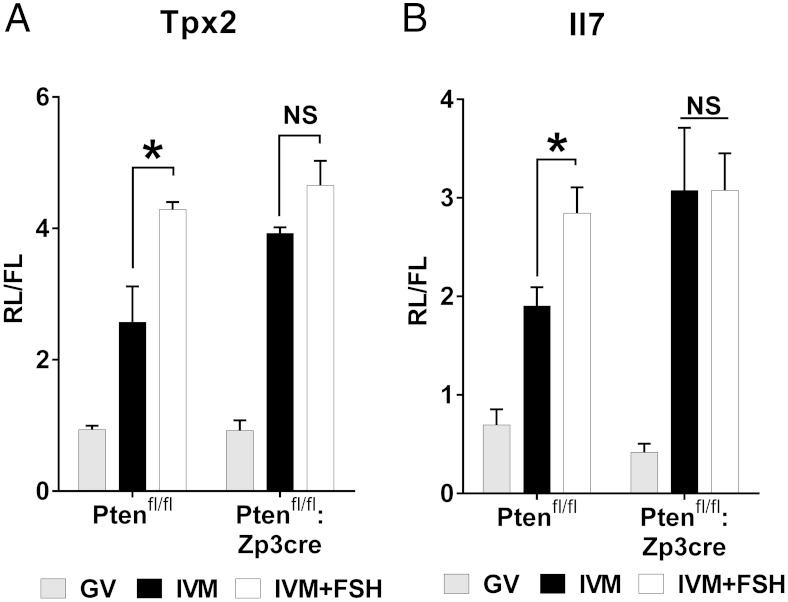
In this model of oocyte AKT constitutive activation, the translation of the Tpx2 and Il7 reporters was already maximal in absence of FSH, and the FSH treatment did not promote any further increase in translation (Figure 6, A and B), as happened in the control littermates. To control whether AKT constitutive activation affects transcript stability, we measured the total amount of the endogenous Il7 and Tpx2 transcripts. No differences were observed between the oocytes from Ptenfl/fl and Ptenfl/fl:Zp3cre mice (Supplemental Figure 1B). The translation of the FL constitutive reporter was also not affected in Pten conditional knockout oocytes. Notably, Pten deletion did not affect the oocyte growth and the time of GV breakdown (Supplemental Figure 5).
Figure 6.
The constitutive activation of AKT in the oocyte is sufficient to induce maximal translation of the Tpx2 (A) and Il7 (B) reporters during oocyte maturation. cRNA was injected in oocytes still enclosed in cumulus cells collected from Ptenfl/fl or Ptenfl/fl:Zp3cre mice and cultured for 16 hours in milrinone (GV) or in the absence (IVM) or presence of FSH (IVM+FSH). At the end of the incubation, oocytes were denuded, homogenized, and the luciferase activity measured in the oocyte extract. The bar graphs represent the ratio (mean ± SEM) between the RL activity, translated under the control of the Tpx2 3′ UTR (A, n = 3) or Il7 3′ UTR (B, n = 4) and the FL activity used for injection normalization. *, Significant differences in the luciferase activity (P < .05) between IVM and IVM+FSH treatments in Ptenfl/fl mice, whereas no differences were observed in the Ptenfl/fl:Zp3cre mice (two way ANOVA followed by Tukey's multiple comparison test).
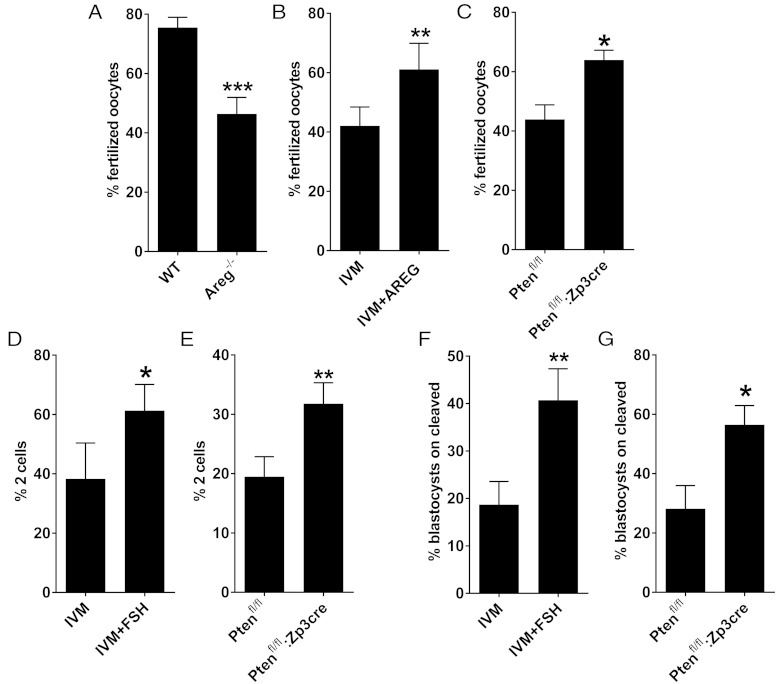
The hypothesis that AKT phosphorylation in the oocyte promotes developmental competence was tested in different experimental conditions. In a first set of experiments, the in vitro fertilization (IVF) rate was investigated in the following: 1) in vivo-matured CEOs (hCG injection) from Areg null or wild-type mice; 2) in vitro-matured CEOs from wild-type mice cultured with or without AREG; and 3) in vitro-matured CEOs from Ptenfl/fl or Ptenfl/fl:Zp3cre cultured in the absence of any growth factor. The fertilization rate of CEOs that matured in an environment depleted of AREG was significantly lower compared with the AREG-exposed group, both in vivo and in vitro (Figure 7, A and B). On the other hand, the constitutive activation of AKT significantly increased the fertilization ability of Ptenfl/fl:Zp3cre CEOs, compared with control littermates (Figure 7C). Having confirmed that the constitutive AKT activation affected the very early stages of zygote formation, the following steps of preimplantation embryo development were monitored. Both the two-cell rate and the blastocyst rate were increased in Ptenfl/fl:Zp3cre oocytes compared with control littermates (Figure 7, E–G), with a pattern that parallels the increase observed when wild-type CEOs were matured in the presence of FSH (Figure 7, D–F).
Figure 7.
The constitutive activation of AKT in the oocyte is sufficient to improve the developmental competence after IVM in the absence of growth factors. CEOs collected from different mice strains and genotypes were matured in different conditions and in vitro fertilized. After IVF the presumptive zygotes were either fixed and the DNA stained to assess the formation of the two pronuclei (A–C) or cultured for an extra 19 hours to assess the formation of two-cell embryos (D and E) or up to E5.0 to assess the blastocyst formation (F and G). A, CEOs were collected from the ampullae of Areg null (Areg−/−) and wild-type (WT) littermate mice 14–17 hours after the injection of hCG. The bar graph represents the fertilization rate (mean ± SEM) in the different genotypes (n = 12). ***, Significant differences (unpaired t test, P < .001). B, CEOs were in vitro matured for 17 hours in the absence (IVM) or presence of AREG (IVM+AREG). The bar graph represents the fertilization rate (mean ± SEM) in the different treatments (n = 6). **, Significant differences (paired t test, P < .01). C, CEOs were collected from Ptenfl/fl and Ptenfl/fl: Zp3cre mice and in vitro matured in the absence of any hormone/growth factor. The bar graph represents the fertilization rate (mean ± SEM) in the different genotypes (n = 5). *, Significant differences (paired t test, P < .05). D, CEOs collected from C57BL/6NxCD1 hybrids were in vitro matured in the absence (IVM) or presence of FSH (IVM+FSH). The bar graph represents the two-cell rate (mean ± SEM) in the different treatments (n = 5). *, Significant differences (paired t test, P < .05). E, CEOs were collected from Ptenfl/fl and Ptenfl/fl:Zp3cre mice and in vitro matured in absence of any hormone/growth factor. The bar graph represents the two-cell rate (mean ± SEM) in the different genotypes (n = 8). **, Significant differences (paired t test, P < .01). Note that Ptenfl/fl and Ptenfl/fl:Zp3cre mice are on a C57BL/6N pure background. This can account for the lower two-cell rate compared with the C57BL/6NxCD1 hybrids. F, CEOs collected from C57BL/6NxCD1 hybrids were in vitro matured in the absence (IVM) or presence of FSH (IVM+FSH). The bar graph represents the blastocyst rate (mean ± SEM) in the different treatments (n = 3). **, Significant differences (paired t test, P < .01). G, CEOs were collected from Ptenfl/fl and Ptenfl/fl:Zp3cre mice and in vitro matured in the absence of any hormone/growth factor. The bar graph represents the blastocyst rate (mean ± SEM) in the different genotypes (Ptenfl/fl, n = 4, and Ptenfl/fl: Zp3cre, n = 3). *, Significant differences (unpaired t test, P < .05).
Discussion
With the present study, we have identified a novel signaling mechanism by which FSH controls the developmental competence of oocytes. Using loss- and gain-of-function genetic models, we demonstrate that FSH signaling intersects with the EGF network in the ovarian follicle to activate the PI3K/AKT cascade in the oocyte during maturation. Through this pathway, FSH regulates the translation of previously stored maternal mRNAs in the oocyte. The resulting accumulation of a subset of proteins in the oocyte is associated with an increased fertilization rate and improved embryo development. These findings provide a molecular rationale for the use of FSH in IVM media to improve the quality of eggs used for assisted reproductive technologies.
Our experiments show that FSH treatment of CEOs induces an increased translation of a subset of maternal mRNAs and an increase in corresponding protein accumulation in mouse oocytes. The total mRNA levels of the corresponding transcripts are not affected by FSH, excluding mRNA stabilization as the cause of the increased protein levels. We propose that FSH exerts its effects through the activation of the EGF network in somatic cells. This conclusion is based on the loss of function of EGFR in which we show that the FSH effects are lost when the EGFR expression is reduced by 80%–85% in cumulus cells. Several reports have shown that FSH stimulation causes an increase in the expression of the EGFR ligands Areg, Ereg, and Btc (betacellulin) in mural granulosa cells and CEOs (15, 39–43), followed by peptide accumulation (15). If the effects of FSH on oocyte translation were exclusively due to de novo expression of EGF-like growth factors mRNAs, followed by propeptide synthesis and finally shedding, one would expect that the time course of AKT activation by AREG in the oocyte would occur in a shorter time interval than the one induced by FSH stimulation. Conversely, we show that the time courses of AKT activation by AREG and FSH treated CEOs are comparable. Moreover, FSH continued to have an effect in the Areg null CEOs, suggesting that AREG release is dispensable for the FSH action. However, it should be noted that EREG and β-cellulin are likely released after FSH stimulation (39–43) and may compensate for the loss of AREG. An additional possibility to consider is that AREG may be dispensable in the isolated CEO model but may be required to mediate the FSH action in the intact follicle in which mural granulosa cells are present. It is also possible that additional signaling pathways are involved in this cascade; for instance, FSH may activate the EGFR through an intracellular pathway as demonstrated in other systems (40, 42, 44). Alternatively, FSH may promote the shedding of a preexisting pool of pro-EGF-like growth factors (45). If these intracellular effects are indeed present, the reported accumulation of EGFR ligands induced by FSH (15) may be a mechanism to prolong signaling through the EGFR.
We show that when the AKT pathway is constitutively activated in the Ptenfl/fl:Zp3cre oocytes, FSH is no longer required to maximally induce oocyte translation in MII. However, translation remains suppressed in Ptenfl/fl:Zp3cre oocytes maintained in GV. This finding is consistent with our previous conclusion (12) that AKT activation is not sufficient to activate translation unless the oocytes have reentered the cell cycle. The constitutive activation of AKT also promotes developmental competence of the oocytes and renders growth factor or FSH addition to the IVM medium unnecessary. Taken together, these observations are consistent with the hypothesis that enhanced translation is associated with improved developmental competence. Studies conducted in several mammalian species show that supplementation of the IVM medium with FSH improves oocyte quality (16, 17, 19–23, 46–48). These findings have promoted the widespread use of FSH as hormonal supplementation in IVM protocols. Also, inclusion of EGF or EGF-like growth factors in the IVM media has been explored because these locally released factors function as a physiological amplifier of the LH signal in the follicle. Indeed, the use of EGF and EGF-like peptides improves embryo development (12, 16, 17, 22, 48–53).
Less clear is whether FSH and EGF-like growth factors function in an additive manner through different or redundant signaling pathways. In mice, De La Fuente et al (17) observed a slight, though not significant, increase in two-cell and blastocyst yield with EGF compared with FSH, whereas Merriman et al (22) did not observe any difference at the two-cell stage but a higher number of developing fetuses when CEOs were treated with FSH. Also in mice, Richani et al (15) did not find any difference in the blastocyst rate at E5.0 comparing FSH, EGF, AREG, EREG, and AREG+EREG treatment while reporting higher blastocyst rate at E6.0 for the EREG group. In a study on porcine oocytes, FSH increases the cleavage rate after parthenogenetic activation, but the percentage of blastocysts on cleaved embryos was higher for AREG (41). Finally, in cows, no significant differences were observed between AREG and FSH (16) or EGF and FSH treatments (48). Therefore, although it is accepted that FSH and EGF-like growth factor supplementation generally has a beneficial effect on developmental competence, it is unclear whether they act through different pathways. The finding that the treatments are usually nonadditive strongly supports our view that FSH effects are mediated by activation of the EGFR.
A midcycle increase in FSH overlaps with the LH surge in mice (54) as well as in humans (24, 30). The biological significance of this increase is debated. It has been proposed that the in vitro effects of FSH during IVM simply mimic the LH effects in vivo (55) by increasing cAMP levels in granulosa cells. Even though FSH activates a cAMP/protein kinase A signal transduction pathway (41), in our hands the activation of this signaling cascade is not sufficient to trigger oocyte AKT phosphorylation and maternal transcripts translation when the EGFR is down-regulated in cumulus cells. At present we cannot assess experimentally whether FSH has any effect distinct from LH on translation in vivo.
Our findings further support the concept that follicular somatic cells are important regulators of the final oocyte maturation critical for the development of the competence to sustain embryo development. In this view, we propose that activation of the oocyte translational program is one of the mechanisms mediating somatic cells promotion of embryo development; furthermore, the downstream effector of the somatic-derived signal is the PI3K/AKT cascade in the oocyte. Importantly, both FSH and AREG increased maternal mRNA translation by inducing the oocyte PI3K/AKT signaling (the present study and reference 12) and promote embryo development. Even though other functions of the cumulus cells in promoting fertilization, especially in the interactions with sperm, should be considered, the central role of the PI3K/AKT cascade and the maternal mRNA translation in promoting the oocyte developmental competence is supported by our IVF experiments. The AKT activation, which is, for instance, uncoupled from the cumulus expansion in Ptenfl/fl:Zp3cre oocytes, is sufficient to induce the same increase in developmental potential as that observed in the presence of AREG or FSH.
In conclusion, our results provide a molecular rationale for the use of FSH during IVM procedures and support the possibility of a cooperative function of the FSH and LH midcycle surges in promoting the acquisition of oocyte developmental competence. This would be in line with the findings that combined FSH/hCG treatment in patients undergoing IVF improves egg quality (31, 56). Importantly, the present study provides further evidence that the regulation of translation and protein accumulation during oocyte maturation is associated with the acquisition of developmental competence. In the same vein, our findings reinforce the idea that understanding the properties and regulation of the translation program executed during the final stages of oocyte maturation will shed light on the molecular aspects of developmental competence and, ultimately, will provide quantitative measurements to predict the fitness of an egg to sustain a pregnancy to term.
Acknowledgments
We thank Dr Joao Pedro Sousa Martins for performing the immunodetection of TEX19.1 protein and Dr Stephan Viville for generously providing the TEX19.1 antibody. We also thank Dr Sahar Houshdaran for the valuable discussion on statistical analysis.
This work was supported by Grant FP7-PEOPLE-2013-IOF GA 624874 MateRNA (to F.F.) and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Cooperative Agreement P50HD055764-06, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to M.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AREG
- amphiregulin
- CEO
- cumulus-enclosed oocyte
- CT
- cycle threshold
- E
- embryonic day
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- EREG
- epiregulin
- FL
- Firefly luciferase
- GV
- germinal vesicle
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- IVM
- in vitro maturation
- MII
- metaphase II
- PI3K
- phosphatidyl-inositol 3-phosphate
- Pten
- phosphate and tensin homolog gene
- RL
- Renilla luciferase
- RLU
- relative light unit
- TEX19.1
- testis expressed gene 19.1
- TPX2
- targeting protein for the Xenopus kinesin xklp2
- UTR
- untranslated region.
References
- 1. Szybek K. In vitro maturation of oocytes from sexually immature mice. J Endocrinol. 1972;54:527–528. [DOI] [PubMed] [Google Scholar]
- 2. Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–536. [DOI] [PubMed] [Google Scholar]
- 3. Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–172. [DOI] [PubMed] [Google Scholar]
- 4. Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276. [DOI] [PubMed] [Google Scholar]
- 5. Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. [DOI] [PubMed] [Google Scholar]
- 6. Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–587. [DOI] [PubMed] [Google Scholar]
- 7. De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. [DOI] [PubMed] [Google Scholar]
- 8. Liu H, Aoki F. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote. 2002;10:327–332. [DOI] [PubMed] [Google Scholar]
- 9. Clarke HJ. Post-transcriptional control of gene expression during mouse oogenesis. Results Probl Cell Differ. 2012;55:1–21. [DOI] [PubMed] [Google Scholar]
- 10. Conti M, Sousa Martins JP, Han SJ, Franciosi F. Translational control in the germ line. In: Post-Transcriptional Mechanisms in Endocrine Regulation. New York: Springer; 2016. [Google Scholar]
- 11. Chen J, Melton C, Suh N, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Torcia S, Xie F, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol. 2013;15:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 14. Peluffo MC, Ting AY, Zamah AM, et al. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27:2430–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richani D, Ritter LJ, Thompson JG, Gilchrist RB. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod. 2013;19:500–509. [DOI] [PubMed] [Google Scholar]
- 16. Sugimura S, Ritter LJ, Sutton-McDowall ML, Mottershead DG, Thompson JG, Gilchrist RB. Amphiregulin co-operates with bone morphogenetic protein 15 to increase bovine oocyte developmental competence: effects on gap junction-mediated metabolite supply. Mol Hum Reprod. 2014;20:499–513. [DOI] [PubMed] [Google Scholar]
- 17. De La Fuente R, O'Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod. 1999;14:3060–3068. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong DT, Zhang X, Vanderhyden BC, Khamsi F. Hormonal actions during oocyte maturation influence fertilization and early embryonic development. Ann NY Acad Sci. 1991;626:137–158. [DOI] [PubMed] [Google Scholar]
- 19. Izadyar F, Zeinstra E, Bevers MM. Follicle-stimulating hormone and growth hormone act differently on nuclear maturation while both enhance developmental competence of in vitro matured bovine oocytes. Mol Reprod Dev. 1998;51:339–345. [DOI] [PubMed] [Google Scholar]
- 20. Abdoon AS, Kandil OM, Otoi T, Suzuki T. Influence of oocyte quality, culture media and gonadotropins on cleavage rate and development of in vitro fertilized buffalo embryos. Anim Reprod Sci. 2001;65:215–223. [DOI] [PubMed] [Google Scholar]
- 21. Schoevers EJ, Kidson A, Verheijden JH, Bevers MM. Effect of follicle-stimulating hormone on nuclear and cytoplasmic maturation of sow oocytes in vitro. Theriogenology. 2003;59:2017–2028. [DOI] [PubMed] [Google Scholar]
- 22. Merriman JA, Whittingham DG, Carroll J. The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod. 1998;13:690–695. [DOI] [PubMed] [Google Scholar]
- 23. Schroeder AC, Downs SM, Eppig JJ. Factors affecting the developmental capacity of mouse oocytes undergoing maturation in vitro. Ann NY Acad Sci. 1988;541:197–204. [DOI] [PubMed] [Google Scholar]
- 24. Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris). 2010;71:132–143. [DOI] [PubMed] [Google Scholar]
- 25. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. [DOI] [PubMed] [Google Scholar]
- 27. El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc Natl Acad Sci USA. 2014;111:16778–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeppesen JV, Kristensen SG, Nielsen ME, et al. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burns KH, Yan C, Kumar TR, Matzuk MM. Analysis of ovarian gene expression in follicle-stimulating hormone beta knockout mice. Endocrinology. 2001;142:2742–2751. [DOI] [PubMed] [Google Scholar]
- 30. Barbieri RL. The endocrinology of the menstrual cycle. Methods Mol Biol. 2014;1154:145–169. [DOI] [PubMed] [Google Scholar]
- 31. Kol S, Humaidan P. LH (as HCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod Biomed Online. 2010;21:590–592. [DOI] [PubMed] [Google Scholar]
- 32. Arimura A, Debeljuk L, Schally AV. Blockade of the preovulatory surge of LH and FSH and of ovulation by anti-LH-RH serum in rats. Endocrinology. 1974;95:323–325. [DOI] [PubMed] [Google Scholar]
- 33. Brunet S, Dumont J, Lee KW, et al. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS One. 2008;3:e3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng Y, Yata A, Klein C, Cho JH, Deguchi M, Hsueh AJ. Oocyte-expressed interleukin 7 suppresses granulosa cell apoptosis and promotes oocyte maturation in rats. Biol Reprod. 2011;84:707–714. [DOI] [PubMed] [Google Scholar]
- 35. Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One. 2011;6:e21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X, Xie F, Zamah AM, Cao B, Conti M. Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod. 2014;91:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jagarlamudi K, Liu L, Adhikari D, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4:e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev. 2013;25:890–899. [DOI] [PubMed] [Google Scholar]
- 40. Chen X, Zhou B, Yan J, et al. Epidermal growth factor receptor activation by protein kinase C is necessary for FSH-induced meiotic resumption in porcine cumulus-oocyte complexes. J Endocrinol. 2008;197:409–419. [DOI] [PubMed] [Google Scholar]
- 41. Prochazka R, Petlach M, Nagyova E, Nemcova L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction. 2011;141:425–435. [DOI] [PubMed] [Google Scholar]
- 42. Wang J, Chen Q, Zhou J, et al. Specific protein kinase C isoforms α and βI are involved in follicle-stimulating hormone-induced mouse follicle-enclosed oocytes meiotic resumption. PLoS One. 2012;7:e45043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamashita Y, Hishinuma M, Shimada M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J Ovarian Res. 2009;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khan DR, Guillemette C, Sirard MA, Richard FJ. Characterization of FSH signalling networks in bovine cumulus cells: a perspective on oocyte competence acquisition. Mol Hum Reprod. 2015;21(9):688–701. [DOI] [PubMed] [Google Scholar]
- 45. Chen Q, Zhang W, Ran H, et al. PKCδ and θ possibly mediate FSH-induced mouse oocyte maturation via NOX-ROS-TACE cascade signaling pathway. PLoS One. 2014;9:e111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin YH, Hwang JL, Seow KM, et al. Effect of incubation with different concentrations and durations of FSH for in vitro maturation of murine oocytes. Reprod Biomed Online. 2011;23:111–117. [DOI] [PubMed] [Google Scholar]
- 47. Modina S, Abbate F, Germana GP, Lauria A, Luciano AM. Beta-catenin localization and timing of early development of bovine embryos obtained from oocytes matured in the presence of follicle stimulating hormone. Anim Reprod Sci. 2007;100:264–279. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi K, Yamashita S, Hoshi H. Influence of epidermal growth factor and transforming growth factor-α on in vitro maturation of cumulus cell-enclosed bovine oocytes in a defined medium. J Reprod Fertil. 1994;100:439–446. [DOI] [PubMed] [Google Scholar]
- 49. Rieger D, Luciano AM, Modina S, Pocar P, Lauria A, Gandolfi F. The effects of epidermal growth factor and insulin-like growth factor I on the metabolic activity, nuclear maturation and subsequent development of cattle oocytes in vitro. J Reprod Fertil. 1998;112:123–130. [DOI] [PubMed] [Google Scholar]
- 50. Wang W, Niwa K. Synergetic effects of epidermal growth factor and gonadotropins on the cytoplasmic maturation of pig oocytes in a serum-free medium. Zygote. 1995;3:345–350. [DOI] [PubMed] [Google Scholar]
- 51. Grupen CG, Nagashima H, Nottle MB. Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro. Reprod Fertil Dev. 1997;9:571–575. [DOI] [PubMed] [Google Scholar]
- 52. Abeydeera LR, Wang WH, Cantley TC, Rieke A, Prather RS, Day BN. Presence of epidermal growth factor during in vitro maturation of pig oocytes and embryo culture can modulate blastocyst development after in vitro fertilization. Mol Reprod Dev. 1998;51:395–401. [DOI] [PubMed] [Google Scholar]
- 53. Goud PT, Goud AP, Qian C, et al. In vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–1644. [DOI] [PubMed] [Google Scholar]
- 54. Ryan KD, Schwartz NB. Changes in serum hormone levels associated with male-induced ovulation in group-housed adult female mice. Endocrinology. 1980;106:959–966. [DOI] [PubMed] [Google Scholar]
- 55. Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68(suppl 1):S71–S76. [DOI] [PubMed] [Google Scholar]
- 56. Lamb JD, Shen S, McCulloch C, Jalalian L, Cedars MI, Rosen MP. Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2011;95:1655–1660. [DOI] [PubMed] [Google Scholar]
- 57. Tarabay Y, Kieffer E, Teletin M, et al. The mammalian specific Tex19.1 gene plays an essential role in spermatogenesis and placenta-supported development. Human Reprod. 2013;28:2201–2214. [DOI] [PubMed] [Google Scholar]