Abstract
The neuropeptide kisspeptin (Kiss1) is integral to the advent of puberty and the generation of cyclical LH surges. Although many complex actions of Kiss1 are known, the mechanisms governing the processing/regulation of this peptide have not been unveiled. The metallo enzyme, endopeptidase 24.15 (thimet oligopeptidase), has been demonstrated to play a key role in the processing and thus the duration of action of the reproductive neuropeptide, GnRH, which signals downstream of Kiss1. Initial in silico modeling implied that Kiss1 could also be a putative substrate for EP24.15. Coincubation of Kiss1 and EP24.15 demonstrated multiple cleavages of the peptide predominantly between Arg29-Gly30 and Ser47-Phe48 (corresponding to Ser5-Phe6 in Kiss-10; Kiss-10 as a substrate had an additional cleavage between Phe6-Gly7) as determined by mass spectrometry. Vmax for the reaction was 2.37±0.09 pmol/min · ng with a Km of 19.68 ± 2.53μM, which is comparable with other known substrates of EP24.15. EP24.15 immunoreactivity, as previously demonstrated, is distributed in cell bodies, nuclei, and processes throughout the hypothalamus. Kiss1 immunoreactivity is localized primarily to cell bodies and fibers within the mediobasal and anteroventral-periventricular hypothalamus. Double-label immunohistochemistry indicated coexpression of EP24.15 and Kiss1, implicating that the regulation of Kiss1 by EP24.15 could occur in vivo. Further studies will be directed at determining the precise temporal sequence of EP24.15 effects on Kiss1 as it relates to the control of reproductive hormone secretion and treatment of fertility issues.
Reproduction is a tightly regulated physiological process requiring integration of the hypothalamic-pituitary gonadal (HPG) axis with multiple sensory, metabolic, and hormonal inputs. Many of these inputs impinge upon GnRH neurons and relay information about the energy balance or stress state of the organism. In addition to a wide range of amino acid-derived neurotransmitters, a number of neuropeptides have facilitatory effects on GnRH neurons and gonadotropin responses which influence the timing of puberty, generation of LH surges and overall activity of the axis (reviewed in Ref. 1).
Kisspeptin (Kiss1) is one such neuropeptide that exerts strong influence over the activity of GnRH neurons and the reproductive axis. Kiss1 was originally identified as a metastatic tumor suppressor (2) and later the ligand for the G protein-coupled receptor 54, (GPR54), now referred to as Kiss1R (3–5). Inactivating mutations in Kiss1R in both humans and mice lead to hypogonadotropic hypogonadism, delayed or absent sexual maturation, and infertility (6, 7). These findings are replicated when the Kiss1 gene is knocked out in mice (8, 9). The Kiss1 gene produces a 145-amino acid peptide, which is then further processed into a 52- or 54-amino acid peptide, depending on the species, that binds to Kiss1R. Proteolytic processing of Kiss1 results in peptides of 14, 13, and 10 amino acids with the terminal decapeptide sequence (YNWNSFGLRF-NH2, hereafter referred to as Kiss-10 with amino acid residue numbering corresponding to the decapeptide), being the shortest peptide required for receptor activation (3–5).
Intracellular and extracellular peptide concentrations are regulated by exo- and endo-peptidases. Presently, there is a gap in the understanding of the mechanisms underlying the termination of Kiss1 signaling. Neuropeptides do not employ a reuptake system as do small molecule neurotransmitters. Once secreted into the extracellular space, the ability of neuropeptides to bind their cognate receptor and initiate signaling pathways remains intact until they undergo proteolytic processing. Cleavage of neuropeptides may result in fragments that have altered binding affinity, ability to bind their cognate receptor, or an altogether different physiological function. Over a decade ago, a single report by Takino et al (10) implies that Kiss1 is cleaved in vitro by several members of the matrix metalloproteinase family at the Gly7-Leu8 peptide bond to yield inactive fragments.
The neuropeptide processing enzyme, endopeptidase 24.15 (EP24.15; EC 3.4.24.15, thimet oligopeptidase), is responsible for the cleavage and subsequent signal modulation of bioactive neuropeptides. EP24.15 is a 78-kDa, zinc-dependent endopeptidase, which is highly expressed in the brain and in reproductive tissues (11–15). The enzyme generally cleaves peptide substrates less than approximately 42 amino acids and, although it does not recognize any specific consensus sequence, EP24.15 exhibits a preference for cleavage on the carboxyl side of basic or aromatic residues (15–18). EP24.15 has already been demonstrated to cleave GnRH (15, 17, 19) and other neuropeptide neurotransmitters (15, 17). Inhibition of EP24.15 results in increased GnRH bioavailability and a subsequent increase in LH and FSH levels, suggesting that EP24.15 may play a key role in the regulation of reproduction (20–22).
Based on the sequence similarities of several known substrates and x-ray crystallographic data that allows in silico modeling of peptide substrates of EP24.15 in a neuropeptide discovery pipeline, we observed that Kiss1 was a potential candidate for cleavage by EP24.15. To experimentally prove this assertion, studies here were designed to examine whether Kiss1 is a physiological substrate for EP24.15 by first determining if, and at what position(s) in the sequence, Kiss1 is cleaved using mass spectrometry, and then determining the enzyme kinetics of the reaction. Anatomical coexpression of Kiss1 and EP24.15 immunoreactivities was performed in rat hypothalamic tissue to determine whether there is overlap in their distribution providing supporting evidence of in vivo regulation of Kiss1 by EP24.15. Here, we report that EP24.15 cleaves Kiss-10 in vitro between Ser5 and Phe6, and Phe6 and Gly7, with kinetic parameters comparable with other known physiological substrates. Furthermore, we demonstrate colocalization of EP24.15 and Kiss1 immunoreactivities in the arcuate nucleus (ArcN) of rats. The evidence presented here suggests that EP24.15 cleaves Kiss1 both in vivo and in vitro to produce inactive peptide fragments and provides a mechanism for Kiss1 signal modulation.
Materials and Methods
Molecular modeling
Computer-aided molecular modeling of EP24.15 was based on the x-ray crystallographic structure coordinates derived from the apo-form of EP24.15 (Research Collaboratory for Structural Bioinformatics-Protein Data Bank, entry 1S4B). In silico molecular visualization of the structures was performed with the PyMOL molecular graphics system v.1.3 (Schrödinger LLC) (23). The Kiss-10 peptide was modeled in PyMOL and through rotational and translational functions placed into the active site cleft of EP24.15.
EP24.15 expression and purification
Recombinant rat EP24.15 protein was expressed in Escherichia coli BL21-DE3 (Stratagene) as previously described (30) using the pGEX2T-GST plasmid (GE Healthcare) containing cDNA encoding for the open reading frame (accession number NM_172075.2) and purified by affinity chromatography on a glutathione-sepharose column (GE Healthcare). After purification, protein was subjected to SDS-PAGE, and the gel was stained with Coomassie blue for visualization of the band representing EP24.15 (Thermo Scientific). Protein concentration was determined using a Bradford assay (24) (Bio-Rad). Homogeneous protein with a purity of more than 98% was stored at −80°C and used in all subsequent experiments.
Peptides
Rat Kiss-52 (metastin; accession number NP_859043.1) was purchased from Phoenix Pharmaceuticals. Kiss1 peptides were custom synthesized (GenScript) corresponding to human Kiss1 residues 112–121 (YNWNSFGLRF-NH2) and 117–121 (FGLRF-NH2; accession number NP_002247.3).
Mass spectrometry
Determination of Kiss1 cleavage and the position of the scissile bond was performed as outlined previously (25). In brief, 2mM Kiss peptides were incubated with 1 μg of EP24.15 at 37°C in 25mM Tris-HCl (pH 7.4), 125mM NaCl, and 0.3mM dithiothreitol for 0–120 minutes. Reactions were terminated by the addition of 2 μL of 5% (vol/vol) formic acid. A portion of the reaction mixture (2 μL) was spotted onto a standard matrix-assisted laser desorption ionization (MALDI) sample holder, along with matrix (2 μL; 10 mg/mL α-cyano-4-hydroxycinnamic acid in 60% acetonitrile, with 0.1% formic acid). MALDI-time of flight (TOF) data were collected from 200 laser shots on a Voyager DE-STR Biospectrometry Workstation (Applied Biosystems) using a 199-Da low mass cutoff to eliminate matrix and salt peaks. The resulting peaks were matched to Kiss1 fragments using Expasy's FindPept tool (http://web.expasy.org/findpept/).
Sequencing confirmation experiments were also performed with the above EP24.15-digested samples using liquid chromatography multidimensional mass spectrometry with an Ultimate 3000 RSLCnano system coupled with a Thermo LTQ Orbitrap Elite. Samples were concentrated with an Acclaim pepmap 100 C18 (300 μm × 5 mm; Thermo Dionex) column before downstream separation with a C18 column, (75 μm × 15 mm; Thermo Dionex). A gradient of 4%–70% B was employed over 60 minutes with mobile phase A (0.1% formic acid in water with 2% acetonitrile) and mobile phase B (0.1% formic acid in acetonitrile with 2% water) at a flow rate of 300 nL/min. External calibration was performed on the LTQ Orbitrap Elite mass spectrometer with a nanospray ion source before running the samples. A MS precursor scan with resolution (Fourier Transform full scan), 120 000 m/Δm50% was set. Top 10 MS2 in the ion trap with 1 microscan was used for product scan. The MS2 threshold was set as 5000. The activation type is collision-induced dissociation. The collision energy was set as 35 with 10 milliseconds of activation time. The isolation width was set as 2 m/z. MS target was 1e6 and MS2 target was 1e4. A charge state of 2+ was selected. Fourier transform mass spectrometry injection time was 600 milliseconds, and ion trap mass spectrometry injection time was 100 milliseconds. De novo sequencing was used in addition to database searches with PEAKS (Bioinformatics Solutions, Inc).
Kinetic measurements
Enzyme kinetics were quantified (n=6) using a HPLC-based assay, as previously described (26), with Kiss-10 as the substrate. Briefly, 20–200 ng of EP24.15 were incubated at 37°C with varying concentrations of Kiss-10 (0μM–100μM) in reaction buffer (25mM Tris/HCl, 125mM NaCl, and 0.3mM dithiothreitol; pH 7.5). Reactions were terminated after 15 minutes by the addition of 100 μL of methanol containing 1% TFA. The appearance of a Kiss-10 product fragment (residues 6–10) was measured via HPLC (Waters) with absorbance detection at 214 nm. Solvents were filtered and degassed before use (solvent A, 0.08% TFA; solvent B, 70% acetonitrile, 0.08% TFA). Samples were eluted at 1.0 mL/min from a Symmetry C18 reverse phase column (Waters) by a linear gradient of 3%–85% solvent B over 25 minutes. Enzyme kinetics were determined from a Kiss-5 standard curve prepared under identical assay conditions (26). Suitable negative controls were included in each assay, including reactions that were quenched with 100 μL of methanol containing 1% TFA at t = 0 minutes or a reaction containing no enzyme. Kinetic parameters (Km and Vmax) were subsequently calculated by the Michaelis-Menten equation using Prism v.5.0 (GraphPad) under first order conditions.
Animals
Male and female Sprague Dawley (Harlan Laboratories or Charles River Laboratories) rats (250–300 g) were housed 2 or 3 per cage in an Association for the Assessment and Accreditation of Laboratory Animal Care accredited facility. A 12-hour light, 12-hour dark cycle was maintained; temperature and humidity were constantly monitored, and food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science. Estrous cycles were monitored daily through examination of vaginal cytology. Female rats were used on the day of metestrus (see Figure 5 below) or proestrus depending on the hypothalamic area being analyzed.
Figure 5.
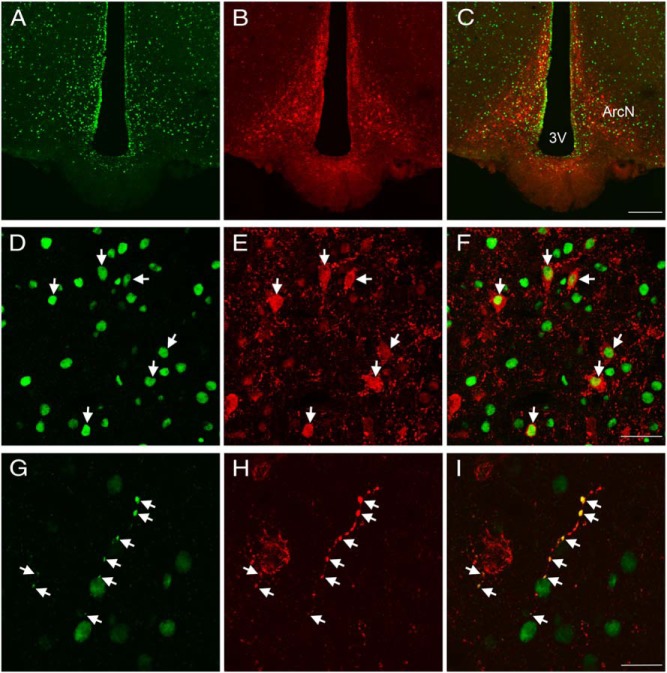
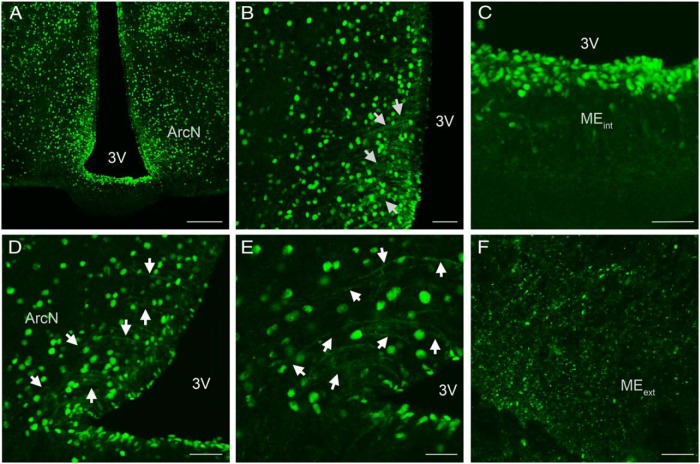
Colocalization of EP24.15 and Kiss1 immunoreactivities in the ArcN of a metestrous female rat. Representative confocal photomicrograph of EP24.15 (green) (A) and Kiss1 (red) immunoreactivity in the ArcN (B). C, Merged image of A and B showing overlap and codistribution of Kiss1 and EP24.15. Numerous Kiss1 and EP24.15-immunoreactive cells are distributed throughout the ArcN (scale bar, 200 μm). Higher magnification of the ArcN demonstrating EP24.15 (D) and Kiss1 immunoreactivity (E). F, Colocalization of EP24.15 and Kiss1 in the ArcN. Arrows highlight regions of colocalization (scale bar, 40 μm). G, Colocalization of EP24.15 and H. Kiss1 in a nerve fiber. I, Merged image of G and H demonstrating coexpression along a “beaded” fiber (scale bar, 20 μm). 3V, third ventricle.
Tissue preparation
Male and female rats were anesthetized with pentobarbital (50 mg/kg, ip) and transcardially perfused with warm (37°C) PBS containing 0.1% procaine and heparin, followed by cold 4% paraformaldehyde in PBS (pH 7.5) at 2 pm. After perfusion, the brains were removed and placed in fixative at 4°C overnight and maintained in PBS at 4°C until sectioning.
Multilabel immunohistochemistry
EP24.15 and Kiss1
Double-label immunohistochemistry was performed on free floating, vibratome-cut (40 μm) sections as described previously (27). In brief, sections were rinsed in PBS (pH 7.5), treated with 1% H2O2 for 20 minutes and further rinsed in 3 × 5-minute PBS washes. The sections were blocked for 3 hours in 10% normal donkey serum (NDS) in PBS-gelatin and then incubated for 72 hours with the affinity purified rabbit 1° antibody against EP24.15 (1:110 000) in 4% NDS, PBS-gelatin. Sections were rinsed in PBS-gelatin and incubated with biotinylated donkey antirabbit 2° antibody (1:1500; Jackson ImmunoResearch) for 1 hour. After washes in PBS-gelatin, the tissues were incubated with avidin-biotin complex (2 μL/mL; Vector Labs) for 30 minutes and rinsed in PBS-gelatin. For amplification of the signal, the tissues were incubated with biotinylated tyramide in 0.01% H2O2/PBS for 10 minutes, rinsed, and incubated with Alexa Fluor 488 streptavidin (1:250; Life Technologies) in PBS-gelatin for 3 hours. After washes in PBS-gelatin, sections were next incubated with a rabbit 1° antibody against Kiss-10 (1:1000, catalog number AB9754; Millipore) in 4% NDS, 0.25% Triton X-100 and PBS-gelatin for 72 hours and rinsed in PBS-gelatin. Lastly, tissues were incubated with Alexa Fluor 594 antirabbit 2° antibody (1:250; Life Technologies) in PBS-gelatin for 3 hours, rinsed in Tris-buffered saline (pH 7.5), and mounted on gelatin-subbed slides and air dried. Coverslips were applied using polyvinyl alcohol-1,4-diazabicyclo[2.2.2]octane.
Biotinylated tyramide amplification permits the use of lower concentrations of 1° antibody than detectable by standard indirect immunofluorescence, and is therefore useful for preventing cross-reactivity when using 1° antibodies obtained from the same species. Nevertheless, antibody specificity was verified by omitting each 1° antibody in separate experiments and observing no signal in the appropriate channel.
The distribution of EP24.15 immunoreactivity and its colocalization with Kiss1 was assessed in the hypothalamus of metestrous (see Figure 5 below) or proestrous (see Figure 6 below) rats using scanning laser confocal microscopy (Olympus Fluoview FV10i). Images were imported into Photoshop (Adobe Systems, Inc) where adjustments to brightness and contrast, as well as superimposition of the images, were performed for representation in the figures.
Figure 6.
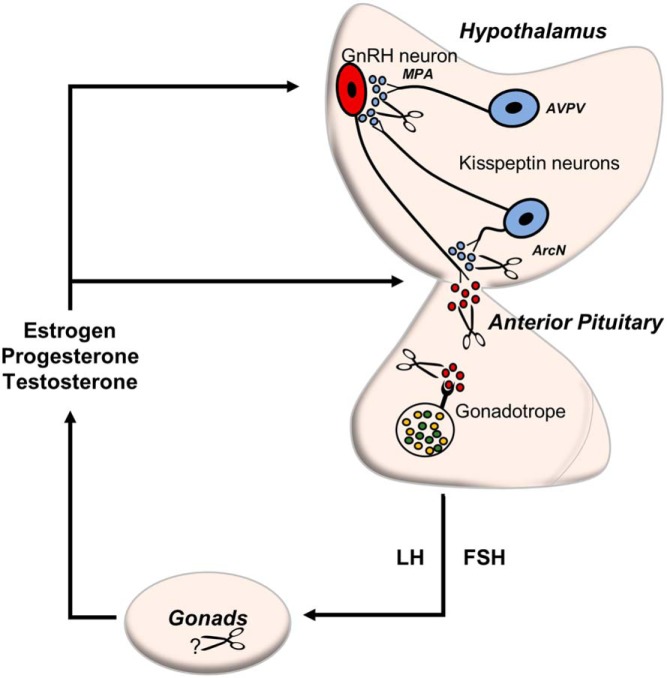
Schematic representation of EP24.15 in the HPG axis. EP24.15 (scissors) modulates the concentration and bioavailability of neuropeptides such as Kiss1 and GnRH by enzymatic processing of these peptides. Depicted here is a diagram proposing different levels where EP24.15 could influence the circuitry of the HPG axis. EP24.15 may cleave Kiss1 at axosomatic and axoaxonal junctures of GnRH neurons in the MPA and ArcN, respectively, in turn affecting GnRH levels into the ME. EP24.15 activity has been identified in the portal vasculature (22) and anterior pituitary (13), where it is positioned to influence the bioactivity of Kiss1 and GnRH. The secretion of the gonadotropins, LH and FSH, promote steroidogenesis in the gonads that feedback to regulate the axis on the level of the hypothalamus and pituitary. EP24.15 is highly expressed in the gonads (11, 13).
EP24.15 and GnRH
To assess the degree of colocalization of EP24.15 and GnRH we used the protocol described above for amplification of EP24.15 immunoreactivity followed by standard secondary antibody immunofluorescence. After staining for EP24.15, sections were incubated at 4°C for 72 hours in primary rabbit anti-GnRH (1:5000, catalog number ab5617; Abcam) in immunocytochemistry buffer with 4% NDS and visualized with Alexa Fluor 594 antirabbit 2° antibody (1:250; Life Technologies).
Primary antibody specificities
The specificity of EP24.15 antibody immunoreactivity (Supplemental Figure 1A) has been vetted previously (14), and here ascertained by the lack of immunoreactive signal after coincubation of the antibody with 10 μg/mL of EP24.15 protein (Supplemental Figure 1B) but not with EP24.16 (Supplemental Figure 1C). The Kiss1 antibody is directed against a 10-amino acid sequence of mouse metastin (Table 1). Characterization was previously executed using RIA to test cross-reactivity with various hypothalamic peptides and immunohistochemistry using antibody preincubated with immunizing peptide (28, 29). Franceschini et al (28) report no cross-reactivity, whereas True et al (29) suggest that there may be minimal cross-reactivity to RFamide-related peptide 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | DOI or Publication Data |
|---|---|---|---|---|---|---|
| Kiss1 | YNWNSFGLRY-NH2 | Anti-Kiss1 | Millipore, AB9754 | Rabbit; polyclonal | 1:1000 | 10.1016/j.neulet.2006.03.039 |
| EP24.15 | Anti-EP24.15 | Dr Marc J. Glucksman | Rabbit; polyclonal | 1:110 000 | 10.1016/S0006-8993 (99)02135-6 | |
| GnRH | Anti-GnRH | Abcam; ab5617 | Rabbit; polyclonal | 1:5000 | 10.1093/hmg/ddr216 |
Results
Kiss1 can potentially interact with the EP24.15 enzyme active site in silico
Enzymatic digestion of substrates by EP24.15 is not predicted by a specific consensus sequence but possesses a broad range of substrate specificity, as a result of the structural flexibility of the active site in the enzyme (30–32). EP24.15 exhibits some preference for cleavage of substrates with large hydrophobic or basic residues near the scissile bond (15–17). In silico structural analyses of the apoenzyme (PDB ID, 1S4B) revealed an open cleft in which short peptide segments may fully extend in an unfolded conformation (Figure 1A) (32). Molecular modeling indicated that Kiss-10 could be accommodated in the cleft with access to the active site (Figure 1B). Based on these characteristics and sequence similarity between known EP24.15 substrates, such as phenylalanine, serine, glycine, or aromatic residues surrounding the scissile bond (Figure 1C), it is reasonable to suggest that Kiss-10 is a putative EP24.15 substrate with binding in the active site.
Figure 1.
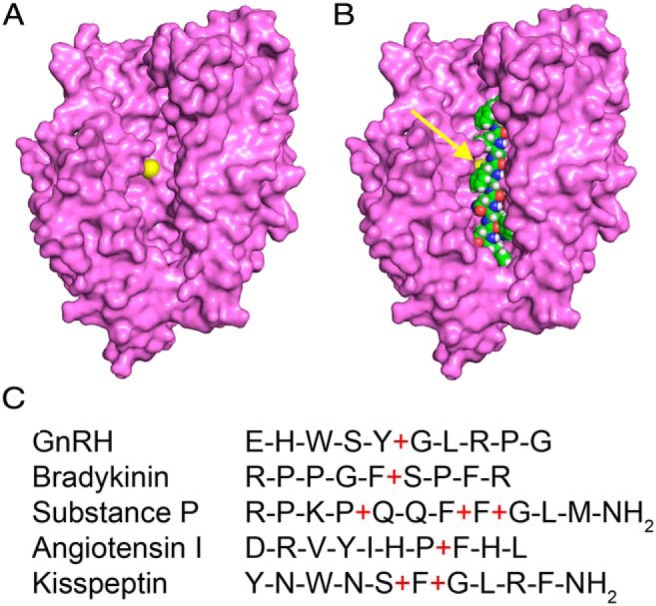
Kiss-10 is a putative substrate of EP24.15 based on in silico structural molecular modeling and substrate sequence similarity. A, The apoenzyme structure of EP24.15 (PDB ID, 1S4B) in an open conformation with the catalytic zinc highlighted in yellow. B, The apoenzyme structure with Kiss-10, highlighted in green, modeled into the active site cleft. C, Sequence similarities between known EP24.15 substrates and Kiss-10. Red plus signs (+) indicate cleavage sites.
Identification of the cleavage site of Kiss1 by EP24.15 digestion
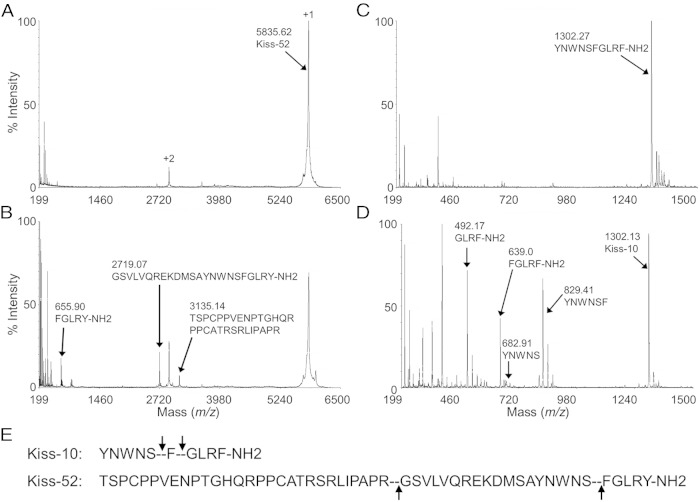
Kiss1/metastin, a 52 residue peptide that binds to Kiss1R, hereafter referred to as Kiss-52, was subjected to enzymatic digestion with EP24.15 (Figure 2, A and B). At t = 0 minutes (Figure 2A), both the +1 and +2 charged “parent” peptides were detected by mass spectrometry as expected at m/z of 5835.5 and 2918, respectively. At t = 15 minutes (Figure 2B) major cleavage fragments appeared at masses of 3135 and 2719.5, representing Kiss-52 (1–29) and (30–52), respectively, with cleavage at Arg29-Gly30 and a mass at 655.8, consistent with cleavage at Ser47-Phe48 (corresponding to the terminal pentapeptide FGLRF-NH2 of Kiss-10).
Figure 2.
Mass spectrometric analysis of enzymatic digestion of Kiss1/metastin and Kiss-10 by EP24.15. A, At t = 0 minutes, both the +1 and +2 charged parent peptides corresponding to the parent peak of Kiss1/metastin are present (arrows). B, At t = 15 minutes, major cleavage fragments appeared at masses of 3135.14 and 2719.07 representing cleavage products at Arg29-Gly30, and 655.90 the C-terminal cleavage at Ser47-Phe48 (corresponding to the terminal pentapeptide FGLRF-NH2). C, At t = 0 minutes, the parent peak corresponds to the intact full-length Kiss-10 (arrows). D, At t = 5 minutes, major cleavage fragments corresponding to: the last 5 amino acids of Kiss-10, FGLRF-NH2, the last 4 amino acids of Kiss-10, GLRF-NH2, and the first 6 amino acids of Kiss-10 YNWNSF with an m/z of 829. The N-terminal fragment, YNWNS, is a small peak due to the ionization by MALDI-TOF mass spectrometry. E, Sequences of Kiss1/metastin and Kiss-10 with their cleavage sites (dashed lines indicated by arrows).
In order to identify potential site(s) of cleavage of Kiss-10, a series of enzymatic digestions were performed by coincubating EP24.15 and Kiss-10. The resulting digestions were analyzed by MALDI-TOF mass spectrometry (Figure 2, C and D). At t = 0 minutes (Figure 2C), the observed mass to charge (m/z) ratio corresponded to intact full-length Kiss-10 (arrow). Coincubation of Kiss-10 and EP24.15 for 5 minutes resulted in 4 identifiable peptides: a diminished full-length Kiss-10 substrate fragment with an m/z of 1302, and appearances of: a peptide fragment with an m/z of 639 corresponding to the last 5 amino acids of Kiss-10, FGLRF-NH2, a peptide fragment with an m/z of 492 corresponding to the last 4 amino acids of Kiss-10, GLRF-NH2, and a peptide fragment of the complementary first 6 amino acids of Kiss-10 YNWNSF with an m/z of 829 (Figure 2D). This data demonstrated that EP24.15 cleaved Kiss-10 between Ser5 and Phe6, and Phe6 and Gly7, and Kiss-52 was also a substrate with cleavage occurring between Arg29 and Gly30, and Ser47 and Phe48 (corresponding to the terminal pentapeptide FGLRF-NH2 of Kiss-10) (Figure 2E).
Kinetic analysis of EP24.15-dependent Kiss1 cleavage
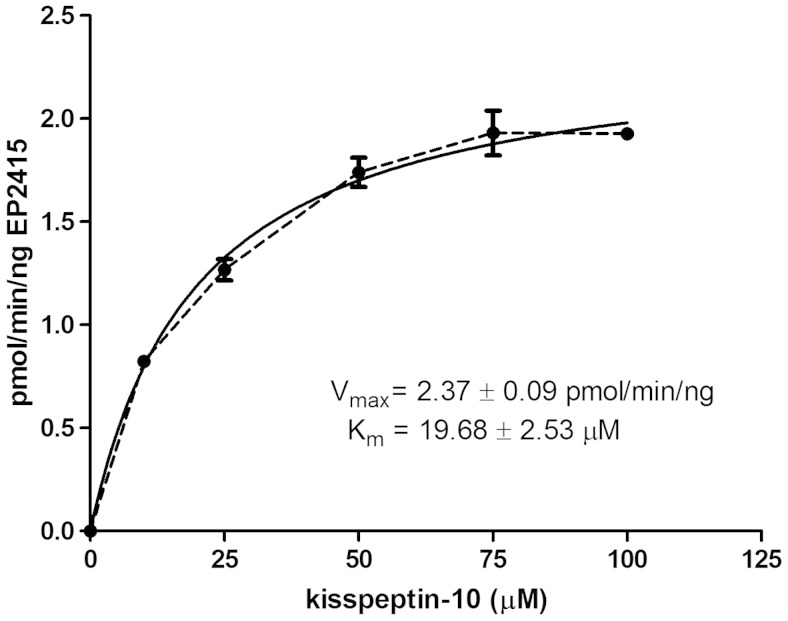
Enzyme kinetic reactions were used to determine whether the cleavage of Kiss-10 by EP24.15 proceeds in a physiologically relevant range. The kinetic parameters, Km and Vmax, were determined from initial velocity measurements at several different substrate concentrations (Figure 3). The Vmax for the reaction was 2.37 ± 0.09 pmol/min · ng with a Km of 19.68 ± 2.53μM, comparable with other known substrates of EP24.15.
Figure 3.
Kinetic assay of EP24.15-dependent cleavage of Kiss-10. Velocity vs substrate concentration (n = 6 independent determinations). The dashed line represents the experimental data, and the solid line represents the best fit curve.
EP24.15 immunoreactivity in the hypothalamus
EP24.15 immunoreactivity was ubiquitously expressed throughout all levels of the hypothalamus (Figure 4 and Supplemental Figures 2 and 3). Although immunoreactivity was apparent within the cell nucleus, staining was also visible within the cytoplasm. At the level of the ArcN EP24.15 immunoreactivity was evident within the ependymal cells lining the third ventricle (Figure 4, A–D) as well as in the proximal portion of tanycytic processes (Figure 4, D and E). Within the median eminence (ME), EP24.15 immunoreactivity was present within the internal and external zone, visualized in cell bodies, fibers, and punctate (Figure 4, A, C, and F, and Supplemental Figure 3). In the rostral hypothalamus, EP24.15 was expressed in cell bodies and fibers and also shared regions of colocalization with GnRH (Supplemental Figure 3). Supplemental Figure 2F (arrow) shows a GnRH cell body with EP24.15 immunoreactivity in the nucleus.
Figure 4.
Confocal microscopy images of EP24.15 immunoreactivity in the ArcN and ME of a male rat. A, Distribution of EP24.15 was evident within the nucleus of the cell as well as in fibers and tanycytes (scale bar, 200 μm). B, Higher magnification of A highlighting EP24.15 immunoreactivity in ependymal cells and tanycytic processes (arrows; scale bar, 50 μm). C, Ependymal cells lining the third ventricle in the internal zone of the ME exhibited EP24.15 immunoreactivity (scale bar, 50 μm). D, EP24.15 immunoreactivity is present within tanycytic processes proximal to the third ventricle (arrows; scale bar, 50 μm). E, Higher magnification of D highlighting EP24.15 immunoreactivity in tanycytic processes (arrows; scale bar, 30 μm). F, EP24.15-immunoreactive punctate is distributed throughout the external ME (scale bar, 20 μm). MEint, internal ME; MEext, external ME; 3V, third ventricle.
Colocalization of EP24.15 and Kiss1 in vivo
Within the hypothalamus Kiss1-immunoreactive neurons were predominately located in the ArcN (Figure 5). Although Kiss1 gene expression is present within the anteroventral-periventricular (AVPV) region (33, 34), immunoreactive cell bodies for Kiss1 are not readily evident (Supplemental Figure 4), although there is a preponderance of Kiss1-immunoreactive fibers. In rodents, these cell populations are differentially regulated by gonadal steroids: elevated levels of estrogen increase Kiss1 gene expression in the AVPV and reduce it in the ArcN (33, 34). To this end, intact female rats in either metestrus or proestrus were used to optimize the amount of Kiss1 immunoreactivity detected in the ArcN and AVPV, respectively, so that the degree of coexpression with EP24.15 could be determined without using colchicine treatment. Kiss1 immunoreactivity was present throughout the ArcN (Figure 5, B and E) with more cells noted in the posterior regions of the ArcN, consistent with recent observations (data not shown) (35). Additionally, Kiss1 immunoreactivity was notably present throughout the neuropil of the ArcN and in beaded axonal-like projections (Figure 5H) characteristic of neuropeptide neurons. EP24.15 immunoreactivity was also dispersed throughout the ArcN, most notably within individual cell nuclei (Figure 5D) although it was also noted in fibers (Figure 5G) and tanycytes (Figure 5A). Within the ArcN, there was considerable overlap and coexpression of Kiss1 and EP24.15 immunoreactivities throughout the extent of the nucleus (Figure 5, C, F, and I). Coexpression of the 2 antigens was demonstrated in the ArcN with EP24.15 immunoreactivity concentrated in the nucleus and Kiss1 immunoreactivity predominantly in the cell soma and fibers (Figure 5, D–F, arrows). Figure 5, G–I, arrows, depicts a fiber in the dorsal ArcN displaying Kiss1 and EP24.15 colocalization. Within the AVPV, Kiss1 immunoreactivity was predominately identified in fibers and punctate (Supplemental Figure 4). EP24.15 immunoreactivity was present within cells scattered throughout the AVPV and lining the third ventricle (Supplemental Figure 4A). When compared with the ArcN, the distribution of EP24.15 immunoreactivity was more sparsely distributed. Again in contrast to the ArcN, there was no apparent colocalization or apposition of Kiss1 and EP24.15 in the rostral hypothalamus (Supplemental Figure 4C).
Discussion
These studies demonstrate both the physiochemical feasibility and the functional neuroanatomical circuitry whereby the actions of Kiss1 may be regulated by the neuropeptide processing enzyme EP24.15. In addition to cleavage of Kiss-10 by EP24.15 in vitro, demonstrated by mass spectrometric studies and analysis of enzyme-substrate kinetics, was the cleavage of Kiss-52 with peptide products at Arg29-Gly30 and Ser47-Phe48 (corresponding to the Kiss-10 terminal pentapeptide FGLRF-NH2). The coexpression of EP24.15 and Kiss1 in the ArcN implies that Kiss1 is a substrate for EP24.15 in vivo. Over the last decade Kiss1 has emerged as a critical component in the regulation of reproductive function in conjunction with the classical actions of GnRH. Even more compelling is the already established role of EP24.15 in regulating GnRH actions (15, 17–21). This present study bridges a gap in knowledge regarding the processing of Kiss1 as a potential means of terminating its postsynaptic actions and controlling extracellular peptide concentrations. It was determined that EP24.15 cleaved Kiss-10 between Ser5 and Phe6 as well as Phe6 and Gly7 with enzymatic rates and affinities similar to those of known EP24.15 substrates (26, 36). Furthermore, regions of colocalization of EP24.15 and Kiss1 within the hypothalamus were identified, suggesting that EP24.15 may cleave Kiss1 in vivo as well as in vitro.
Computer-aided structural analyses of the EP24.15 apoenzyme revealed a large cleft in the active site where a peptide could bind (32). The computational molecular model in Figure 1 demonstrates that, based on size, Kiss-10 would be able to fit completely into the cleft and access the active site where substrate binding and catalysis are known to occur. It is likely that upon substrate binding the enzyme undergoes a conformational change to induce strain on the peptide backbone to facilitate hydrolysis of the peptide bond. EP24.15 is known to cleave a variety of peptide substrates including, but not limited to, GnRH, neurotensin, angiotensin I and II, dynorphin A1–8, and somatostatin (15, 17, 19, 37). Sequence comparison of known EP24.15 substrates revealed the presence of large hydrophobic residues, such as Phe, at the P1 or P1′ position relative to the scissile bond. Mass spectrometric and kinetic analyses of the products of the EP24.15 and Kiss-52 reactions identified 2 major cleavage sites resulting in product peaks that corresponded to the terminal 5 amino acids of Kiss-10, FGLRF-NH2, the C-terminal 4 amino acids of Kiss-10, GLRF-NH2, and the complementary peptide of the first 6 amino acids of Kiss-10: YNWNSF (Figure 2D). Based on structural and spectrometric data it is, therefore consistent that EP24.15 cleaves Kiss1 on the amino-terminal side of both Phe6 and Gly7 of Kiss-10.
The focus of most experimental paradigms is on Kiss-10, the shortest peptide sequence that encapsulates the functional aspects of full-length Kiss1 with respect to receptor binding and the form utilized in the vast majority of experimental studies and trials (3–5). Using Kiss-10 as a substrate there is an additional cleavage site noted between the Phe6 and Gly7, identical to substance P and very similar to GnRH (Tyr-Gly cleaved by EP24.15). Mutational analysis or truncations of Kiss-10 reveal greatly reduced receptor affinity and activation (38–40). Interestingly, the Ser5 and Phe6 residues are essential for Kiss1R receptor activation (38, 40). From these current studies, it can be assumed that cleavage of Kiss-10 by EP24.15 results in decreased binding affinity for the receptor and hence termination of its signaling mechanisms. However, it would be remiss to suggest that EP24.15 is the only enzyme that regulates Kiss1 activity. It is likely that several peptidases, including the zinc-dependent matrix metalloproteinases as Takino et al (10) identified, participate in the digestion of Kiss1. A homologous zinc metallopeptidase to EP24.15, EP24.16 (neurolysin; EC 3.4.24.16), shares specificity for many of the same neuropeptide substrates. Indeed, EP24.16 does not discernably cleave metastin and Kiss-10 is a very poor substrate for EP24.16 with processing of the peptide requiring enzyme concentrations at least 25-fold higher than EP24.15 (data not shown).
In order for Kiss1 to be a suitable substrate for EP24.15 in vivo, the enzyme must not only be coincident spatially but also cleave the peptide at rates that are physiologically appropriate. Kinetic data demonstrated that EP24.15 cleaves Kiss1 with parameters consistent with the known EP24.15 substrates GnRH and neurotensin (26, 36). Furthermore, regions of EP24.15 and Kiss1 coexpression were identified in the hypothalamus of female rats. Although distribution of the individual immunostaining patterns for Kiss1 and for EP24.15 separately are consistent with previous immunohistochemical reports (12, 22, 41–43) this is the first study to demonstrate the anatomical association of Kiss1 and EP24.15 immunoreactivities within the hypothalamus. Coexpression of Kiss1 and EP24.15 throughout the ArcN suggests that the enzyme and putative substrate are indeed in close proximity and presents a functional circuit in vivo whereby the activity of Kiss1 could be modulated within the ArcN as well as the ME by EP24.15. Compared with the ArcN, there were no clear examples of apparent colocalization in the AVPV which may be a direct result of the reduced ability to detect Kiss1-immunoreactive cell bodies within this region. Previous studies were also unable to detect Kiss1 immunoreactivity in cell bodies of the AVPV in female rats (41, 43), although Kiss1 mRNA levels in this region have been identified (33, 44). Therefore, the possibility of EP24.15 and Kiss1 colocalization in the AVPV cannot be excluded. Kiss1 neurons in the AVPV have projections that extend to the cell bodies of GnRH neurons in the medial preoptic area (MPA) (45). Not surprisingly, EP24.15 immunoreactivity is apparent in the MPA and exhibits colocalization with GnRH neurons (Supplemental Figure 2). Regions of colocalization of GnRH and EP24.15 immunoreactivity are also present in both the internal and external zone of the ME (Supplemental Figure 3). As previously mentioned, EP24.15 immunoreactivity is also present within tanycytes adjacent to the third ventricle. Tanycytes are specialized cells that act as critical regulators of neuroendocrine secretion into the portal vasculature. In particular, tanycytes are associated with the release of GnRH into the perivascular space via structural remodeling of tanycytic-terminal contacts (46, 47). This structural remodeling has been furthered in recent studies by Parkash et al (47) which demonstrate that members of the semaphorin protein family modulate estrous cycle-induced alterations in the morphology of tanycytes and GnRH nerve terminals. These changes comprise the elongation of GnRH axons as well as outgrowth of tanycytic endfeet, both of which are associated with appropriate secretion of reproductive hormones. To add to this neuroendocrine circuitry, d'Anglemont de Tassigny et al (48) demonstrated that application of Kiss1 to organotypic cultures of mediobasal hypothalamus directly stimulates GnRH secretion nerve terminals. Thus, this may suggest that EP24.15 could potentially regulate both Kiss1 and GnRH concentrations before their release into the perivascular space. It can be inferred from this data that EP24.15 cleaves both Kiss1 and GnRH at nerve terminals in both the MPA and ME, providing multilayered regulatory mechanisms for these neuropeptides within the mediobasal hypothalamus and HPG axis (Figure 6).
Although Kiss1 immunoreactivity demonstrated patterns typical for that of neuropeptides, immunoreactivity for EP24.15 was present in fibers and tanycytes, as well as in the soma. Although cytoplasmic staining was observed, strong immunoreactivity was present in the nuclear compartment, as previously reported (12, 14, 42). The presence of EP24.15 within other cellular compartments (fibers, tanycytes) supports the postulation that the enzyme modulates extracellular levels of Kiss1. Neuropeptides, unlike classical neurotransmitters, are processed through the regulated secretory pathway. They are stored in secretory granules and released into the synaptic cleft in response to a secretagogue free to bind their cognate receptor. In order for neuropeptide processing enzymes, like EP24.15, to act upon their substrates they must be localized to the same subcellular or extracellular space. Although evidence implicates transport of EP24.15 and Kiss1 along a nerve fiber (Figure 5, G–I), it is unknown whether they are indeed localized to the same secretory vesicles. Studies by Garrido et al (49) identify EP24.15 in vesicles containing ACTH, which supports that EP24.15 may be targeted to vesicles of the regulated secretory pathway. Additionally, EP24.15 is localized to the lipid bilayer (42, 50) and may be associated with, and released from, lipid rafts (51, 52). Thus, it is probable that EP24.15 interacts with neuropeptide substrates released into the extracellular space.
Kiss1 is associated with a myriad of actions within the hypothalamus, many of which are associated with the timing of puberty, regulation of ovulatory cycles, ovulation, and the development of polycystic ovarian syndrome (reviewed in Ref. 53). This association of EP24.15 and Kiss1 is important as it suggests that yet another peptide associated with reproductive hormone secretion, in addition to GnRH, may be regulated by EP24.15 affording exquisite fine tuning of hormone levels required for the orchestration of reproduction. Furthermore, it has been suggested that EP24.15 itself may be under hormonal regulation (54, 55). This begins to elucidate a network for the intricate control of neuropeptide concentrations via EP24.15. Previous studies have shown that inhibiting the action of EP24.15 promotes an increase in the plasma levels of LH and FSH proposed to be a result of increased GnRH bioavailability (20–22). At the time of those initial studies, Kiss1 had not yet been identified. Taking the present study into consideration, it is likely that the increase in LH and FSH levels after inhibition of EP24.15 may be in response to elevations in both GnRH and Kiss1 bioavailability through decreased degradation of both neuropeptides. Presently, Kiss1 and its analogs are being explored as therapeutic agents for reproductive disorders (56, 57) and in vitro fertilization (58). EP24.15-dependent cleavage of Kiss, along with the actions of other endopeptidases, may be responsible for establishing the half-life of Kiss1. Therefore, inhibition of EP24.15 may result in a longer half-life of Kiss1, which warrants further study. Additionally, the degradation of Kiss1 by EP24.15 is now another variable to be considered when designing studies involving the use of Kiss-10 or related analogs; Kiss-10 is more frequently used than the full-length Kiss1 in in vitro and in vivo laboratory studies.
In conclusion, this study provides evidence that EP24.15 cleaves Kiss-10 in vitro and immunohistochemical data suggests that EP24.15 may cleave Kiss-10 in vivo as well. Understanding the regulation of Kiss, given its role in the regulation of reproduction, is critical to the development of novel therapeutic targets for the treatment of infertility. Further understanding the role of EP24.15 in hypothalamic peptide metabolism will provide insight into the intricate circuitry within the mediobasal hypothalamus and provide additional targets for modulating hyper- or hypo-activity of various neuroendocrine axes, including reproductive function.
Acknowledgments
We thank Dr Xinli Yang of the Midwest Proteome Center and Ms Gina DeJoseph and Ms Melanie Kramer for technical assistance.
This work was supported by the National Institutes of Health Grant OD010662 (to M.J.G.) and by a pilot grant from the Rosalind Franklin University of Medicine and Science (J.H.U. and M.J.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ArcN
- arcuate nucleus
- AVPV
- anteroventral-periventricular
- EP24.15
- endopeptidase 24.15
- GnRH
- gonadotropin releasing hormone
- HPG
- hypothalamic-pituitary gonadal
- Kiss1
- kisspeptin
- Kiss1R
- Kisspeptin receptor
- LH
- luteinizing hormone
- MALDI
- matrix-assisted laser desorption ionization
- ME
- median eminence
- MPA
- medial preoptic area
- MS
- mass spectrometry
- NDS
- normal donkey serum
- TFA
- trifluoroacetic acid
- TOF
- time of flight.
References
- 1. Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324(1–2):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–1737. [DOI] [PubMed] [Google Scholar]
- 3. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. [DOI] [PubMed] [Google Scholar]
- 4. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. [DOI] [PubMed] [Google Scholar]
- 5. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. [DOI] [PubMed] [Google Scholar]
- 6. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 7. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. d'Anglemont de Tassigny X, Fagg LA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. [DOI] [PubMed] [Google Scholar]
- 10. Takino T, Koshikawa N, Miyamori H, et al. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22(30):4617–4626. [DOI] [PubMed] [Google Scholar]
- 11. Pineau C, McCool S, Glucksman MJ, Jégou B, Pierotti AR. Distribution of thimet oligopeptidase (E.C. 3.4.24.15) in human and rat testes. J Cell Sci. 1999;112:3455–3462. [DOI] [PubMed] [Google Scholar]
- 12. Healy DP, Orlowski M. Immunocytochemical localization of endopeptidase 24.15 in rat brain. Brain Res. 1992;571(1):121–128. [DOI] [PubMed] [Google Scholar]
- 13. Pierotti AR, Lasdun A, Ayala JM, Roberts JL, Molineaux CJ. Endopeptidase-24.15 in rat hypothalamic/pituitary/gonadal axis. Mol Cell Endocrinol. 1991;76:95–103. [DOI] [PubMed] [Google Scholar]
- 14. Massarelli EE, Casatti CA, Kato A, et al. Differential subcellular distribution of neurolysin (EC 3.4.24.16) and thimet oligopeptidase (EC 3.4.24.15) in the rat brain. Brain Res. 1999;851:261–265. [DOI] [PubMed] [Google Scholar]
- 15. Chu TG, Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985;116(4):1418–1425. [DOI] [PubMed] [Google Scholar]
- 16. Horsthemke B, Bauer K. Substrate specificity of an adenohypophyseal endopeptidase capable of hydrolyzing luteinizing hormone-releasing hormone: preferential cleavage of peptide bones involving the carboxyl terminus of hydrophobic and basic amino acids. Biochemistry. 1982;21(5):1033–1036. [DOI] [PubMed] [Google Scholar]
- 17. Orlowski M, Michaud C, Chu TG. A soluble metalloendopeptidase from rat brain. Purification of the enzyme and determination of specificity with synthetic and natural peptides. Eur J Biochem. 1983;135:81–88. [DOI] [PubMed] [Google Scholar]
- 18. Camargo AC, Gomes MD, Reichl AP, et al. Structural features that make oligopeptides susceptible substrates for hydrolysis by recombinant thimet oligopeptidase. Biochem J. 1997;324:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molineaux CJ, Lasdun A, Michaud C, Orlowski M. Endopeptidase-24.15 is the primary enzyme that degrades luteinizing hormone-releasing hormone both in vitro and in vivo. J Neurochem. 1988;51:624–633. [DOI] [PubMed] [Google Scholar]
- 20. Lasdun A, Reznik S, Molineaux CJ, Orlowski M. Inhibition of endopeptidase 24.1 5 slows the in vivo degradation of luteinizing hormone-releasing hormone. J Pharmacol Exp Ther. 1989;251(2):439–447. [PubMed] [Google Scholar]
- 21. Lasdun A, Orlowski M. Inhibition of endopeptidase 24.15 greatly increases the release of luteinizing hormone and follicle stimulating hormone in response to luteinizing hormone/releasing hormone. J Pharmacol Exp Ther. 1990;253(3):1265–1271. [PubMed] [Google Scholar]
- 22. Wu TJ, Pierotti AR, Jakubowski M, et al. Endopeptidase EC 3.4.24.15 presence in the rat median eminence and hypophysial portal blood and its modulation of the luteinizing hormone surge. J Neuroendocrinol. 1997;9:813–822. [DOI] [PubMed] [Google Scholar]
- 23. PyMol Molecular Graphics System, version 1.3r1. 2010; Schrödinger LLC.
- 24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 25. Scholle MD, Kriplani U, Pabon A, Sishtla K, Glucksman MJ, Kay BK. Mapping protease substrates by using a biotinylated phage substrate library. Chem Biochem. 2006;7(5):834–838. [DOI] [PubMed] [Google Scholar]
- 26. Cummins PM, Pabon A, Margulies EH, Glucksman MJ. Zinc coordination and substrate catalysis within the neuropeptide processing enzyme endopeptidase EC 3.4.24.15. Identification of active site histidine and glutamate residues. J Biol Chem. 1999;274(23):16003–16009. [DOI] [PubMed] [Google Scholar]
- 27. Urban JH, Leitermann RJ, DeJoseph MR, Somponpun SJ, Wolak ML, Sladek CD. Influence of dehydration on the expression of neuropeptide Y Y1 receptors in hypothalamic magnocellular neurons. Endocrinology. 2006;147:4122–4131. [DOI] [PubMed] [Google Scholar]
- 28. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 29. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray K, Hines CS, Rodgers DW. Mapping sequence differences between thimet oligopeptidase and neurolysin implicates key residues in substrate recognition. Protein Sci. 2002;11(9):2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruce LA, Sigman JA, Randall D, et al. Hydrogen bond residue positioning in the 599–611 loop of thimet oligopeptidase is required for substrate selection. FEBS J. 2008;275(22):5607–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ray K, Hines CS, Coll-Rodriguez J, Rodgers DW. Crystal structure of human thimet oligopeptidase provides insight into substrate recognition, regulation, and localization. J Biol Chem. 2004;279(19):20480–20489. [DOI] [PubMed] [Google Scholar]
- 33. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–1783. [DOI] [PubMed] [Google Scholar]
- 34. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 35. Overgaard A, Ruiz-Pino F, Castellano JM, Tena-Sempere M, Mikkelsen JD. Disparate changes in kisspeptin and neurokinin B expression in the arcuate nucleus after sex steroid manipulation reveal differential regulation of the two KNDy peptides in rats. Endocrinology. 2014;155(10):3945–3955. [DOI] [PubMed] [Google Scholar]
- 36. Tullai JW, Cummins PM, Pabon A, et al. The neuropeptide processing enzyme EC 3.4.24.15 is modulated by protein kinase A phosphorylation. J Biol Chem. 2000;275(47):36514–36522. [DOI] [PubMed] [Google Scholar]
- 37. Dando PM, Brown MA, Barrett AJ. Human thimet oligopeptidase. Biochem J. 1993;249:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niida A, Wang Z, Tomita K, et al. Design and synthesis of downsized metastin (45–54) analogs with maintenance of high GPR54 agonistic activity. Bioorg Med Chem Lett. 2006;16(1):134–137. [DOI] [PubMed] [Google Scholar]
- 40. Gutiérrez-Pascual E, Leprince J, Martínez-Fuentes AJ, et al. In vivo and in vitro structure-activity relationships and structural conformation of kisspeptin-10-related peptides. Mol Pharmacol. 2009;76(1):58–67. [DOI] [PubMed] [Google Scholar]
- 41. Desroziers E, Mikkelsen J, Simonneaux V, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22(10):1101–1112. [DOI] [PubMed] [Google Scholar]
- 42. Fontenele-Neto JD, Massarelli EE, Gurgel Garrido PA, Beaudet A, Ferro ES. Comparative fine structural distribution of endopeptidase 24.15 (EC3.4.24.15) and 24.16 (EC3.4.24.16) in rat brain. J Comp Neurol. 2001;438:399–410. [DOI] [PubMed] [Google Scholar]
- 43. Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45:85–90. [DOI] [PubMed] [Google Scholar]
- 44. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prevot V, Croix D, Bouret S, et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94(3):809–819. [DOI] [PubMed] [Google Scholar]
- 47. Parkash J, Messina A, Langlet F, et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat Commun. 2015;6:6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garrido PA, Vandenbulcke F, Ramjaun AR, et al. Confocal microscopy reveals thimet oligopeptidase (EC 3.4.24.15) and neurolysin (EC 3.4.24.16) in the classical secretory pathway. DNA Cell Biol. 1999;18(4):323–331. [DOI] [PubMed] [Google Scholar]
- 50. Oliveira V, Garrido PA, Rodrigues CC, et al. Calcium modulates endopeptidase 24.15 (EC 3.4.24.15) membrane association, secondary structure and substrate specificity. FEBS J. 2005;272(12):2978–2992. [DOI] [PubMed] [Google Scholar]
- 51. Jeske NA, Glucksman MJ, Roberts JL. EP24.15 is associated with lipid rafts. J Neurosci Res. 2003;74:468–473. [DOI] [PubMed] [Google Scholar]
- 52. Jeske NA, Glucksman MJ, Roberts JL. Metalloendopeptidase EC3.4.24.15 is constitutively released from the exofacial leaflet of lipid rafts in GT1–7 cells. J Neurochem. 2004;90(4):819–828. [DOI] [PubMed] [Google Scholar]
- 53. Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cyr NE, Kua LH, Bruce LA, Chadwick JG, Tetel MJ, Wolfson AJ. Nuclear thimet oligopeptidase is coexpressed with oestrogen receptor α in hypothalamic cells and regulated by oestradiol in female mice. J Neuroendocrinol. 2010;22(8):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bruce LA, Cyr NE, Qiao JW, Defries CC, Tetel MJ, Wolfson AJ. Neuropeptidase activity is down-regulated by estradiol in steroid-sensitive regions of the hypothalamus in female mice. Neuropeptides. 2012;46(4):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jayasena CN, Nijher GM, Chaudhri OB, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;94(11):4315–4323. [DOI] [PubMed] [Google Scholar]
- 57. Sonigo C, Bouilly J, Carré N, et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122(10):3791–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abbara A, Jayasena C, Comninos A, et al. Kisspeptin: a novel physiological trigger for oocyte maturation in in-vitro fertilisation treatment. Lancet. 2014;383:S17. [Google Scholar]