Abstract
Clinical studies indicate alternate-day, intermittent fasting (IMF) protocols result in meaningful weight loss in obese individuals. To further understand the mechanisms sustaining weight loss by IMF, we investigated the metabolic and neural alterations of IMF in obese mice. Male C57/BL6 mice were fed a high-fat diet (HFD; 45% fat) ad libitum for 8 weeks to promote an obese phenotype. Mice were divided into four groups and either maintained on ad libitum HFD, received alternate-day access to HFD (IMF-HFD), and switched to ad libitum low-fat diet (LFD; 10% fat) or received IMF of LFD (IMF-LFD). After 4 weeks, IMF-HFD (∼13%) and IMF-LFD (∼18%) had significantly lower body weights than the HFD. Body fat was also lower (∼40%–52%) in all diet interventions. Lean mass was increased in the IMF-LFD (∼12%–13%) compared with the HFD and IMF-HFD groups. Oral glucose tolerance area under the curve was lower in the IMF-HFD (∼50%), whereas the insulin tolerance area under the curve was reduced in all diet interventions (∼22%–42%). HPLC measurements of hypothalamic tissue homogenates indicated higher (∼55%–60%) norepinephrine (NE) content in the anterior regions of the medial hypothalamus of IMF compared with the ad libitum-fed groups, whereas NE content was higher (∼19%–32%) in posterior regions in the IMF-LFD group only. Relative gene expression of Npy in the arcuate nucleus was increased (∼65%–75%) in IMF groups. Our novel findings indicate that intermittent fasting produces alterations in hypothalamic NE and neuropeptide Y, suggesting the counterregulatory processes of short-term weight loss are associated with an IMF dietary strategy.
Calorie restriction is the most widely prescribed and self-imposed strategy for treating excessive weight gain and obesity (1–3). In the United States, most common commercial programs for calorie reduction include reducing daily caloric intake by portion control, low-calorie meals, and/or meal-replacement options (1). Rather than reducing daily total caloric intake, intermittent fasting (IMF) has received attention as a possible approach for long-term weight loss (4). Although varying in the period of fasting (eg, alternate day fasting or once/twice a week fasting days), IMF protocols have a similar advantage in that bouts of unrestricted eating occur after fasting periods (5, 6). Several human studies have indicated that a short-term (ie, 8–24 wk) IMF protocol results in weight loss (ie, 3%–8%) in overweight or obese subjects (5–13). Weight loss occurs over several weeks because, despite overeating on refeeding days, individuals do not fully compensate for the calorie-deficit realized on the fasting days (6). One appealing feature of IMF protocols is that dieters do not have to count calories during the bouts of unrestricted eating (4). However, one common obstacle to the long-term adherence to IMF is intense feelings of hunger during the fasting periods (14). These subjective feelings of hunger can be mitigated by reducing the period of fasting or providing a small meal (10).
The influence of IMF on the hypothalamic control of energy homeostasis in obesity provides an investigative avenue from which research-based strategies to reduce hunger during fasting periods may be elucidated. In normal-weight individuals, energy homeostasis, and subsequently perceived hunger, is tightly controlled through various peripheral and central signaling factors. The hypothalamus is considered one of the central regulatory regions in this regard and responds directly to peripheral signals as well as to inputs from hindbrain noradrenergic nuclei (A1 and A2) (15). Within the hypothalamus, the arcuate nucleus (ARC), the paraventricular nucleus (PVN), and the ventromedial nucleus (VMH) are involved in energy homeostasis (16, 17). The ARC contains orexigenic neuron populations, including neuropeptide Y (NPY)/agouti-related protein (AgRP)-expressing neurons, and anorexigenic neuron populations, including proopiomelanocortin (POMC)-expressing neurons (16, 17). The PVN contains both CRH- and TRH-expressing neurons. Elevations in CRH and TRH levels have been shown to decrease food intake and reduce body weight (18, 19). Among the circulating peripheral modulators of energy homeostasis are a number of peptide hormones whose receptors can be found in these hypothalamic nuclei. One example is ghrelin, which is a gastrointestinal peptide that stimulates feeding and promotes positive energy balance (16). Ghrelin exerts its orexigenic response by stimulating NPY/AgRP neurons and simultaneously inhibiting the POMC neurons (17). Alternatively, the adipokine leptin functions to decrease food intake by inhibiting NPY/AgRP while stimulating POMC neurons (17). Although the actions of these peripheral and central signals are well defined in nonobese and lean animals, the role of these signals are diminished or attenuated in states of excess weight gain and obesity.
Obesity results in distinct neural and metabolic alterations that support overconsumption and weight gain (20–22). For instance, leptin and insulin resistance, dysregulation of hypothalamic neuropeptides, and reduced satiety signals are some of the broad physiological impairments that accompany diet-induced obesity (DIO) (20, 23–26). As such, physiological changes that result in lower body weight in nonobese or lean phenotypes do not accurately represent the mechanisms of weight loss in obesity. Despite the wealth of animal studies examining how IMF improves markers for aging (27, 28), cognitive performance (29–31), and immune responses (32, 33), there are no studies in obese animals to determine how IMF promotes weight loss. An understanding of the neural and metabolic alterations that promote weight loss by IMF in obese animals can provide greater insight into developing research-based modifications to IMF protocols to reduce hunger, increase long-term compliance, and enhance maintenance of weight loss.
The goal of this study was to examine the central and peripheral changes in response to IMF in a DIO model. C57 male mice at postnatal date (PND) 49 were fed a high-fat diet (HFD; 45% fat) ad libitum for 8 weeks. After this 8-week period, mice were either maintained on an ad libitum HFD, received IMF of HFD (IMF-HFD), switched to an ad libitum low-fat diet (LFD; 10% fat), or received IMF of LFD (IMF-LFD). Although other DIO protocols have used an extended period of high-fat feeding (≥12 wk) (34), the rationale for the 8-week initial HFD feeding study was to model the target population of overweight and obese individuals that have reported the most beneficial weight loss with intermittent fasting protocols. We hypothesized that, despite being on a HFD, mice fed IMF-HFD would display improved glucose metabolism, enhanced metabolic profiles, and distinct monoamine signaling in the hypothalamus comparably with LFD and IMF-LFD groups.
Materials and Methods
Animals
Male C57BL/6 mice (n = 64) were purchased from The Jackson Laboratory. At PND 49, all were fed an ad libitum, HFD (4.73 kcal/g, 45% fat, 20% protein, 35% carbohydrate; D12451) for 8 weeks. Mice were then equally divided by body weight and transitioned to one of four experimental groups as follows: ad libitum HFD, IMF of HFD (IMF-HFD), ad libitum LFD (3.85 kcal/g, 10% fat, 20% protein, 70% carbohydrate; D12450B), or IMF of LFD (IMF-LFD). All diets were obtained from Research Diets. IMF mice were food deprived every other 24-hour period beginning at 9:00 am (fasting day), 2 hours into the light cycle. On fasting days, all animals were weighed, food intake was recorded, and cages were changed. Mice were pair housed and maintained on a 12-hour light, 12-hour dark cycle; lights on from 7:00 am to 7:00 pm. All procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University.
Body composition and respiratory exchange ratio (RER)
Body composition was assessed using the EchoMRI 3-in-1 body composition analyzer (Echo Medical Systems) in all mice. The Comprehensive Lab Animal Monitoring System (Columbus Instruments), an indirect calorimeter, was used to measure O2 consumption (v.O2), CO2 production (v.CO2), and RER (v.CO2/v.O2). Mice were maintained on their respective feeding protocols and housed in the system for 48 hours, beginning on a fast day for IMF-HFD and IMF-LFD mice. The second 24-hour epoch (feeding day for IMF mice) was used for analysis.
Oral glucose and insulin tolerance tests
An oral glucose tolerance test (OGTT) and an insulin tolerance test were performed on all groups. For IMF-HFD and IMF-LFD animals, these were performed on fasting days and food was not replaced after testing. Six hours prior to the OGTT, all mice were placed in clean cages, weighed, and food deprived. At the start of the test, mice were placed in Plexiglas restrainers, and a tail nick was performed to obtain a baseline glucose reading using a glucometer (AlphaTRAK 2). Immediately thereafter, mice were gavaged with a bolus of glucose (2.0 g/kg body weight) and placed in an individual clean cage without food and water. Blood samples were collected from the tail in their individual cages at 15, 30, 60, 90, 120, and 180 minutes after the gavage. After 180 minutes, all mice were returned to their home cages, water was replaced, and food was returned to HFD and LFD animals. After sufficient recovery (2–3 d), an insulin tolerance test was performed after a fast in a similar manner as the OGTT with an ip injection of insulin (0.75 U/kg). Blood samples were collected from the tail in their individual cages at 15, 30, 60, 90, and 120 minutes after the injection.
Plasma hormones
After a 5-hour fast (fast day for IMF-HFD and IMF-LFD), animals were euthanized by decapitation. Blood was collected; a protease inhibitor, 4-(2-asminoethyl) benzenesulfonyl fluoride hydrochloride, at 1 mg/mL, was added to each sample, and samples were maintained on ice until centrifugation at 3000 rpm for 10 minutes at 4°C. Plasma was stored at −80°C until analysis. Insulin, ghrelin (active), and leptin were determined by multiplex assay (EMD Millipore). A RIA was performed to determine plasma corticosterone (sensitivity: 25 ng/mL; MP Biomedicals) levels.
Biogenic amines
Brain samples were dissected from the anterior (containing the anterior hypothalamus and the paraventricular hypothalamus) and posterior portions of the medial hypothalamus (containing the ARC and the VMH). Biogenic amines for each brain section were extracted and analyzed as previously described by reverse-phase HPLC (Dionex Ultimate 3000; Thermo Fisher Scientific) with electrochemical detection (Coulochem III; Thermo Fisher Scientific) (35). An acetonitrile-based phosphate buffer mobile phase (MD-TM; Thermo Fisher Scientific) was used for all experiments. The internal standard, 3,4-dihydroxybenzylamine, was added to all samples prior to extraction. Quantification of norepinephrine (NE), epinephrine (E), dopamine (DA), and serotonin (5-HT), plus metabolites homovanillic acid (HVA) and 5-hydroxyindoleacetic acid, was determined by Chromeleon 7.1 software (ThermoFisher). Values were expressed as picograms divided by wet tissue weight (milligrams) of each sample.
Tissue dissections for quantitative real-time PCR (qPCR)
Hypothalamic nuclei were microdissected for RNA extraction and gene expression analysis. The PVN, ARC, and VMH were cut into 1-mm coronal slices using a brain matrix (Ted Pella, Inc), anterior (bregma: −0.70 to −1.34 mm) and posterior (bregma: −1.35 to −1.94 mm) (36). The brain blocks were transferred to RNAlater (Life Technologies, Inc) and stored overnight at 4°C. Samples were dissected from slices using a dissecting microscope. The dissected tissue was stored at −80°C. Total RNA was extracted from the PVN, VMH, and ARC using Ambion RNAqueous-Micro kits (Life Technologies, Inc). The total RNA was also deoxyribonuclease I treated, using the extraction kits, at 37°C for 30 minutes to minimize any genomic DNA contamination. The RNA quantity and quality were determined using a NanoDrop ND-2000 spectrophotometer (ThermoFisher, Inc).
Quantitative real-time PCR
cDNA was synthesized from 200 ng of total RNA using Superscript III reverse transcriptase (Life Technologies, Inc), 4 μL 5× buffer, 25 mM MgCl2, 10 mM deoxynucleotide triphosphate (CLONTECH Laboratories, Inc), 100 ng random hexamer primers (Promega Corp), 40 U/μL Rnasin (Promega), and 100 mM dithiothreitol in diethylpyrocarbonate-treated water (Gene Mate; Bioexpress, Inc) in a total volume of 20 μL. Reverse transcription was conducted using the following protocol: 5 minutes at 25°C, 60 minutes at 50°C, and 15 minutes at 70°C. The cDNA was diluted to 1:20 with nuclease-free water (Gene Mate; Bioexpress) for a final cDNA concentration of 0.5 ng/μL and stored at −20°C. The basal hypothalamus test tissue RNA was used for positive and negative controls (no reverse transcriptase) and processed simultaneously with the experimental samples.
All primers were designed to span exon-exon junctions and synthesized by Life Technologies using Clone Manager 5 software (Sci Ed Software). See Table 1 for a listing of all the primer sets used for qPCR. For qPCR, 4 μL of cDNA template (an equivalent of 2 ng total RNA) was amplified using Sso Advanced SYBR Green (Bio-Rad Laboratories, Inc) on a CFX-Connect real-time PCR instrument (Bio-Rad Laboratories). Standard curves for each primer pair were prepared using serial dilutions of basal hypothalamus cDNA in duplicate to determine the efficiency [E = 10(−1/m) − 1, m = slope] of each primer pair. All efficiencies expressed as percent efficiency were approximately equal (one doubling per cycle, 90%–100%). The relative mRNA expression data were analyzed using the δδcycle threshold (CT) method (37, 38). The amplification protocol for all the genes was as follows: initial denaturing at 95°C for 3 minutes followed by 40 cycles of amplification at 94°C for 10 seconds (denaturing), 60°C for 45 seconds (annealing), and completed with a dissociation step for melting point analysis with 60 cycles of 95°C for 10 seconds, 65°C–95°C (in increments of 0.5°C) for 5 seconds and 95°C for 5 seconds. The reference genes used were Actb and Gapdh. Positive and negative controls were added to each amplification run, which included a water blank. Quantification values were generated only from samples showing a single product at the expected melting point.
Table 1.
Primer Sequences Used for qPCR
| Gene Name | Product Length | Primer Sequence | Base Pair Number | Accession Number |
|---|---|---|---|---|
| Adra1a | 187 | Forward: TCTGCTGGCTGCCATTCTTC | 1638–1657 | NM_013461 |
| Reverse: CACTGGATTCGCAGCACATTC | 1805–1824 | |||
| Adra1b | 84 | Forward: CTTCATCGCTCTCCCACTTG | 1174–1193 | NM_007416 |
| Reverse: TAGCCCAGCCAGAACACT | 1240–1257 | |||
| Adra2b | 130 | Forward: GCAGAGGTCTCGGAGCTAA | 905–923 | NM_009633.3 |
| Reverse: GCCTCTCCGACAGAAGATA | 1016–1034 | |||
| Adra2c | 154 | Forward: CTCATGGCCTACTGGTACTTC | 1657–1677 | NM_007418.3 |
| Reverse: TGCGCTTCAGGTTGTACTC | 1792–1810 | |||
| Agrp | 146 | Forward: CTCCACTGAAGGGCATCAGAA | 287–307 | NM_007427.2 |
| Reverse: ATCTAGCACCTCCGCCAAA | 414–432 | |||
| Actb | 63 | Forward: GCCCTGAGGCTCTTTTCCA | 849–867 | NM_007393.3 |
| Reverse: TAGTTTCATGGATGCCACAGGA | 890–911 | |||
| Crh | 86 | Forward: AGGAGGCATCCTGAGAGAAGT | 152–173 | NM_205769.2 |
| Reverse: CATGTTAGGGGCGCTCTC | 906–923 | |||
| Gapdh | 98 | Forward: TGACGTGCCGCCTGGAGAAA | 778–797 | NM_008084.2 |
| Reverse: AGTGTAGCCCAAGATGCCCTTCAG | 852–875 | |||
| Ghsr | 122 | Forward: CAGGGACCAGAACCACAAAC | 1003–1022 | NM_177330 |
| Reverse: AGCCAGGCTCGAAAGACT | 1107–1124 | |||
| Glp1r | 190 | Forward: TTCAAGCTGTATCTGAGCATAG | 806–827 | NM_021332 |
| Reverse: AGATGACACGGATGAAGATAAG | 974–995 | |||
| Npy | 182 | Forward: ACTGACCCTCGCTCTATCTC | 106–125 | NM_023456 |
| Reverse: TCTCAGGGCTGGATCTCTTG | 268–287 | |||
| Pomc | 200 | Forward: GGAAGATGCCGAGATTCTGC | 145–164 | NM_008895 |
| Reverse: TCCGTTGCCAGGAAACAC | 327–344 | |||
| Trh | 238 | Forward: TTCGGCTTAACGTCTTC | 150–170 | NM_009426.3 |
| Reverse: CTTCGTCGTAACTGGTATCC | 369–387 |
Final relative quantitation was done using the comparative CT method (37, 38). The data were reported as relative mRNA expression. To determine the CT for each transcript, the threshold was consistently set at the lowest point of the exponential curve where the slope of the curve was the steepest for all plates. The relative linear quantity of target molecules was calculated using the formula 2−δδCT. All gene expression data were expressed as an n-fold difference relative to the HFD group.
Statistical analyses
The data are presented as mean ± SEM. A multi-factorial ANOVA or multi-factorial ANOVA with repeated measures was performed to determine schedule, diet, time, time × schedule, time × diet, diet × schedule, and time × diet × schedule effects. An analysis of covariance with body weight as a covariate was also performed on the RER measurements (39). A Newman-Keuls post hoc was performed unless otherwise specified. All statistical analyses were performed using Statistica 7.1 software (StatSoft) and significance was set at α = .05.
Results
IMF feeding reduces body weight, fat mass, and caloric intake comparable to LFD
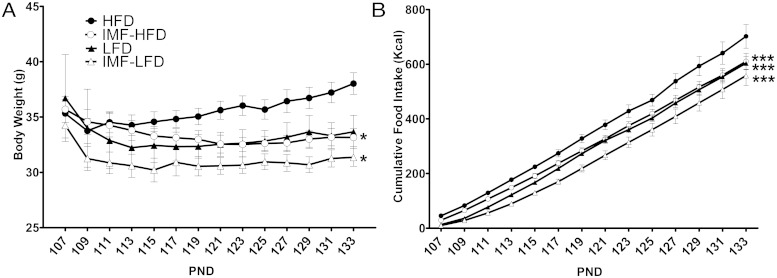
For all groups (HFD, LFD, IMF-HFD, and IMF-LFD), we measured body weight, food intake, and body composition. For body weight, there were significant effects of diet [F (1, 28) = 13.2, P < .01], schedule [F (1, 28) = 11.2, P < .01], and time × diet × schedule [F (13, 364) = 2.4, P < .01]. As demonstrated in Figure 1A, at PND 133 (4 weeks after the diet interventions) the IMF-HFD and IMF-LFD groups had lower (P < .05) body weights than did the HFD group. Total caloric intake was measured over the course of the experimental period. For cumulative caloric intake, there were significant effects of diet [F (1, 12) = 24.5, P < .001], schedule [F (1, 12) = 13.0, P < .01], time [F (13, 156) = 1528.4, P < .001], and time × schedule [F (13, 156) = 3.5, P < .001]. At the end of the 4 weeks, all groups had lower cumulative intakes than the HFD group (P < .001) (Figure 1B).
Figure 1.
Male mice were placed on a HFD (45% fat) to promote DIO. After 8 weeks, mice continued on the HFD (control group; n = 8), were placed on an alternate-day calorie deprivation IMF protocol with HFD (IMF-HFD; n = 8), switched to a control LFD (10% fat; n = 8), or placed on placed on IMF protocol with LFD (IMF-LFD; n = 8). Data are represented as means ± SEM. A, Body weights of mice at the end of 4 weeks of the diet intervention. B, Average cumulative food intake (kilocalories) over 4 weeks. *, Difference (P < .05) from HFD; ***, difference from HFD (P < .001).
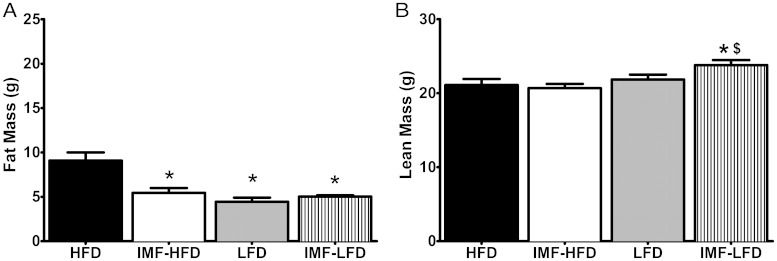
For fat mass (grams), there were significant effects of diet [F (1, 28) = 18.9, P < .001], schedule [F (1, 28) = 6.8, P < .05], and diet × schedule [F (1, 28) = 13.1, P < .01]. All groups had lower fat mass than the HFD group (P < .001) (Figure 2A). For lean mass, there was only an effect of diet [F (1, 28) = 7.8, P < .01], whereas the IMF-LFD had higher lean mass than the IMF-HFD and HFD groups (P < .05 for both) (Figure 2B).
Figure 2.
Body composition as assessed by EchoMRI in all groups at the end of 4 weeks of the diet intervention. Data are represented as means ± SEM. A, Fat mass (grams). B, Lean body mass (grams). ***, Difference from HFD (P < .001); *, difference (P < .05) from HFD; $, difference (P < .05) from IMF-HFD.
IMF increases RER
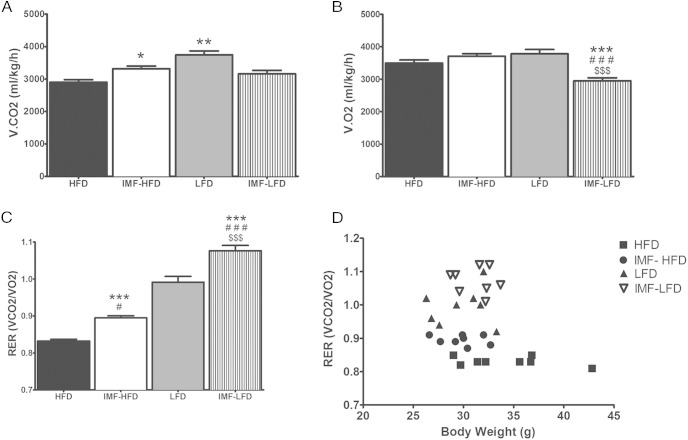
For v.CO2, there were significant effects of diet [F (1, 28) = 11.9, P < .01] and diet × schedule [F (1, 28) = 25.6, P < .001]. Both the LFD and IMF-LFD groups had higher v.CO2 than the HFD group (P < .01 and P < .05, respectively) (Figure 3A). For v.O2, there were significant effects of diet [F (1, 28) = 5.5, P < .05], schedule [F (1, 28) = 9.3, P < .01], and diet × schedule [F (1, 28) = 26.6, P < .001]. Additionally, v.O2 was lower in the IMF-LFD group compared with all other groups (P < .001) (Figure 3B). RER analysis indicated a diet effect [F (1, 28) = 243.4, P < .001] and a schedule effect [F (1, 28) = 45.1, P < .001]. RER was elevated in the IMF-HFD group relative to HFD (P < .001) and lower relative to LFD (P < .05), whereas the IMF-LFD group was elevated in respect to all other groups (P < .001) (Figure 3C). Because body weight can influence energy metabolism, RER was analyzed by an analysis of covariance with body weight as a covariate. Accounting for body weight, there was a diet effect [F (1, 27) = 209, P < .0001] and a schedule effect [F (1, 27) = 39.7, P < .0001]. All groups were different from HFD (P < .05). The RER was plotted as a function of body weight to illustrate the effect of diet and schedule (Figure 3D).
Figure 3.
RER measured by indirect calorimetry (24 h; fed day) in all groups at the end of 4 weeks of the diet intervention. Data are represented as means ± SEM. A, v.CO2. B, v.O2 C, RER. D, RER data as a function of body weight. *, Difference (P < .05) from HFD; **, difference (P < .01) from HFD; #, difference (P < .05) from LFD; ###, difference (P < .001) from LFD; $$$, difference (P < .001) from IMF-HFD.
Glucose and insulin tolerances are altered by an IMF schedule of feeding
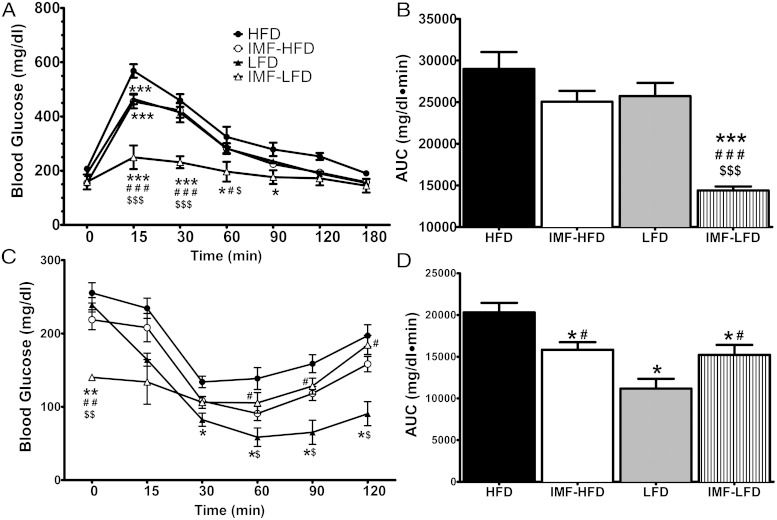
Glucose tolerance was determined over 180 minutes after an oral bolus of glucose. For glucose tolerance, there were significant effects of diet [F (1, 28) = 40.1, P < .001], schedule [F (1, 28) = 47.2, P < .001], and time × diet × schedule [F (6, 168) = 5.3, P < .001]. At 15 minutes, all groups had lower blood glucose levels compared with the HFD (P < .001). The IMF-LFD was also lower than the IMF-HFD and LFD (P < .001). At 30 and 60 minutes, the IMF-LFD group maintained lower blood glucose than all other groups (P < .001 and P < .05 for all, respectively). At 90 minutes, the IMF-LFD group had lower blood glucose levels than the HFD group only (P < .05) (Figure 4A). The area under the curve (AUC) analysis showed an overall reduction in oral glucose tolerance in the IMF-LFD mice compared with all other groups (P < .001) (Figure 4B).
Figure 4.
OGTTs and insulin tolerance tests in all groups at the end of 4 weeks of the diet intervention. Data are represented as means ± SEM. A, Blood glucose (milligrams per deciliter) response to an oral bolus of glucose (2 g/kg) over 180 minutes. Values for IMF-HFD and LFD overlap. B, AUC of the glucose tolerance test. C, Blood glucose response to an ip injection of insulin (0.75 U/kg) over 120 minutes. D, AUC of insulin tolerance test. *, Difference (P < .05) from HFD; **, difference (P < .01) from HFD; ***, difference (P < .001) from HFD; #, difference (P < .05) from LFD; ###, difference (P < .001) from LFD; ##, difference (P < .01) from LFD; $, difference (P < .05) from IMF-HFD; $$, difference (P < .01) from IMF-HFD; $$$, difference (P < .001) from IMF-HFD.
Insulin tolerance was measured after an ip injection of insulin over 120 minutes. For insulin tolerance, there were significant effects of diet [F (1, 28) = 27.3, P < .001], diet × schedule [F (1, 28) = 9.9, P < .01], and time × diet × schedule [F (5, 140) = 10.0, P < .001]. The IMF-LFD group had lower baseline glucose than all other groups (P < .01), but for 60, 90, and 120 minutes, the IMF-LFD was elevated compared with the LFD group (P < .05). For all time points, except for baseline and 15 minutes, the LFD was lower than the HFD group (P < .05). In addition, the LFD group also had lower blood glucose than the IMF-HFD group at 60, 90, and 120 minutes (P < .05 for all) (Figure 4C). The AUC analysis showed a reduction as a consequence of the intermittent schedule; the IMF-HFD and IMF-LFD were lower than HFD and LFD groups, respectively (P < .05 for both). Also, the LFD group had a lower AUC than the HFD group (P < .05) (Figure 4D).
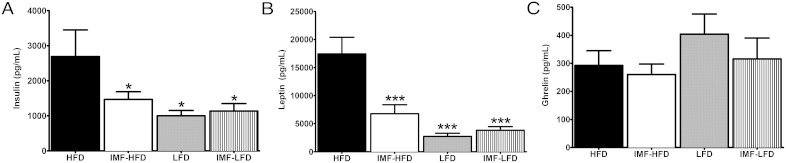
IMF and HFD influence terminal plasma levels of Insulin and leptin but not ghrelin or corticosterone
Plasma levels of hormones were assessed by a multiplex assay. For plasma insulin levels, there was an effect of diet [F (1, 28) = 5.8, P < .05]. Insulin was significantly lower in all groups compared with the HFD group (P < .05) (Figure 5A). Likewise, for leptin concentrations, there were effects of diet [F (1, 28) = 25.9, P < .001], schedule [F (1, 28) = 7.6, P < .05], and diet × schedule [F (1, 28) = 11.531, P < .01]. Plasma leptin was lower in all groups compared with the HFD group (P < .001) (Figure 5B). There were no effects of diet, schedule, or diet × schedule on terminal plasma ghrelin (Figure 5C). Similarly, we did not observe any effects on terminal corticosterone (data not shown), suggesting that the IMF protocols did not induce a stress response.
Figure 5.
Terminal levels of plasma hormones in all groups at the end of 4 weeks of the diet intervention. Data are represented as means ± SEM. A, Insulin B, Leptin. C, Ghrelin. *, Difference (P < .05) from HFD; ***, difference (P < .001) from HFD.
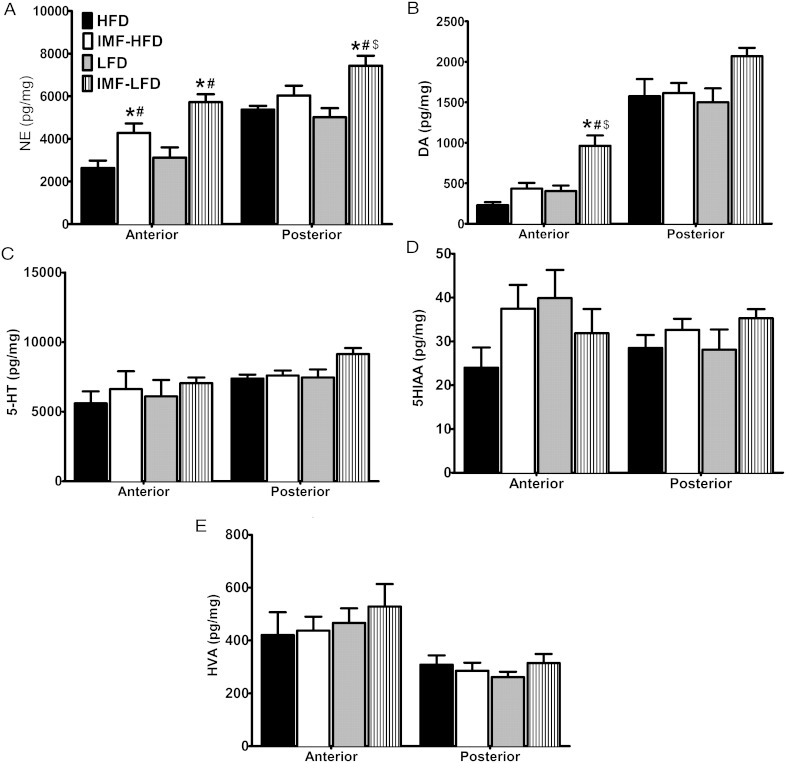
Medial hypothalamic norepinephrine and dopamine increase in response to IMF
Biogenic amines were measured in the anterior and posterior medial hypothalamus by HPLC. These regions are inclusive of the PVN and ARC/VMH, respectively. For NE content in the anterior medial hypothalamus, there were effects of diet [F (1, 27) = 5.4, P < .05] and schedule [F (1, 27) = 26.0, P < .001]. There was an elevation as a consequence of the intermittent schedule. The IMF-HFD and IMF-LFD groups were higher than HFD and LFD groups, respectively (P < .05 for both) (Figure 6A [left panel]). In the posterior medial hypothalamus, there were effects of schedule [F (1, 27) = 15.1, P < .001] and diet × schedule [F (1, 27) = 4.9, P < .05]. NE was increased in the IMF-LFD compared with all other groups (P < .05) (Figure 6A [right panel[). For DA content in the anterior medial hypothalamus, there were effects of diet [F (1, 26) = 18.6, P < .001], schedule [F (1, 26) = 22.1, P < .001], and diet × schedule [F (1, 26) = 4.7, P < .05]. DA concentrations were significantly higher in the anterior medial hypothalamus of the IMF-LFD animals than all other groups (P < .001) (Figure 6B [left panel]). For 5-HT, 5-hydroxyindoleacetic acid, and HVA, there were no effects of diet or schedule in either hypothalamic region (Figure 6, C–E).
Figure 6.
Biogenic amines were measured by HPLC in the anterior and posterior medial hypothalamus in all groups at the end of 4 weeks of the diet intervention (n = 8 per group). A, NE. B, DA. C, 5-HT. D, 5HIAA. E, HVA. Data are represented as means ± SEM. *, Difference (P < .05) from HFD; #, difference (P < .05) from LFD; $, difference (P < .05) from IMF-HFD.
NPY and POMC mRNA expression in the ARC and adrenergic receptor in the PVN of the hypothalamus respond to an intermittent schedule of feeding
Gene expression in the ARC, PVN, and VMH was measured by qPCR. For ARC Npy expression, there was an effect of schedule [F (1, 26) = 21.7, P < .001]. There was an elevation as a consequence of the intermittent schedule. The IMF-HFD and IMF-LFD groups had significantly greater Npy expression than both the HFD and LFD groups (P < .05 for all) (Table 2). For ARC Pomc expression, there was a diet effect [F (1, 27) = 14.8, P < .001] and schedule effect [F (1, 27) = 8.3, P < .01]. Pomc expression was lower in all groups compared with the HFD group (P < .05) (Table 2). For ARC GH secretagogue receptor (GHSR) gene expression, there was an effect of schedule [F (1, 27) = 9.4, P < .01]. The IMF-HFD and IMF-LFD groups demonstrated higher levels of Ghsr gene expression than the HFD group (P < .05) (Table 2). Conversely, there were no significant differences in ARC expression of Agrp, glucagon-like peptide 1 receptor (Glp1r), or the adrenergic receptors, Adra1a, Adra1b, or Adra2c (Table 2). In the PVN, there was an effect of schedule on Adra1a [F (1, 26) = 8.3, P < .01] and Adra1b [F (1, 26) = 5.1, P < .05] (Table 3). For the expression of Adra1a, the IMF-HFD and LFD were significantly lower than the HFD group (P < .05). For the expression of Adra1b, the LFD had lower levels than the HFD group (P < .05) (Table 3). Gene expressions of PVN Adra2c, Crh, Trh, and Glp1r were not significantly different between groups (Table 3). In the VMH, the expressions of Glp1r, Adra1a, Adra1b, Adra2b, and Adra2c showed no significant effects of either diet or schedule of the diet (Table 4).
Table 2.
Relative Gene Expression in the ARC of DIO Mice After 4 Weeks on a HFD, IMF-HFD, LFD, or IMF-LFD Feeding Schedule (n = 8/Group)
| Gene | HFD | IMF-HFD | LFD | IMF-LFD |
|---|---|---|---|---|
| Npy | 0.94 ± 0.10 | 1.93 ± 0.27a,b | 1.15 ± 0.11 | 2.04 ± 0.22a,b |
| Pomc | 1.02 ± 0.07 | 0.81 ± 0.06a | 0.74 ± 0.06a | 0.57 ± 0.07a |
| Agrp | 1.06 ± 0.13 | 1.33 ± 0.19 | 1.00 ± 0.13 | 1.28 ± 0.16 |
| Glp1r | 1.06 ± 0.14 | 1.31 ± 0.19 | 1.01 ± 0.13 | 1.28 ± 0.17 |
| Ghsr | 1.02 ± 0.08 | 1.73 ± 0.17a | 1.41 ± 0.20 | 1.74 ± 0.17a |
| Adra1a | 1.06 ± 0.13 | 1.34 ± 0.20 | 1.01 ± 0.13 | 1.30 ± 0.17 |
| Adra1b | 1.06 ± 0.13 | 1.31 ± 0.20 | 1.00 ± 0.13 | 1.28 ± 0.17 |
| Adra2c | 1.03 ± 0.10 | 1.13 ± 0.24 | 0.95 ± 0.17 | 0.79 ± 0.10 |
Abbreviations: Adra1A, α-adrenergic receptor 1A; Adra1B, α-adrenergic receptor 1B; Adra2C, α-adrenergic receptor 2C; GLP-1R, glucagon-like peptide 1 receptor. All gene expression data were expressed as an n-fold difference relative to the mean of the HFD group. Data are represented as means ± SEM.
P < .05 from HFD.
P < .05 from LFD.
Table 3.
Relative Gene Expression in the PVN of DIO Mice After 4 Weeks on a HFD, IMF-HFD, LFD, or IMF-LFD Feeding Schedule (n = 8/Group)
| Gene | HFD | IMF-HFD | LFD | IMF-LFD |
|---|---|---|---|---|
| Glp1r | 0.93 ± 0.14 | 1.06 ± 0.08 | 0.81 ± 0.12 | 0.95 ± 0.09 |
| Adra1a | 1.01 ± 0.06 | 0.83 ± 0.04a | 0.79 ± 0.04a | 0.89 ± 0.05 |
| Adra1b | 1.07 ± 0.13 | 0.78 ± 0.04 | 0.73 ± 0.07a | 0.84 ± 0.07 |
| Adra2c | 1.05 ± 0.11 | 0.80 ± 0.08 | 0.89 ± 0.08 | 0.83 ± 0.08 |
| Crh | 1.15 ± 0.16 | 0.84 ± 0.14 | 0.99 ± 0.13 | 1.10 ± 0.17 |
| Trh | 1.04 ± 0.11 | 1.12 ± 0.15 | 1.09 ± 0.14 | 1.40 ± 0.12 |
Abbreviations: Adra1A, α-adrenergic receptor 1A; Adra1B, α-adrenergic receptor 1B; Adra2C, α-adrenergic receptor 2C; GLP-1R, glucagon-like peptide 1 receptor. All gene expression data were expressed as an n-fold difference relative to the mean of the HFD group. Data are represented as means ± SEM.
P < .05 from HFD.
Table 4.
Relative Gene Expression in the VMH of DIO Mice After 4 Weeks on a HFD, IMF-HFD, LFD, or IMF-LFD Feeding Schedule (n = 8/Group)
| Gene | HFD | IMF-HFD | LFD | IMF-LFD |
|---|---|---|---|---|
| Glp1r | 1.04 ± 0.11 | 1.18 ± 0.10 | 0.93 ± 0.05 | 1.07 ± 0.10 |
| Adra1a | 1.00 ± 0.03 | 1.03 ± 0.07 | 0.95 ± 0.07 | 0.97 ± 0.05 |
| Adra1b | 1.04 ± 0.11 | 1.01 ± 0.07 | 1.03 ± 0.11 | 0.84 ± 0.07 |
| Adra2c | 1.02 ± 0.07 | 0.98 ± 0.07 | 1.17 ± 0.17 | 1.17 ± 0.17 |
Abbreviations: Adra1A, α-adrenergic receptor 1A; Adra1B, α-adrenergic receptor 1B; Adra2C, α-adrenergic receptor 2C; GLP-1R, glucagon-like peptide 1 receptor. All gene expression data were expressed as an n-fold difference relative to the mean of the HFD group. Data are represented as means ± SEM.
Discussion
Several clinical studies have indicated that IMF is an effective weight-loss treatment for some obese and overweight populations (7, 9, 11, 40). However, there have not been any preclinical studies examining the effects of this diet strategy in animal models of obesity. The IMF protocol used in our study was an alternate day fasting regimen with repeated 24-hour intervals of food deprivation followed by 24-hour ad libitum food access. Our study sought to further understand the neural and metabolic consequences of an IMF protocol in adult male DIO mice. In particular, our study promoted an obese phenotype by exposing mice to ad libitum high-fat feeding for 8 weeks before beginning the IMF protocol or low fat/low calorie diet switch (LFD). One group of mice was maintained on the HFD throughout the study (12 wk total), which was the control group in these experiments. Indeed, most IMF protocols in humans have been validated in overweight, class I obese (body mass index [BMI] ≤ 34.9 kg/m2), or class II obese (BMI ≤ 39.9 kg/m2) individuals (7–10, 40). However, most of the subjects in these studies were either overweight or class I obese (40).
To uncover the neural and metabolic changes that promote weight loss by IMF, our measurements were taken after significant body weight loss was achieved. This was achieved at the 4-week time point in the present set of experiments. At 4 weeks, body weights were significantly lower in IMF-HFD (∼13% reduction) and IMF-LFD (∼18% reduction) groups compared with the HFD group. It is important to note that all three groups (IMF-HFD, LFD, and IMF-LFD) consumed statistically similar cumulative caloric intakes over the 4-week period (∼15%–20% reduction compared with the HFD group). Although the study did not have a pair-fed control group, there was complete overlap in cumulative caloric intakes between the IMF-HFD and LFD diet groups (ie, calorie matched). As a result, there was a reduction in fat mass in all groups compared with the HFD group. This was also reflected in reduced terminal plasma leptin levels by approximately 65% in all groups compared with the HFD group. Thus, it appears that IMF of a HFD is similarly effective at reducing caloric intake, and therefore fat accumulation, as a low-fat/low-calorie diet.
One interesting finding in our study was that the IMF-LFD had higher lean mass than the HFD and IMF-HFD groups. Although the cause for this increase in lean mass is unknown, the retention of lean mass has been reported in humans undergoing a modified IMF protocol for 7 weeks (6). In a study by Klempel et al (6), overweight or obese subjects (BMI 30–39.9 kg/m2; n = 32 completers) were randomly assigned to receive a high-fat diet (45% fat) or lower-fat diet (25% fat). To reduce feelings of hunger, subjects were able to consume 25% of their energy needs on fast days. At the completion of the study, subjects in either the high-fat or low-fat IMF regimen lost weight from baseline (∼4.5%), but there was no difference in amount of weight loss or body composition between diets (6). The retention (or elevation in lean mass) is in contrast to the findings observed by Chausse et al (2014) (41) in nonobese male Sprague Dawley rats exposed to an alternate fasting protocol for 3 weeks. In that study, IMF produced a reduction in epididymal fat mass; it also resulted in a reduction of soleus and plantaris muscle mass compared with ad libitum feeding of the same AIN-93 diet (13.8% protein, 76% carbohydrates, 10.2% fat).
Chausse et al (2014) (41) also found similar relative RER levels between ad libitium and IMF (on fed days) rats, whereas our findings indicate an increase in RER on fed days with IMF with respect to diet (ie, IMF-HFD was increased relative to the HFD and IMF-LFD was increased relative to the LFD). In addition to the difference in rodent species and diets, one prominent difference in the design of our study and that of Chausse et al (2014) (41) was that our mice were placed on an HFD for 8 weeks prior to the intermittent fasting regimen. Future studies will be designed to determine whether the metabolic changes associated with DIO promote the retention of lean mass during an IMF regimen.
Another major finding of the present study was that oral glucose tolerance was slightly improved with an IMF of an HFD. Although there was not a difference in the AUC, 15 minutes after the oral glucose load, blood glucose levels were lower in the IMF-HFD and LFD groups. Possibly related to the retention in lean mass, the IMF-LFD had lower glucose levels, over time and when expressed as AUC, after the oral glucose load. Improvements with IMF and a LFD were also observed in the insulin tolerance tests. Notably, terminal plasma insulin levels were reduced by approximately 45% in all groups compared with the obese HFD group. Improvements in insulin levels have been noted with long-term IMF protocols in overweight and obese women (5). In a 24-week study, overweight or obese women (BMI 30 ± 5 kg/m2) were randomly divided into either a continuous calorie restriction of 25% or an IMF protocol of two consecutive fasting days (75% calorie restriction) per week. At 6 months, weight reduction was 7% in both groups, but the IMF subjects had significantly improved fasting insulin (5.2 vs 6.3 μU/mL) and lower homeostasis model assessment scores (1.1 vs 1.3 μU/mmol · L) than continuously calorie-restricted subjects (5). Reductions in plasma levels of insulin levels also have been noted with IMF protocols in nonobese rodents (27–29, 33, 42). Therefore, combination of IMF regimen with a lower-fat diet could be beneficial for the glucose homeostatic impairments associated with obesity. Because we did not measure free fatty acids, lipoproteins, or markers for fat oxidation, it is unclear how an IMF regimen with a lower-fat diet improves lipid impairments associated with obesity.
The increase in hypothalamic NE content as a consequence of the IMF schedule is another major finding of our study. Hypothalamic NE content was measured in the anterior section of the medial hypothalamus, inclusive of the PVN. In the PVN, there was a decrease in the gene expression of α1A receptor in the IMF-HFD and LFD groups, whereas the expression of α1B receptor was decreased in the LFD group compared with the HFD group. In the posterior section of the hypothalamus, inclusive of the ARC and VMH, NE content was elevated in the IMF-LFD group compared with all other groups. A predominant source of NE for hypothalamic nuclei are from a group of neurons located in caudal hindbrain (43). Hypothalamic NE levels have been associated with the neuroendocrine response to stress and reproductive behaviors (15). With respect to feeding behavior, elevated hypothalamic NE is critically involved in the hyperphagia that accompanies conditions of acute glucoprivation (44). In addition, orexigenic NPY and AgRP neurons in the ARC of the hypothalamus, which project to the PVN, are activated and NPY/AgRP gene expression levels are increased after glucoprivation with the nonmetabolized glucose analog, 2-deoxyglucose (2-DG) (45, 46). Using immunotoxic lesions of the NE and epinephrine (E) neurons (by using saporin-conjugated to antibody for dopamine β-hydroxylase) that project to ARC, Fraley and Ritter (47) demonstrated the critical role of NE/E neurons to glucoprivic response. In particular, Fraley and Ritter found that immunotoxin lesions of NE/E-projecting neurons in the medial hypothalamus abolished the hyperphagic response to 2-DG. In addition, the authors demonstrated NPY and AgRP basal levels were elevated, but did not alter expression levels, in response to 2-DG after an immunotoxin lesion of NE/E (47). Long-term bouts (14 d) of glucoprivation in intact animals have demonstrated the feeding response is attenuated over time, but NPY levels in the ARC remain elevated at day 14 (48). Because our measurements were taken at the 4-week time point during the initial phases of weight loss, one issue that needs examination is how maintenance of the weight loss achieved by IMF alters hypothalamic NE target regions. In addition, we need to determine whether elevated hypothalamic NE is directly involved with the glucose homeostasis improvements.
Findings from our study indicate that Npy relative mRNA levels in the ARC, similar to NE in the PVN, were elevated in response to an intermittent fasting protocol (regardless of diet) compared with HFD and LFD conditions. Although not reaching statistical significance, Agrp relative mRNA levels in the ARC demonstrated a similar trend as Npy. Interestingly, relative mRNA expression levels of the hypothalamic, anorexigenic precursor polypeptide Pomc were elevated in all groups compared with the HFD group. This is in agreement with previous findings that arcuate Pomc gene expression is reduced in animals fed a HFD (20) and those prone to DIO (49). Our findings suggest that hypothalamic NE content and Npy mRNA are elevated after a 5-hour fast as a consequence of the 4-week exposure to an IMF regimen. Acute bouts of food deprivation have been shown to increase hypothalamic Npy mRNA expression (50, 51), an effect that may potentially be augmented by the repetitive nature of an IMF protocol. The role of NE in the PVN and interactions with orexigenic peptide, NPY, are certainly mechanisms that require further attention. In addition, the elevation of Npy and NE as a consequence of the entrainment, not weight loss, of the intermittent feeding paradigm is another possibility that needs further investigation. Because our measurements of NE content and Npy gene expression were performed on regions rather than distinct nuclei, future experiments will use targeted, more mechanistic approaches to uncover the role of NE and NPY in the weight loss and glucose regulatory alterations that accompany IMF.
Another finding of our study was that DA content in the posterior region of the medial hypothalamus was increased in the IMF-LFD compared with the IMF-HFD and LFD groups. In a study by Martin et al (29), regional monoamine content was measured after 6 months on different dietary feeding protocols, including IMF, in male and female nonobese rats. Although hypothalamic regions were not examined, there were differences in the catecholamine metabolite 3,4-dihydroxyphenylacetic acid in the cerebellum of male and female rats exposed to the IMF schedule (29). Female rats exposed to a 40% caloric restriction for 6 months had increased performance in a behavioral cognitive task, which was accompanied by a decrease in DA and increase in serotonin content in the hippocampus compared with ad libitum-fed rats (29). Thus, the significance of the increase in DA in the posterior medial hypothalamus in DIO mice exposed to the IMF-LFD schedule is unclear. In addition, another limitation of our methods is that measurements of biogenic amine tissue content do not provide an accurate index of biogenic amine turnover or steady-state concentrations (52).
An obstacle for IMF regimens is the feelings of hunger that accompany prolonged bouts of food deprivation (14). Ghrelin is a gastrointestinal hormone that is elevated during periods of fasting and associated with subjective feelings of hunger in nonobese adults (53). Fasting increases Ghsr in the hypothalamus (54), whereas DIO via a HFD has been shown to induce ghrelin resistance in ARC NPY/AgRP neurons (55). In addition, although the actions of peripheral ghrelin are thought to be primarily through the afferent vagal nerve to the hindbrain (56), there exist ghrelin-containing cells within the hypothalamus and specifically the ARC (57, 58). Interestingly, hypothalamic ghrelin release decreases in glucoprivic states, such as with fasting or 2-DG administration, whereas the opposite effect is observed with peripheral ghrelin release (59). In our study, terminal ghrelin levels were not elevated, but the relative gene expression of the ghrelin receptor, Ghsr, was increased in the ARC of animals placed on the IMF schedules (ie, IMF-HFD and IMF-LFD). Future work is needed to understand the feed-forward increase in Ghsr gene expression, subjective feelings of hunger, and the weight loss associated with IMF protocols.
Because our study design used pair-housed mice, one limitation of our findings is that diet-specific, calorie-restricted groups were not included in our study. Information gathered from a restricted access to diet, but not exposed to prolonged periods of food deprivation, would provide insight into whether fasting has benefits over simple calorie restriction. As it stands, our studies do not resolve the ongoing debate as to whether IMF provides an added benefit beyond daily caloric restriction (4). Keeping in mind the limitation of extrapolating animal studies and fasting periods to clinical practice, these studies do provide a starting point for research-based human studies to examine the efficacy of long-term IMF regimens for weight loss in certain populations of overweight or obese individuals.
Acknowledgments
Technical assistance with the feeding protocols was provided by Brandon Smith, Ami Patel, Thissa Thambugala, Lauren Palena, and Brittany Wilhite. The authors wish to thank Drs Judith Storch and Sara Campbell for the use of the CLAMS, EchoMRI, and Millipore Lumenix System, respectively.
This work was supported by Grants NJ06156 (to N.T.B.) and NJ06107 (to T.A.R.) from the United States Department of Agriculture—National Institute of Food and Agriculture and by National Institutes of Health Public Health Grants R00DK083457, R00DK83457-S1, and P30ES005022 (to T.A.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related protein
- ARC
- arcuate nucleus
- AUC
- area under the curve
- BMI
- body mass index
- CT
- cycle threshold
- DA
- dopamine
- 2-DG
- 2-deoxyglucose
- DIO
- diet-induced obesity
- E
- epinephrine
- GHSR
- GH secretagogue receptor
- HFD
- high-fat diet
- 5-HT
- serotonin
- HVA
- homovanillic acid
- IMF
- intermittent fasting
- LFD
- low-fat diet
- NE
- norepinephrine
- NPY
- neuropeptide Y
- OGTT
- oral glucose tolerance test
- PND
- postnatal date
- POMC
- proopiomelanocortin
- PVN
- paraventricular nucleus
- qPCR
- quantitative real-time PCR
- RER
- respiratory exchange ratio
- v.CO2
- CO2 production
- v.O2
- O2 consumption
- VMH
- ventromedial nucleus.
References
- 1. Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Julia C, Peneau S, Andreeva VA, et al. Weight-loss strategies used by the general population: how are they perceived? PloS One. 2014;9:e97834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnstone A. Fasting for weight loss: an effective strategy or latest dieting trend? Int J Obes (Lond). 2015;39:727–733. [DOI] [PubMed] [Google Scholar]
- 5. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62:137–143. [DOI] [PubMed] [Google Scholar]
- 7. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;21:1370–1379. [DOI] [PubMed] [Google Scholar]
- 8. Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutr J. 2015;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. [DOI] [PubMed] [Google Scholar]
- 11. Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. [DOI] [PubMed] [Google Scholar]
- 15. Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. [DOI] [PubMed] [Google Scholar]
- 16. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. [DOI] [PubMed] [Google Scholar]
- 17. Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, Barrios V, Arilla Ferreiro E. Peptides and food intake. Front Endocrinol. 2014;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi YH, Hartzell D, Azain MJ, Baile CA. TRH decreases food intake and increases water intake and body temperature in rats. Physiol Behav. 2002;77:1–4. [DOI] [PubMed] [Google Scholar]
- 19. Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res. 1988;443:63–69. [DOI] [PubMed] [Google Scholar]
- 20. Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000;875:89–95. [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Bertone ER, Stanek EJ, 3rd, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. [DOI] [PubMed] [Google Scholar]
- 22. Molnar D, Jeges S, Erhardt E, Schutz Y. Measured and predicted resting metabolic rate in obese and nonobese adolescents. J Pediatr. 1995;127:571–577. [DOI] [PubMed] [Google Scholar]
- 23. Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–456. [DOI] [PubMed] [Google Scholar]
- 24. Moran TH. Gut peptide signaling in the controls of food intake. Obesity. 2006;51(suppl 4):250S–253S. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 26. Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA. 2003;100:6216–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. Nutr J. 2003;133:1921–1929. [DOI] [PubMed] [Google Scholar]
- 29. Martin B, Pearson M, Kebejian L, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasconcelos AR, Yshii LM, Viel TA, et al. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflamm. 2014;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PloS One. 2013;8:e66069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varady KA, Roohk DJ, Bruss M, Hellerstein MK. Alternate-day fasting reduces global cell proliferation rates independently of dietary fat content in mice. Nutrition. 2009;25:486–491. [DOI] [PubMed] [Google Scholar]
- 33. Wan R, Ahmet I, Brown M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. [DOI] [PubMed] [Google Scholar]
- 35. Verpeut JL, Walters AL, Bello NT. Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. Nutr Res. 2013;33:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates, Compact. The Coronal Plates and Diagrams. 3rd ed Amsterdam: Elsevier Academic Press; 2008. [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δδC[T]) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 38. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tschop MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–311. [DOI] [PubMed] [Google Scholar]
- 41. Chausse B, Solon C, Caldeira da Silva CC, et al. Intermittent fasting induces hypothalamic modifications resulting in low feeding efficiency, low body mass and overeating. Endocrinology. 2014;155:2456–2466. [DOI] [PubMed] [Google Scholar]
- 42. Martin B, Pearson M, Brenneman R, et al. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PloS One. 2008;3:e2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–R235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. [DOI] [PubMed] [Google Scholar]
- 45. Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport. 2000;11:117–121. [DOI] [PubMed] [Google Scholar]
- 46. Fraley GS, Dinh TT, Ritter S. Immunotoxic catecholamine lesions attenuate 2DG-induced increase of AGRP mRNA. Peptides. 2002;23:1093–1099. [DOI] [PubMed] [Google Scholar]
- 47. Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. 2003;144:75–83. [DOI] [PubMed] [Google Scholar]
- 48. Ozawa Y, Arima H, Watanabe M, et al. Repeated glucoprivation delayed hyperphagic responses while activating neuropeptide Y neurons in rats. Peptides. 2011;32:763–769. [DOI] [PubMed] [Google Scholar]
- 49. Betley JN, Xu S, Cao ZF, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. [DOI] [PubMed] [Google Scholar]
- 51. Palou M, Sanchez J, Rodriguez AM, Priego T, Pico C, Palou A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: relationship with circulating leptin, insulin and glucose. Cell Physiol Biochem. 2009;23:115–124. [DOI] [PubMed] [Google Scholar]
- 52. Estes KS, Simpkins JW. Age-related alteration in catecholamine activity within microdissected brain regions of ovariectomized Fischer 344 rats. J Neurosci Res. 1984;11:405–417. [DOI] [PubMed] [Google Scholar]
- 53. Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- 54. Kim MS, Yoon CY, Park KH, et al. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–1320. [DOI] [PubMed] [Google Scholar]
- 55. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. [DOI] [PubMed] [Google Scholar]
- 56. Date Y, Shimbara T, Koda S, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. [DOI] [PubMed] [Google Scholar]
- 57. Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. [DOI] [PubMed] [Google Scholar]
- 58. Lu S, Guan JL, Wang QP, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett. 2002;321:157–160. [DOI] [PubMed] [Google Scholar]
- 59. Sato T, Fukue Y, Teranishi H, Yoshida Y, Kojima M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-d-glucose administration. Endocrinology. 2005;146:2510–2516. [DOI] [PubMed] [Google Scholar]