Abstract
Prenatally testosterone (T)-treated sheep present metabolic disruptions similar to those seen in women with polycystic ovary syndrome. These females exhibit an increased ratio of small to large adipocytes, which may be the earliest event in the development of adult insulin resistance. Additionally, our longitudinal studies suggest the existence of a period of compensatory adaptation during development. This study tested whether 1) in utero cotreatment of prenatally T-treated sheep with androgen antagonist (flutamide) or insulin sensitizer (rosiglitazone) prevents juvenile insulin resistance and adult changes in adipocyte size; and 2) visceral adiposity and insulin sensitivity are both unaltered during early adulthood, confirming the predicted developmental trajectory in this animal model. Insulin sensitivity was tested during juvenile development and adipose tissue distribution, adipocyte size, and concentrations of adipokines were determined during early adulthood. Prenatal T-treated females manifested juvenile insulin resistance, which was prevented by prenatal rosiglitazone cotreatment. Neither visceral adiposity nor insulin sensitivity differed between groups during early adulthood. Prenatal T-treated sheep presented an increase in the relative proportion of small adipocytes, which was not substantially prevented by either prenatal intervention. A large effect size was observed for increased leptin concentrations in prenatal T-treated sheep compared with controls, which was prevented by prenatal rosiglitazone. In conclusion, gestational alterations in insulin-glucose homeostasis likely play a role in programming insulin resistance, but not adipocyte size distribution, in prenatal T-treated sheep. Furthermore, these results support the notion that a period of compensatory adaptation of the metabolic system to prenatal T exposure occurs between puberty and adulthood.
The growing fetus is vulnerable to subtle changes in the intrauterine milieu during critical periods of development. Existing evidence suggests that adverse conditions altering the environment in which the fetus develops are associated with early onset of adult pathologies in the offspring (1). In particular, metabolic disorders including obesity, diabetes, cardiovascular disease, and polycystic ovary syndrome (PCOS) may be linked to adaptive responses to early environmental cues in utero.
Steroid hormones play an important role during development, influencing cell differentiation into organ systems (2–5). Prenatal exposure to testosterone (T) excess in rodents (6), monkeys (7), and sheep (8) results in adult reproductive and metabolic disorders that parallel those seen in women with PCOS. Despite a controlled nutrient intake to prevent excessive weight gain, female sheep prenatally exposed to T excess exhibit insulin resistance during adulthood (9), which is associated with disrupted insulin signaling in metabolic tissues (10). Prenatal T treatment was also found to induce insulin resistance during the infantile (11) and early juvenile development in female sheep (9). During postpubertal time points, however, prenatal T-treated sheep manifested increased insulin sensitivity and reduced visceral adiposity, suggesting the existence of a period of compensatory adaptation (12). Interestingly, prenatal T-treated sheep also exhibited a greater relative proportion of small adipocytes in both the visceral and sc adipose depots postpubertally (12). In obese human subjects, recent studies indicate that insulin resistance may result from failure of a subset of small adipocytes to mature into fully differentiated adipocytes that have an increased lipid storage capacity, culminating in ectopic lipid accumulation and consequent lipotoxicity (13, 14). Therefore, the increase in the relative proportion of small adipocytes may play a role in the development of adult insulin resistance in prenatal T-treated sheep. In addition, because increased visceral adiposity is also implicated in the development of insulin resistance (15, 16), changes in adipose tissue deposition may be involved in the dysregulation of glucose-insulin homeostasis seen in these animals.
The components mediating metabolic alterations in prenatal T-treated sheep remain unknown. Gestational T excess increases not only maternal T but also fetal T and estradiol levels in sheep (17), indicating that programming of metabolic disruptions may occur via androgenic or estrogenic mediation. Supporting a role in adipocyte development, androgens have been demonstrated to inhibit adipogenesis (18) as well as subsequent differentiation of preadipocytes into mature adipocytes (19). Additionally, gestational T treatment leads to maternal hyperinsulinemia (20), thus supporting a potential metabolic mediation. In favor of this premise, disruptions in insulin signaling in adipose tissue were found to prevent adipocyte differentiation resulting in insulin resistance in rodents (21–23). This study tested whether prenatal treatment with an androgen antagonist (flutamide) or an insulin sensitizer (rosiglitazone) would prevent the changes in insulin sensitivity and adipocyte morphology seen in female sheep treated with T during gestational day (GD)30–GD90. Because prenatal T-treated sheep exhibit improved insulin sensitivity and decreased visceral adiposity at a postpubertal age (12), this study also examined whether this adaptation would persist further into adulthood before the establishment of insulin resistance in later adult life (9). This is of clinical relevance, because disruptions in adipocyte morphology and adipose tissue distribution may be the earliest events in the development of insulin resistance in this sheep model of PCOS-like phenotype.
Materials and Methods
All animal-related procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health Guide for Use and Care of Animals.
Animals and prenatal interventions
Information regarding prenatal T treatment, husbandry, and nutrition has been described in detail previously (24). Briefly, adult ewes were group-fed daily with 0.5 kg of shelled corn and 1.0–1.5 kg of alfalfa hay per animal beginning ∼3 weeks before breeding. After mating to rams with proven fertility, all ewes were housed in pasture and group-fed daily with 1.25 kg of alfalfa hay per animal. Pregnant females were assigned randomly to 4 treatment groups: control (C) (n = 7), gestational treatment with T (T group, n = 11), gestational cotreatment with T and flutamide (TF), an androgen antagonist (n = 9), and gestational cotreatment with T and rosiglitazone (TR), an insulin sensitizer (n = 6). For generation of T females, pregnant Suffolk sheep were treated twice weekly with 100 mg of T propionate suspended in 2 mL of corn oil (∼1.2 mg/kg, im; Sigma-Aldrich Co) between GD30 and GD90 (term pregnancy, ∼147 d). This treatment results in circulating concentrations of T in pregnant sheep and umbilical artery similar to those seen in intact adult males and 60-day-old male fetuses, respectively (17). C females received im injections of vehicle (corn oil) between GD30 and GD90. Female sheep in the TF group were cotreated with T and flutamide (15 mg/kg·d, sc; Sigma-Aldrich) between GD30 and GD90, whereas TR females were cotreated with T and rosiglitazone (8 mg/d, orally; Avandia, GlaxoSmithKline). To examine whether oral administration of flutamide was as effective as repeated long-term daily injections in preventing virilization (hallmark of excessive prenatal androgen action), 1 TF ewe received flutamide orally in the same dose (15 mg/kg·d) and duration (GD30–GD90) as that administered sc to other TF females. When twin births were involved, only 1 offspring from each mother was used in the study. All lambs were supplemented with a pelleted diet (Shur-Gain; Nutreco Canada, Inc) containing 3.6-MCal/kg digestible energy and 18% crude protein. Female lambs were weaned at ∼8 weeks of age and maintained outdoors at the University of Michigan Sheep Research Facility (Ann Arbor, MI; 42°, 18′N). We have previously investigated the preovulatory LH surge dynamics during the first breeding season in a subset of the animals used in the present study and observed that prenatal T treatment results in LH surge defects, which were prevented by cotreatment with androgen antagonist but not with an insulin sensitizer (25).
At ∼6 weeks (juvenile) and 13 months of age (postpubertal), iv glucose tolerance tests (IVGTTs) were performed to determine the impact of prenatal treatments on glucose-insulin homeostasis. At ∼20 months of age (early adulthood), ewes were subjected to a hyperinsulinemic-euglycemic clamp and computed tomography (CT) scanning for assessment of insulin sensitivity and adipose tissue distribution, respectively. Additionally, blood samples were collected for determination of circulating concentrations of adiponectin and leptin. At ∼22 months of age, estrous cycle was synchronized to avoid a potential influence of reproductive cycle stage on adipocyte morphology (26). Two injections of prostaglandin F2α (20 mg, im; Lutalyse, Pfizer Animal Health) were administered 11 days apart, and animals were ovariectomized 24 hours after the second injection (presumptive natural follicular phase). Visceral adipose tissue was collected concomitantly with ovariectomy from a subset of the animals (C, n = 5; T, n = 5; TF, n = 8; TR, n = 6). In addition, visceral fat was collected from C females that were postnatally treated with flutamide (15 mg/kg·d, orally; C+F, n = 4) from 8 weeks until ∼22 months of age, when ovariectomy and visceral adipose tissue collection were performed.
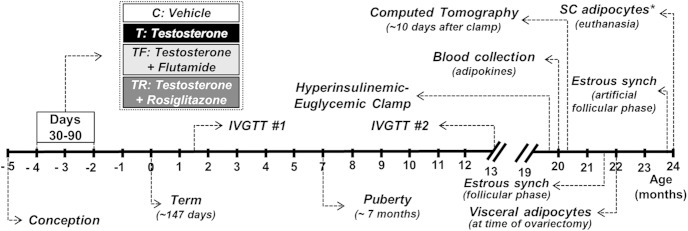
At ∼24 months of age, all females were treated for 7 days with a controlled internal drug release progesterone implant (Zoetis Animal Health). Sixteen hours after removal of progesterone implants, females were treated with 4 3-cm-long estradiol implants to simulate ovarian steroid levels during the normal estrous cycle (27). Twenty-four hours after insertion of estradiol implants (artificial follicular phase), ewes were euthanized by administration of barbiturate overdose (10–15 mL, iv; Fatal Plus, Vortech Pharmaceuticals) and sc adipose tissue was harvested (C, n = 6; T, n = 5; TF, n = 8; TR, n = 6). Visceral adipose tissue was also collected from a subset of these animals during euthanasia (n = 3) to determine whether adipocyte size differed between tissue collected during the presumptive natural follicular phase at time of ovariectomy (22 mo of age) and during the artificial follicular phase at time of euthanasia (24 mo of age). Figure 1 illustrates the temporal sequence of experimental procedures conducted in this study.
Figure 1.
Schematic showing the temporal sequence of experimental procedures in this study. IVGTTs were performed at ∼6 weeks and 13 months of age. At ∼20 months of age, females were subjected to computed tomography (CT) and hyperinsulinemic-euglycemic clamp for assessment of adipose tissue distribution and insulin sensitivity, respectively. At ∼22 months of age, all females were treated with 2 injections of prostaglandin F2α 11 days apart and ovariectomy was performed 24 hours after the second injection (presumptive follicular phase). Collection of visceral adipose tissue for adipocyte morphometric analysis was performed concomitantly with ovariectomy. Approximately 70 days after ovariectomy (24 mo of age), females received a progesterone implant for 7 days followed by an estradiol implant. Forty hours after insertion of the estradiol implant (artificial follicular phase), ewes were euthanized and sc tissue was harvested for adipocyte morphometric analysis. *, visceral adipose tissue was also collected from a subset of the animals (n = 3) at time of euthanasia.
Glucose tolerance test
To investigate the impact of prenatal interventions on glucose-insulin homeostasis in juvenile and postpubertal females, IVGTTs were performed at ∼6 weeks and 13 months of age, respectively, as previously described (9). Briefly, females (C, n = 6; T, n = 5; TF, n = 6; TR, n = 6) were fasted for 48 hours before the IVGTT. Basal insulin and glucose concentrations were measured in samples taken at 15, 10, 5, and 1 minute before glucose administration (300 mg/kg, iv; 50% dextrose-sterile solution; Hospira Worldwide, Inc). Additionally, insulin and glucose levels were determined in samples collected at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 20, 30, 45, 60, 90, 120, 150, and 180 minutes after glucose administration.
Hyperinsulinemic-euglycemic clamp
Hyperinsulinemic-euglycemic clamp was performed at ∼20 months of age, as previously described (12). Briefly, a subset of the animals (C, n = 6; T, n = 5; TF, n = 6; TR, n = 6) was fasted for 48 hours before the clamp procedure and bilateral indwelling jugular catheters were placed. Insulin (Novolin R; Novo Nordisk, Inc) was infused at a constant rate of 4 mU/kg·min for 3 hours. Starting 15 minutes before insulin infusion, blood samples were collected at 5-minute intervals for the assessment of glucose concentrations using a glucometer (Accu-Check; Roche Diagnostics Corp). Starting at 15 minutes after insulin infusion, glucose (iv, 50% dextrose-sterile solution; Hospira Worldwide, Inc) was administered at a rate to restore and maintain euglycemia (based on 5-min glucose readings). In addition, blood samples were collected at 10-minute intervals in heparinized sodium fluoride/potassium oxalate tubes and plasma was stored at −20°C until concentrations of insulin and glucose were determined. The first 3 samples collected before insulin infusion were used for determining basal fasting concentrations of insulin and glucose. The insulin sensitivity index was calculated as the mean glucose infusion rate/mean basal fasting insulin as reported previously (12, 28). The steady-state period was defined as the interval between 90 and 150 minutes after the beginning of insulin infusion, when changes in the glucose infusion rate required to maintain euglycemia were less than 20%.
CT scan
Adiposity distribution was determined in a subgroup of animals (C, n = 6; T, n = 5; TF, n = 6; TR, n = 6) using a multislice CT scanner (GE Brightspeed CT system, 16 slices; General Electric) from the Diagnostic Imaging Service in the Veterinary Medical Center at Michigan State University. Ewes were anesthetized with xylazine (0.1–0.2 mg/kg, im) and placed on the CT scanner in sternal recumbency with their forelegs bent and their hind legs extended. Skeletal landmarks were identified by transverse (axial) scan and used for generating the multiplanar CT images. Scan procedures were performed in all animals using the same parameters (1.25-mm-thick slices, 120 kVp, and 300–330 mA). Images were constructed using a standard algorithm, and adipose tissue volume was determined in the region from the 10th thoracic to the first lumbar vertebrae (mean number of slices analyzed per ewe, 190.7 ± 1.8). Identification of adipose tissue was based on an attenuation range between −50 and −150 Hounsfield units. Images were analyzed using an image analysis software (Analyze 6.0; AnalyzeDirect). Differentiation of the sc (outside the peritoneal cavity) from the visceral compartment (inside the peritoneal cavity) was performed manually using the peritoneum, ribs, vertebrae, and muscular fascia as landmarks.
Hormone and glucose assays
Concentrations of insulin during the IVGTT and hyperinsulinemic-euglycemic clamp procedures were assessed by RIA (insulin RIA kit; MP Biomedicals) with a mean sensitivity of 2.7 μU/L (n = 13 assays), and inter- and intraassay coefficients of variation (CVs) of 6.1% and 8.2%, respectively. Concentrations of glucose were determined by the glucose oxidase method (Pointe Scientific, Inc) and mean inter- and intraassay CV for the assays (n = 30 assays) were 4.1% and 2.1%, respectively.
At ∼20 months of age, circulating concentrations of adiponectin and leptin were determined in blood samples collected from a subset of the animals (C, n = 6; T, n = 5; TF, n = 8; TR, n = 5) after estrus synchronization (presumptive natural follicular phase) with prostaglandin F2α (20 mg, im; Lutalyse, Pfizer Animal Health). Adiponectin concentrations were determined in a single assay using a commercial ELISA kit for detection of ovine adiponectin (Cusabio Biotech USA). Recovery of added mass and parallelism between serial dilutions of samples with the standard curve were performed to validate the ELISA. The sensitivity of the assay was 3.5 ng/mL, with intraassay CV of 5.3%. Concentrations of leptin were determined in a single assay using a highly specific ovine leptin RIA (29). The sensitivity of the assay was 0.1 ng/mL with an intraassay CV of 1.6%.
Adipocyte morphometric analysis
For determining adipocyte size distribution, adipose tissue (visceral: C, n = 5; T, n = 5; TF, n = 8; TR, n = 6; sc: C, n = 6; T, n = 5; TF, n = 8; TR, n = 6) was minced and suspended in phosphate buffer saline containing 4% bovine serum albumin and 5 mg/mL of collagenase A (Roche Diagnostics). After incubation in an orbital shaker for one hour at 40°C, dissociated adipose tissue was filtered through a nylon strainer (mesh, 250 μm), and the upper layer of the suspension containing the adipocytes was reconstituted in fresh medium. Suspension containing adipocytes was then transferred to siliconized glass slides and covered with a siliconized glass slip. Images of adipocytes were captured using a bright-field microscope (Leica DMR) attached to a digital camera (Spot RT camera; Diagnostic Instruments), and the same magnification and camera settings were used for all images. Uniform microspheres with 98 μm diameter (Bangs Laboratories) were used as reference for size determination. Adipocyte diameter was evaluated by computerized image analysis (Image Pro Analyzer, version 7.01; Media Cybernetics).
Adipocyte morphometric analysis was also performed in frozen visceral and sc adipose tissue collected from a subset of the same animals (C, n = 6; T, n = 5; TF, n = 6; TR, n = 5). For this analysis, ∼0.25 g of frozen tissue was embedded in optimal cutting temperature medium (Tissue-Tek O.C.T. Compound; VWR Scientific), cut into 10-μm sections, and stained with hematoxylin and eosin following standard procedures. Images were digitally scanned using Aperio ScanScope (Aperio Technologies) at ×20 magnification. Four sections per animal for each adipose depot (visceral and sc) were analyzed via manual circling (∼200 cells/section). Adipocyte area was determined using the Aperio ImageScope Software (Aperio Technologies).
Statistical analysis
Mean glucose infusion rate and insulin sensitivity index during the hyperinsulinemic-euglycemic clamp, as well as insulin and glucose concentrations during the IVGTT were analyzed by mixed-model analyses for repeated measures using the MIXED procedure of SAS (version 9.3; SAS Institute, Inc). Mean body weight at time of CT scan, volume of adipose tissue (sc, visceral, and total), and concentrations of adiponectin and leptin were analyzed using one-way ANOVA with Tukey's post hoc test (SAS). For mean glucose, insulin, and insulin to glucose ratio, a power analysis was also carried out using the effect size test (30, 31), which allows comparison of the means with respect to the magnitude of difference when the sample size is small. Statistical results of the effect size test are reported as a Cohen's d value, and 0.2, 0.5, and 0.8 were considered as small, medium, and large effect sizes, respectively.
To assess the effects of treatment on adipocyte diameter and area, an empirical cumulative distribution function was calculated for each measurement and the difference between groups was tested using a permutation test based on Kolmogorov-Smirnov statistics with pairwise comparisons (PASW Statistics for Windows; IBM) as described previously (12). For size distribution of visceral adipocytes, C and C+F females were combined into a single group (C#, n = 9), because distribution of adipocyte diameter did not differ between these 2 groups (Supplemental Figure 1). In addition, because both sc and oral administration of flutamide prevented the phenotypic virilization induced by prenatal T treatment, the TF female that received flutamide orally was included in the TF group. Results are presented as mean ± SEM.
Results
Effects of gestational T excess and prenatal interventions on glucose-insulin homeostasis
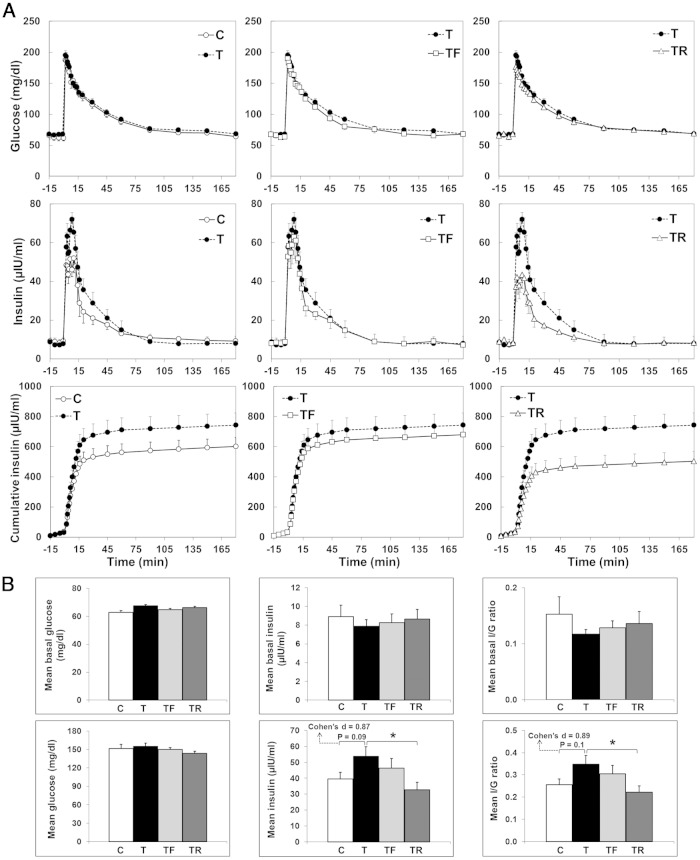
Insulin and glucose dynamics during the IVGTT performed at ∼6 weeks of age are presented in Figure 2. Although comparison of mean concentrations of insulin during the first 60 minutes of the IVGTT between C and T females did not reach statistical significance in the ANOVA test (P = .09), effect size analysis showed a large effect size between C and T groups (Cohen's d = 0.87). TR significantly reduced (P < .05) the mean concentrations of insulin to C levels. Although comparison of mean insulin to glucose ratio between C and T females also did not reach statistical significance in the ANOVA test (P = .10), a large effect size (Cohen's d value = 0.89) was observed between C and T groups. Moreover, prenatal cotreatment with rosiglitazone reduced (P < .05) the mean insulin to glucose ratio in TR females to C levels.
Figure 2.
Effects of gestational T excess and prenatal interventions on insulin-glucose dynamics in juvenile (∼6 wk of age) female sheep. A, mean (±SEM) circulating concentrations of glucose, insulin, and cumulative insulin concentrations during the IVGTT. B, upper panels, Summary bar graphs for mean basal glucose, insulin, and insulin to glucose ratio. B, lower panels, Summary bar graphs for mean glucose, insulin, and insulin to glucose ratio during the first 60 minutes of the IVGTT. *, P < .05.
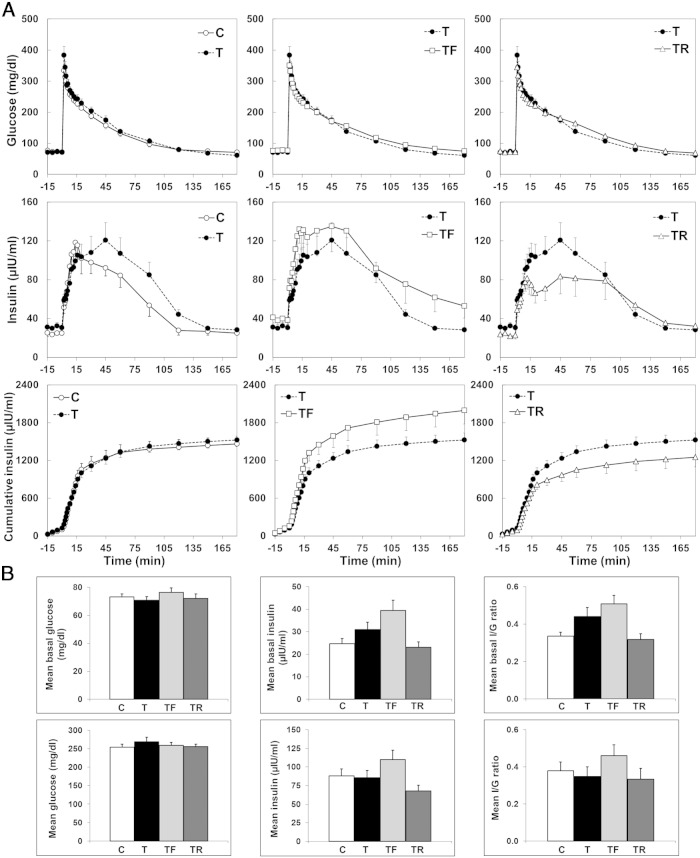
Insulin and glucose dynamics during the IVGTT performed at ∼13 months of age are shown in Figure 3. Mean glucose, insulin, and insulin to glucose ratio both before (basal) and after glucose infusion did not differ between C and T females. Furthermore, prenatal interventions with either flutamide or rosiglitazone did not significantly alter the insulin and glucose concentrations in females prenatally exposed to T excess.
Figure 3.
Effects of gestational T excess and prenatal interventions on insulin-glucose dynamics in postpubertal (∼13 mo of age) female sheep. A, Mean (±SEM) circulating concentrations of glucose, insulin, and cumulative insulin concentrations during the IVGTT. B, upper panels, Summary bar graphs for mean basal glucose, insulin, and insulin to glucose ratio. B, lower panels, Summary bar graphs for mean glucose, insulin, and insulin to glucose ratio during the first 60 minutes of the IVGTT.
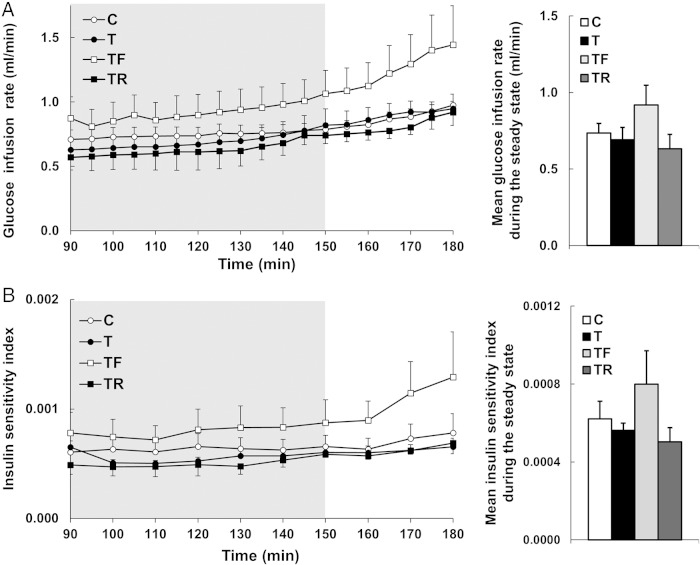
Mean glucose infusion rate and insulin sensitivity index between 90 and 180 minutes from the beginning of insulin infusion during the hyperinsulinemic-euglycemic clamp are shown in Figure 4. At ∼20 months of age (early adulthood) when the clamp was performed, neither the mean glucose infusion rate nor the mean insulin sensitivity index during the steady-state period significantly differed between groups.
Figure 4.
Effects of gestational T excess and prenatal interventions on insulin sensitivity in female sheep. A, Mean (±SEM) glucose infusion rate from 90 to 180 minutes from the beginning of insulin infusion during the hyperinsulinemic-euglycemic clamp is shown on the left. The gray shaded area represents the steady-state period. The overall mean (±SEM) glucose infusion rate calculated during the steady-state period is depicted on the bar graph on the right. B, Mean (±SEM) insulin sensitivity index from 90 to 180 minutes from the beginning of insulin infusion during the hyperinsulinemic-euglycemic clamp is shown on the right. The overall mean (±SEM) insulin sensitivity index calculated during steady-state period is represented by the bar graph on the right.
Effects of gestational T excess and prenatal interventions on adipose tissue distribution
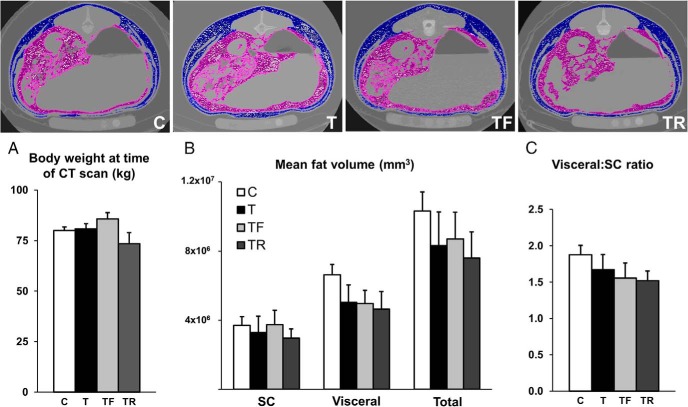
Transverse CT scan images from representative animals are shown in Figure 5. Body weight at the time of CT scan (20 mo of age) (Figure 5A) did not significantly differ between groups. In addition, mean sc, visceral, total adipose tissue volume (Figure 5B), and ratio of sc to visceral adipose tissue volume (Figure 5C) did not differ among groups.
Figure 5.
Effects of gestational T excess and prenatal interventions on adipose tissue distribution in female sheep. Upper panels, Comparable CT scan images from 1 representative animal from each experimental group. Visceral and sc adipose tissue depots are highlighted in purple and blue, respectively. A, Mean (±SEM) body weight at the time of CT scan. B, Mean (±SEM) sc, visceral, and total adipose tissue volume. C, Mean (±SEM) ratio of visceral to sc adipose tissue volume.
Effects of gestational T excess and prenatal interventions on adipocyte size
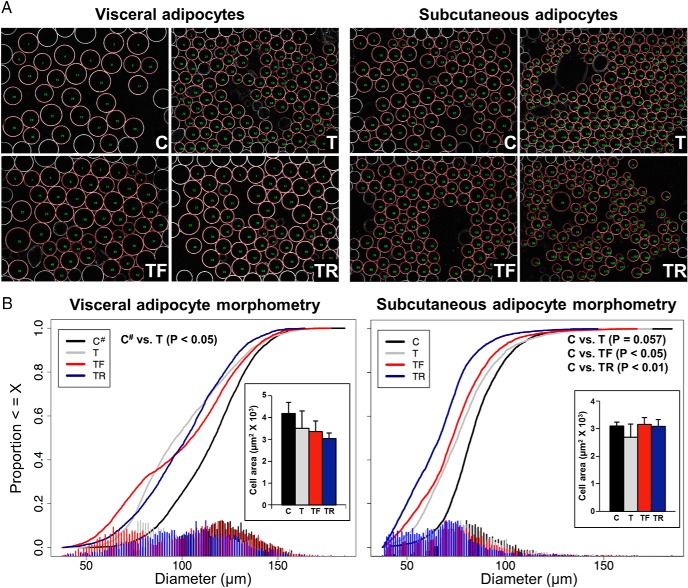
Images of visceral and sc adipocytes identified by computerized image analyses from representative animals are shown in Figure 6A. The mean numbers of adipocytes subjected to morphometric analysis in dissociated visceral and sc adipose tissue were 473.5 ± 25.9 and 551.7 ± 8.2 cells/animal, respectively. In both adipose tissue depots, the adipocyte diameter distribution was shifted (visceral, P < .05; sc, P = .057) to the left (smaller) in prenatal T-treated compared with C females (Figure 6B). Prenatal interventions with either flutamide or rosiglitazone only partially prevented the leftward shift in distribution of visceral adipocyte diameter induced by prenatal T (Figure 6B), with TF and TR distribution curves not differing from either C# or T. In the sc adipose depot, both prenatal interventions failed to prevent a shift (P < .05) to the left (smaller) in the adipocyte diameter distribution compared with the C group (Figure 6B), with TF and TR distribution curves not differing from T. Statistical analysis confirmed that adipocyte diameter distribution did not differ between visceral adipose tissue collected from C animals at ovariectomy (natural follicular phase) and at euthanasia (artificial follicular phase) (Supplemental Figure 1).
Figure 6.
Effects of gestational T excess and prenatal interventions on adipocyte morphometry in female sheep. A, Images of visceral (left panels) and sc (right panels) adipocytes identified by computerized image analysis (red circles) from 1 representative animal from each experimental group. B, Cumulative cell diameter distribution of adipocytes in dissociated visceral (left) and sc (right) adipose tissue. Mean (±SEM) adipocyte area determined in frozen sections of visceral (left) and sc (right) fat depots is depicted in the insets. C#, C combined with C+F (postnatal flutamide).
Results from morphometric analyses of adipocytes in frozen sections are summarized in Figure 6B, insets, and images from representative animals used for the analyses are presented in the Supplemental Figure 2. The mean numbers of adipocytes analyzed in the visceral and sc adipose tissues were 812.3 ± 61.1 and 1055.0 ± 77.5 cells/animal, respectively. Although the direction of prenatal T-induced changes in adipocyte area was similar to that of dissociated cells (smaller adipocytes in T compared with C females), the mean adipocyte area in frozen sections of visceral and sc fat depots did not differ significantly between groups.
Effects of gestational T excess and prenatal interventions on circulating concentrations of adiponectin and leptin
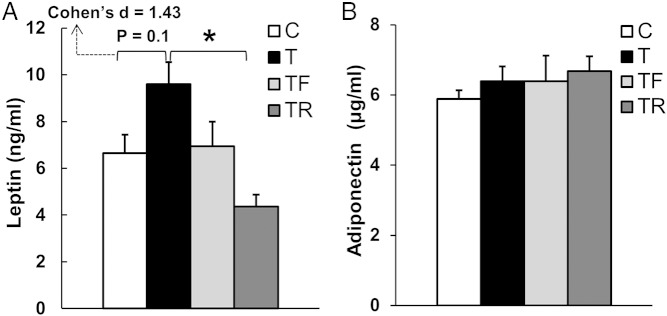
Although comparison of mean circulating concentrations of leptin at ∼20 months of age between C and T females did not reach statistical significance in the ANOVA test (P = .10), effect size analysis showed a large effect size (Cohen's d = 1.43) between C and T groups (Figure 7A). Prenatal cotreatment with rosiglitazone reduced (P < .05) the mean concentrations of leptin in females prenatally exposed to T excess to C levels. Circulating concentrations of adiponectin at ∼20 months of age did not differ between groups (Figure 7B).
Figure 7.
Effects of gestational T excess and prenatal interventions on circulating concentrations of leptin and adiponectin in female sheep. A, Mean (±SEM) circulating concentrations of leptin at ∼20 months of age. B, Mean (±SEM) circulating concentrations of adiponectin at ∼20 months of age. *, P < .05.
Discussion
Our results demonstrate that prenatal treatment with an insulin sensitizer prevented the development of insulin resistance in juvenile female sheep prenatally exposed to T excess, thus indicating that gestational alterations in glucose-insulin homeostasis likely play a role in programming these metabolic defects. Nevertheless, prenatal treatment with an androgen antagonist or an insulin sensitizer failed to considerably prevent the reduction in adipocyte size induced by gestational exposure to T excess. These findings suggest that changes in adipocyte morphometry seen in this sheep model of PCOS phenotype might be programmed via the estrogenic effects of T (facilitated by the conversion of T to estradiol), or alternatively by both androgen and insulin signaling pathways acting in synergy. In addition, the observation that visceral adiposity and insulin sensitivity are both normal during early adulthood, a time point when changes in adipocyte size were evident, corroborates the notion that alterations in adipocyte morphology precede the onset of adult metabolic dysfunctions in these animals. Finally, together with our previous findings of age-specific changes in insulin sensitivity in prenatal T-treated sheep (9, 11, 12), these results further support the idea of a compensatory adaptation of the metabolic system to prenatal exposure to T excess.
Effects of gestational T excess and prenatal interventions on adipocyte size
The dogma in the field has been that adipocyte hypertrophy is associated with the development of insulin resistance and diabetes risk (32–34). Nevertheless, recent studies investigating adipocyte size distribution in obese but otherwise healthy subjects have demonstrated that the proportion of small vs large adipocytes was actually higher in insulin-resistant compared with insulin-sensitive subjects (14, 35). Thus, it has been postulated that insulin resistance in obese patients may stem, in part, from a failure of a subset of small adipocytes to mature into fully differentiated adipocytes with increased lipid storage capacity (13). Consequently, excess free fatty acids may be deposited in nonadipose tissues (ie, liver and muscle), leading to lipotoxicity, oxidative endoplasmic reticulum stress, and related insulin resistance (36–38). Therefore, shifts in adipocyte size distribution in either direction (smaller or larger) appear to have detrimental consequences on peripheral insulin sensitivity.
Similar to insulin-resistant obese subjects (35), female sheep prenatally exposed to excess T exhibited an increased ratio of small to large adipocytes at postpubertal age (12), a period that precedes the onset of adult insulin resistance. Because both androgens and insulin play a role in regulating adipocyte development (18, 19, 21–23) and prenatal T treatment results in alterations in the maternal and fetal metabolic/steroid milieu (20), we hypothesized that androgens and/or insulin mediate the increase in the ratio of small to large adipocytes in prenatal T-treated sheep. The present observations that the prenatal blockage of androgen action with an androgen antagonist or improving insulin sensitivity with an insulin sensitizer failed to substantially prevent these changes in adipocyte size distribution raise the possibility that the effects of prenatal exposure to excess T on adipocyte size may be mediated by the estrogenic effect of T. Studies using adipocyte (39) and bone marrow stromal (40) cell lines as well as transgenic mouse models (41) have indicated that estrogens have inhibitory effects on preadipocyte differentiation and overall adipocyte size. Moreover, ovariectomy leads to adipocyte hypertrophy in female mice, which is prevented by estrogen replacement (42). Importantly, we have previously observed that prenatal T treatment increases circulating concentrations of estradiol in the ovine fetus (20). Another possibility is that androgens and insulin may act in synergy to mediate T effects on increasing the proportion of smaller adipocytes. Future studies evaluating the effects of estrogen antagonists or concomitant treatment with androgen antagonist and insulin sensitizer are necessary to clarify these possibilities.
Reports on the effects of androgen excess on adipocyte size in different animal models are conflicting. Similar to our observations in sheep, prenatal T excess increased the relative proportion of small sc adipocytes in female rhesus monkeys, which was associated with impaired preadipocyte differentiation into mature adipocytes (43). In contrast, prenatal and early postnatal treatment with dihydrotestosterone increased adipocyte size in adult female mice (44) and Wistar rats (45), respectively. Although this discrepancy may be attributed to species-specific differences, the fact that exposure to dihydrotestosterone, a nonaromatizable androgen, did not decrease adipocyte size in rodents further supports the premise that these alterations are programmed via estrogenic effects of T.
In regards to the different techniques used to determine adipocyte size, although the direction of changes was similar in both approaches (smaller adipocytes in prenatal T-treated females), the basis for the discrepancy between the statistical significance of treatment effects in dissociated adipocytes (significant) and frozen sections (nonsignificant) is unclear. One possibility is that cell rupture and/or shrinkage due to freezing may have affected the measurement of adipocyte size in frozen sections as reported previously (46).
Effects of gestational T excess and prenatal interventions on visceral adiposity
Multivariate analysis studies have demonstrated that visceral, and not sc adipose tissue, is significantly correlated with development of insulin resistance, increased risk for type 2 diabetes, and cardiovascular diseases (15, 47–49). In women with PCOS, increased abdominal adiposity worsens their metabolic and reproductive defects (50, 51), whereas loss of visceral fat is associated with resumption of ovulation (52). Prenatal androgen exposure has been reported to alter the overall mass and distribution of adipose tissue in several animal models of PCOS. For instance, prenatal T treatment increased visceral adiposity in postpubertal rats (53) and adult female monkeys (54). In the current study, however, prenatal T treatment did not alter visceral adiposity in female sheep during early adult life. The basis for the discrepancy in visceral adiposity between these animal models is unclear, but it may include species-specific differences, timing of T treatment relative to organ system development, T dosage, and/or the life stage in which adiposity is assessed. In this regard, we have previously observed that prenatal T treatment actually reduced visceral adiposity in postpubertal sheep (12). Whether visceral adiposity is increased in prenatal T-treated sheep during later adult life when insulin sensitivity is markedly reduced (9) remains to be determined. Because 1) reduced estrogen levels are associated with increased visceral fat deposition and accrual in perimenopausal women (55, 56), 2) prenatal T-treated monkeys manifest increased visceral adiposity in their premenopausal years (54), and 3) prenatal T-treated sheep exhibit premature reproductive senescence (57), visceral adiposity is likely to be increased in prenatal T-treated sheep during later adulthood.
Effects of gestational T excess and prenatal interventions on glucose-insulin homeostasis
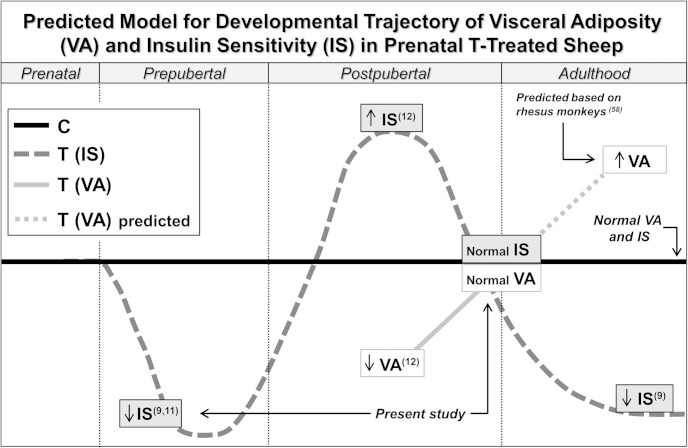
Our previous longitudinal studies investigating glucose-insulin homeostasis in prenatal T-treated sheep have indicated that insulin sensitivity fluctuates throughout development. During infantile (11) and early juvenile life (9), these females exhibit a significant reduction in insulin sensitivity. Conversely, during postpubertal development, prenatal T-treated sheep demonstrate remarkable improvements in insulin sensitivity, displaying greater insulin sensitivity index than controls during a euglycemic-hyperinsulinemic clamp (12). At later adulthood, however, prenatal exposure to T excess results in the reestablishment of insulin resistance in female sheep (9). Based on these observations, we postulated that a period of compensatory adaptation of classical insulin target tissues to prenatal T exposure occurs between juvenile and early adult life in sheep. Our present findings of insulin resistance during juvenile development and normal insulin sensitivity during early adulthood corroborate the predicted trajectory of insulin sensitivity in prenatal T-treated sheep (Figure 8) (58). In agreement, prenatal T excess has also been found to induce tissue- and age-specific changes in the expression of key members of the insulin-signaling cascade. In adult female sheep (3 y of age), prenatal T excess prevented the insulin-stimulated phosphorylation (activation) of protein kinase B in muscle and liver (59), which also supports our proposed developmental trajectory.
Figure 8.
Predicted model for developmental changes in visceral adiposity (VA) and insulin sensitivity (IS) in female sheep prenatally exposed to T excess. This model is supported by reports of insulin resistance during early life in prenatal T-treated sheep (9, 11 and present study), followed by reduced VA and elevated IS at postpubertal age (12), normal VA and IS during early adulthood (present study), and reestablishment of insulin resistance later in adult life (9). Although VA has not been investigated at later time points, observations in prenatal T-treated female rhesus monkeys (58) suggest that VA is likely to be increased during later adulthood in prenatal T-treated sheep. Altogether, findings in this sheep model of PCOS-like phenotype are suggestive of the existence of a period of compensatory adaptation of the adipose and other metabolic tissues to prenatal T exposure. T, prenatal T-treated. Numbers in superscript indicate the reference source.
The findings that prenatal cotreatment with rosiglitazone prevented the development of insulin resistance in prenatal T-treated sheep suggest that gestational alterations in insulin levels play a role in programming these metabolic defects. Importantly, we have previously observed that gestational T treatment in sheep results in maternal hyperinsulinemia, which is prevented by rosiglitazone cotreatment (20). Studies in rodents indicate that other conditions leading to gestational hyperinsulinemia such as maternal obesity (60) and protein restriction (61) are also associated with the development of insulin resistance in the offspring. In future studies, it will be important to determine whether prenatal rosiglitazone treatment prevents the development of insulin resistance in prenatal T-treated sheep also during later adulthood.
Effects of gestational T excess and prenatal interventions on circulating concentrations of adiponectin and leptin
The mechanisms underlying the proposed compensation of metabolic tissues to prenatal T excess in female sheep are unclear. Leptin, an adipose tissue-derived hormone, has been demonstrated to increase glucose metabolism and insulin sensitivity in rodents (62, 63). Thus, the large effect size for increased concentrations of leptin in prenatal T-treated sheep during early adulthood, a time point in which insulin sensitivity has been restored to normal levels, suggests that this might be a compensatory response to the insulin resistance seen earlier during juvenile development in these females. The observation that prenatal cotreatment with rosiglitazone prevented both the juvenile insulin resistance as well as the increase in leptin levels during early adulthood further supports this possibility. Although adiponectin has also been proposed as an important regulator of insulin sensitivity (64), our findings do not indicate that changes in adiponectin levels are involved in the proposed compensatory response of metabolic tissues to prenatal T excess in female sheep.
Validity of prenatal interventions
Because prenatal interventions failed to markedly prevent the changes seen in adipocyte size distribution in prenatal T-treated sheep, it is important to consider the possibility that the doses of flutamide (androgen antagonist) and rosiglitazone (insulin sensitizer) used in the present study were inadequate to achieve the desired effects. For the androgen antagonist, the daily dose of flutamide (15 mg/kg) administered to pregnant ewes in this study has been previously shown to block the effects of both exogenous and endogenous androgens on phenotypic virilization in males and prenatal T-treated female sheep (65). Regarding the daily dose of rosiglitazone (8 mg/kg), biochemical and enzyme analyses have found this treatment dose, which is within the range used to treat women with PCOS (66, 67), to not affect hepatic function or overall health status in sheep (57). More importantly, this dose of rosiglitazone normalized the insulin to glucose ratio during gestation (20) and restored insulin sensitivity (57) in prenatal T-treated sheep. Therefore, the increased ratio of small to large adipocytes seen in prenatal T-treated sheep is unlikely to be mediated, at least exclusively, by androgen or insulin actions on adipocytes during fetal development. However, because gestational T treatment results in hyperinsulinemia and functional hyperandrogenism in the female offspring (8), it is possible that increased insulin and/or androgen signaling during early postnatal life may play a role in the development/maintenance of these changes in adipocyte size distribution.
Translational relevance
Considering that prenatal T-treated female sheep manifest reproductive and metabolic disruptions that closely resemble those seen in women with PCOS, such as oligo-anovulation, LH hypersecretion, multifollicular ovarian morphology, and reduced insulin sensitivity during adulthood (8), findings from the current study are likely to be of translational relevance. In particular, several of the metabolic alterations characterized in this model appear to parallel those seen in lean women with PCOS. For instance, our observations of normal visceral adiposity in prenatal T-treated sheep are consistent with reports in lean PCOS women (68). Investigations of insulin sensitivity in lean women with PCOS are contradictory. An earlier study reported that lean PCOS women presented a 50% reduction in insulin sensitivity compared with lean Cs (69). However, a more recent study did not observe any significant difference in insulin sensitivity between lean PCOS and healthy women (70). Because of the relatively limited data on glucose-insulin homeostasis in lean women with PCOS, additional studies are required to determine whether a similar pattern of fluctuation in insulin sensitivity as that seen in prenatal T-treated sheep also occurs in these women throughout adult life.
In summary, the present findings indicate that gestational alterations in glucose-insulin homeostasis mediate, at least in part, the development of insulin resistance in prenatal T-treated sheep. Our observations also suggest that the increase in the relative proportion of small adipocytes seen in these females may be the earliest event in the development of metabolic dysfunctions in this sheep model of PCOS. Finally, findings that visceral adiposity and insulin sensitivity are both normal during early adult life support the idea that a period of compensatory adaptation of metabolic tissues to prenatal T exposure occurs between puberty and adult life.
Acknowledgments
We thank Mr Douglas Doop and Gary McCalla for their valuable assistance in breeding, lambing, and careful animal care. We also thank Dr Bachir Abi Salloum, Mr Rohit Sreedharan, and Mrs Carol Herkimer for the help provided with prenatal treatment and RIAs; Dr Wen Ye (School of Public Health, University of Michigan) for performing the statistical analyses; Mr Robert Malinowski for assisting with the CT scan Analyze software; and Dr Duane Keisler (Division of Animal Sciences, University of Missouri) for performing the leptin measurements.
Present address for A.V.-L.: Department of Animal Science, Michigan State University, East Lansing, MI 48824.
This work was supported by the National Institutes of Health Grant P01 HD44232.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- C
- control
- CV
- coefficient of variation
- GD
- gestational day
- IVGTT
- iv glucose tolerance test
- PCOS
- polycystic ovary syndrome
- T
- testosterone
- TF
- gestational cotreatment with T and flutamide
- TR
- gestational cotreatment with T and rosiglitazone.
References
- 1. Barker D. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. [DOI] [PubMed] [Google Scholar]
- 2. Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. [DOI] [PubMed] [Google Scholar]
- 3. McEwen B. Steroid hormones: effect on brain development and function. Horm Res Paediatr. 1992;37:1–10. [DOI] [PubMed] [Google Scholar]
- 4. Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130:38–53. [PubMed] [Google Scholar]
- 5. Gorski RA. Sexual differentiation of the brain: a model for drug-induced alterations of the reproductive system. Environ Health Perspect. 1986;70:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology. 2010;151:5165–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Recabarren SE, Padmanabhan V, Codner E, et al. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endoc. 2005;289:801–806. [DOI] [PubMed] [Google Scholar]
- 12. Veiga-Lopez A, Moeller J, Patel D, et al. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Müller G. Let's shift lipid burden–from large to small adipocytes. Eur J Pharmacol. 2011;656:1–4. [DOI] [PubMed] [Google Scholar]
- 14. McLaughlin T, Deng A, Yee G, et al. Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia. 2010;53:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pouliot MC, Després JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. [DOI] [PubMed] [Google Scholar]
- 16. Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. [DOI] [PubMed] [Google Scholar]
- 17. Veiga-Lopez A, Steckler TL, Abbott DH, et al. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh R, Artaza JN, Taylor WE, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with β-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abi Salloum B, Veiga-Lopez A, Abbott DH, Burant CF, Padmanabhan V. Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology. 2015;156:2323–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. [DOI] [PubMed] [Google Scholar]
- 22. Laustsen PG, Michael MD, Crute BE, et al. Lipoatrophic diabetes in Irs1−/−/Irs3−/− double knockout mice. Genes Dev. 2002;16:3213–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J Clin Invest. 2003;112:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. [DOI] [PubMed] [Google Scholar]
- 25. Padmanabhan V, Veiga-Lopez A, Herkimer C, et al. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve LH surge dynamics in prenatal testosterone-treated sheep. Endocrinology. 2015;156:2678–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varlamov O, Chu MP, McGee WK, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154:4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans NP, Dahl GE, Mauger DT, Padmanabhan V, Thrun LA, Karsch FJ. Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system. Endocrinology. 1995;136:5511–5519. [DOI] [PubMed] [Google Scholar]
- 28. Sano H, Matsunobu S, Abe T, Terashima Y. Combined effects of diet and cold exposure on insulin responsiveness to glucose and tissue responsiveness to insulin in sheep. J Anim Sci. 1992;70:3514–3520. [DOI] [PubMed] [Google Scholar]
- 29. Delavaud C, Bocquier F, Chilliard Y, Keisler DH, Gertler A, Kann G. Plasma leptin determination in ruminants: effect of nutritional status and body fatness on plasma leptin concentration assessed by a specific RIA in sheep. J Endocrinol. 2000;165:519–526. [DOI] [PubMed] [Google Scholar]
- 30. Cohen J. Power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 31. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. [DOI] [PubMed] [Google Scholar]
- 32. Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. [DOI] [PubMed] [Google Scholar]
- 33. Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joe AW, Yi L, Even Y, Vogl AW, Rossi F. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. [DOI] [PubMed] [Google Scholar]
- 35. McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–1715. [DOI] [PubMed] [Google Scholar]
- 36. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome – an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. [DOI] [PubMed] [Google Scholar]
- 37. Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. [DOI] [PubMed] [Google Scholar]
- 38. Sørensen TI, Virtue S, Vidal-Puig A. Obesity as a clinical and public health problem: is there a need for a new definition based on lipotoxicity effects? Biochim Byophys Acta. 2010;1801:400–404. [DOI] [PubMed] [Google Scholar]
- 39. Lea-Currie YR, Monroe D, Mcintosh MK. Dehydroepiandrosterone and related steroids alter 3T3-L1 preadipocyte proliferation and differentiation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;123:17–25. [DOI] [PubMed] [Google Scholar]
- 40. Okazaki R, Inoue D, Shibata M, et al. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) α or β. Endocrinology. 2002;143:2349–2356. [DOI] [PubMed] [Google Scholar]
- 41. Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229:1127–1135. [DOI] [PubMed] [Google Scholar]
- 42. Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keller E, Chazenbalk GD, Aguilera P, et al. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155:2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mannerås L, Cajander S, Holmäng A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. [DOI] [PubMed] [Google Scholar]
- 46. Fakler T, O'Brian Smith E, McNeel RL, Mersmann HJ. Evaluation of alternative methods to prepare porcine adipocytes for measurement with an electronic particle number and size determination apparatus. J Anim Sci. 1996;74:2385–2393. [DOI] [PubMed] [Google Scholar]
- 47. Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. [DOI] [PubMed] [Google Scholar]
- 48. Rendell M, Hulthén UL, Törnquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–749. [DOI] [PubMed] [Google Scholar]
- 49. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. [DOI] [PubMed] [Google Scholar]
- 50. Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91:1332–1335. [DOI] [PubMed] [Google Scholar]
- 51. Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15:477–488. [DOI] [PubMed] [Google Scholar]
- 52. Kuchenbecker WK, Groen H, van Asselt SJ, et al. In women with polycystic ovary syndrome and obesity, loss of intra-abdominal fat is associated with resumption of ovulation. Hum Reprod. 2011;26:2505–2512. [DOI] [PubMed] [Google Scholar]
- 53. Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:E262–E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:1206–1210. [DOI] [PubMed] [Google Scholar]
- 55. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151:4007–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bruns CM, Baum ST, Colman RJ, et al. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome. Int J Obes. 2007;31:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu C, Padmanabhan V. Developmental programming: prenatal testosterone excess decreases insulin sensitivity in muscle and liver but not fat via AKT signaling pathway in female sheep. Program of the 97st Annual Meeting of The Endocrine Society, San Diego, CA, 2015 (Abstract THR-623). [Google Scholar]
- 60. Samuelsson AM, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance. Hypertension. 2008;51:383–392. [DOI] [PubMed] [Google Scholar]
- 61. Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R368–R373. [DOI] [PubMed] [Google Scholar]
- 62. Ogawa Y, Masuzaki H, Hosoda K, et al. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. [DOI] [PubMed] [Google Scholar]
- 63. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. [DOI] [PubMed] [Google Scholar]
- 64. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. [DOI] [PubMed] [Google Scholar]
- 65. Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Legro RS, Zaino RJ, Demers LM, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402:E1–E10. [DOI] [PubMed] [Google Scholar]
- 67. Roy KK, Baruah J, Sharma A, et al. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Arch Gynecol Obstet. 2010;281:939–944. [DOI] [PubMed] [Google Scholar]
- 68. Barber TM, Golding SJ, Alvey C, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:999–1004. [DOI] [PubMed] [Google Scholar]
- 69. Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81:2854–2864. [DOI] [PubMed] [Google Scholar]
- 70. Vrbíková J, Cibula D, Dvoráková K, et al. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:2942–2945. [DOI] [PubMed] [Google Scholar]