Abstract
Introduction
There are limited data from randomized controlled clinical trials on the outcomes of biologics after discontinuation of a different systemic therapy. To determine the efficacy of adalimumab in patients who previously received systemic therapy (including failed therapy), we performed a pooled post hoc analysis of Psoriasis Area and Severity Index (PASI) response data from three double-blind, placebo-controlled clinical trials in patients with moderate to severe psoriasis.
Methods
Patients from the M02-528, REVEAL, and CHAMPION studies who were previously exposed to systemic treatment were categorized based on their response. The efficacy of adalimumab compared with placebo was analyzed at the end of the double-blind treatment period for the overall pooled intent-to-treat population (N = 1469) and subgroups that received (n = 780) or did not respond to (n = 229) previous systemic pretreatments.
Results
Rates for an improvement of ≥75 % from baseline in the PASI score (PASI75 response) were significantly greater (p < 0.001) at week 16 in patients treated with adalimumab compared with patients who received placebo in the overall (72.1 vs. 8.0 %, respectively), previously treated (72.7 vs. 8.5 %), and previously failed treatment (70.4 vs. 8.1 %) groups. PASI75 response rates were similar in the overall group and in patients who did not respond to methotrexate, cyclosporine, or psoralen plus ultraviolet A therapy. Improvements of ≥90 or ≥100 % from baseline PASI score were also higher with adalimumab vs. placebo in previously treated patients. Adverse events were similar among subgroups.
Conclusions
Adalimumab was efficacious for the treatment of moderate to severe psoriasis regardless of prior exposure to systemic therapies or failure of those prior therapies.
ClinicalTrials.gov identifiers
Key Points
| Patients with psoriasis treated with adalimumab show significant improvement. |
| Patients with previous exposure to systemic therapies demonstrated improvement comparable to those without prior exposure to systemic therapies. |
| Patients who did not respond to systemic therapies demonstrated improvement comparable to the general psoriasis population. |
Introduction
Current treatment guidelines suggest that psoriasis may be treated with topical, ultraviolet (UV)-based, traditional systemic, or biologic therapies, depending on specific patient and disease characteristics [1–3]. The majority of patients with psoriasis who are prescribed biologic therapy have previously received treatment with other systemic therapies, including methotrexate (MTX), cyclosporine, and psoralen plus UVA (PUVA) therapy. It is of clinical relevance to understand whether patients who have received prior systemic therapy, especially patients who did not respond to previous treatment, will respond to biologics to the same degree as patients who are naive to systemic treatments.
Systemic therapies for the treatment of moderate to severe psoriasis include MTX, which acts as an immunosuppressant and is often a first-line systemic therapy, and cyclosporine, which is a short-term treatment alternative owing to its potential for nephrotoxicity [4]. A third option is PUVA, which uses naturally occurring, photosensitizing psoralen compounds to sensitize cells to the effects of UVA light and form psoralen-DNA crosslinks upon exposure, thus preventing DNA replication [5]. Biologics approved for the treatment of psoriasis have included agents that modulate T cells (e.g., alefacept and efalizumab [both withdrawn from the market]) and inhibitors of tumor necrosis factor (TNF; e.g., adalimumab, etanercept, and infliximab), interleukin-12 and interleukin-23 (e.g., ustekinumab) [6–8], and interleukin-17 (e.g., secukinumab) [9]. Apremilast is a recently approved small-molecule inhibitor of phosphodiesterase 4 [10]. However, there are limited published clinical trial data on the outcomes of systemic biologics after discontinuing a different prior systemic therapy, particularly following failure of the previous therapy.
The efficacy of adalimumab in the treatment of psoriasis was demonstrated in two large, randomized, placebo-controlled phase III trials. In the REVEAL trial, a significantly greater percentage of patients receiving adalimumab achieved an improvement of ≥75 % from baseline in the Psoriasis Area and Severity Index score (PASI75 response) compared with patients receiving placebo (71 vs. 7 %) [11]. In the CHAMPION trial, the superiority of adalimumab over MTX and placebo was demonstrated by a significantly greater percentage of patients receiving adalimumab achieving PASI75 (79.6 %) compared with patients receiving MTX (35.5 %) and placebo (18.9 %) [12].
The results of several small studies suggest that adalimumab may be an appropriate treatment option for patients who have not achieved an adequate response to prior systemic treatments, underscoring the need for confirmation with additional data [13–18]. In this post hoc analysis, we assessed the efficacy of adalimumab vs. placebo in patients with psoriasis who received systemic therapy (biologics, non-biologics, and/or oral PUVA) before enrolling in the clinical trial, and included patients who experienced failure (i.e., lack of improvement or worsening of psoriasis) with one or more prior therapies.
Methods
Data Sources
Pooled data from three double-blind, placebo-controlled efficacy and safety studies of adalimumab for the treatment of moderate to severe psoriasis (N = 1469) were used for this post hoc analysis. Patients from the placebo and adalimumab (80 mg at week 0, then 40 mg every other week [eow] starting at week 1) treatment groups were included in the analysis. Patients who had received a systemic biologic, non-biologic, or oral/topical PUVA were identified; additionally, patients who had not responded to prior treatments were also identified. Assessment of prior treatment outcomes was based on patient self-report rather than medical records or assessment scales. Prior treatment was classified as treatment failure if the patient described his or her psoriasis as the “same” or “worse” after the treatment.
Study Design and Patients
The designs of the three studies used for this pooled post hoc analysis have been described previously [11, 12, 19]. Study M02-528 [19] was a 12-week phase II study in which 148 patients were randomized in a 1:1:1 manner, with 147 patients receiving the following treatments: adalimumab 80 mg at week 0, followed by 40 mg eow starting at week 1 (n = 45); adalimumab 80 mg at weeks 0 and 1, followed by 40 mg weekly starting at week 2 (n = 50); or placebo (n = 52). PASI responses were evaluated at weeks 1, 2, 4, 8, and 12. The CHAMPION study [12] was a 16-week phase III trial in which 271 patients were randomized in a 2:2:1 manner to receive adalimumab 80 mg at week 0, followed by 40 mg eow starting at week 1 (n = 108); MTX (not included in this analysis) 7.5–25.0 mg weekly starting at week 0 (n = 110); or placebo (n = 53). PASI responses were evaluated at weeks 1, 2, 4, 8, 12, and 16. The REVEAL study [11] was a 16-week (Period A) phase III trial in which 1212 patients were randomized in a 2:1 manner to receive adalimumab 80 mg at week 0, followed by 40 mg eow starting at week 1 (n = 814) or placebo (n = 398). PASI responses were evaluated at weeks 4, 8, 12, and 16.
Inclusion and exclusion criteria were similar for each study, which facilitated combining data from the separate study populations. Patients included adults with moderate to severe plaque psoriasis that affected ≥10 % of body surface area (≥5 % for Study M02-528), who had a Physician Global Assessment (PGA) of at least “moderate” disease severity for the CHAMPION and REVEAL studies, and a PASI score of ≥10 for the CHAMPION study or a PASI score ≥12 for the REVEAL study. Patients who had previously used systemic TNF antagonists were excluded from both studies; patients who had previously used MTX were excluded from the CHAMPION study. Washout periods for prior treatments were 2 weeks for topicals and phototherapy, 4 weeks for non-biologic systemic therapies (including PUVA in the REVEAL study), 6 weeks for efalizumab in the REVEAL study, and 12 weeks for biologic therapies.
Study Assessments
The primary efficacy endpoint was PASI75 response at week 16 (except for Study M02-528, which was a 12-week study). The PASI score (range 0–72) assesses the severity of lesions with respect to erythema, induration, and desquamation and the body surface area involved on the head, torso, and upper and lower extremities. Improvements of ≥90 and ≥100 % from baseline in PASI score (PASI90 and PASI100, respectively) were similarly assessed.
Information was collected on the prior use of systemic treatments for psoriasis and patients’ self-reported recollection of their response to treatment. For the M02-528 study, information about prior psoriasis treatments and their effectiveness was collected for the previous 12 months; for the CHAMPION and REVEAL studies, information about all lifetime psoriasis treatment was collected, and response to treatments from the previous 12 months was recorded. Within each category of prior treatment, multiple prior treatments per patient may have been used. For this post hoc analysis, patients who recalled that their psoriasis was the “same or worse” with a prior therapy were considered to have had a lack of response.
Statistical Analyses
Efficacy data from the first 16 weeks (12 weeks for M02-528) of the double-blind period of each study were pooled and analyzed. Efficacy analyses were conducted using the intent-to-treat population, and nonresponder imputation was used for missing PASI data (i.e., patients with missing PASI data were counted as nonresponders). The Fisher exact test was used to calculate p-values for differences between treatment groups.
Results
Patients
Baseline demographics and clinical characteristics were similar between treatment groups; the majority of patients were male and white, and the mean disease duration was 18–19 years (Table 1). The clinical characteristics of the treatment groups included in this analysis were typical of patients with moderate to severe plaque psoriasis. Psoriasis treatments received within the previous 12 months were comparable between the placebo and adalimumab treatment groups, with the majority of patients receiving treatment with topical therapies (Table 2). The rates of responses to prior therapies (i.e., failure [“same or worse”] or improvement) were similar in the placebo and adalimumab groups in the trials analyzed here (Table 3).
Table 1.
Baseline demographics and clinical characteristics
| Placebo (n = 503) | Adalimumab (n = 966) | |
|---|---|---|
| Age, mean ± SD, years | 45 ± 13 | 44 ± 13 |
| Male, % | 65 | 67 |
| White, % | 91 | 92 |
| Body weight, mean ± SD, kg | 93 ± 23 | 92 ± 23 |
| Psoriasis duration, mean ± SD, years | 19 ± 11 | 18 ± 12 |
| BSA affected, mean ± SD, % | 26 ± 15 | 27 ± 16 |
| PASI score, mean ± SD | 19 ± 7 | 19 ± 7 |
| Psoriatic arthritis, % | 28 | 27 |
BSA body surface area, PASI Psoriasis Area and Severity Index, SD standard deviation
Table 2.
Psoriasis treatment within the previous 12 months
| Type of treatmenta, n (%) | Placebo (n = 503) | Adalimumab (n = 966) |
|---|---|---|
| Topical | 380 (75.5) | 743 (76.9) |
| Phototherapy | 89 (17.7) | 176 (18.2) |
| Systemic non-biologic | 121 (24.1) | 232 (24.0) |
| Acitretin | 20 (4.0) | 33 (3.4) |
| Tazarotene | 17 (3.4) | 17 (1.8) |
| Cyclosporine | 18 (3.6) | 32 (3.3) |
| Methotrexate | 45 (8.9) | 94 (9.7) |
| Other | 31 (6.2) | 76 (7.9) |
| Systemic biologic | 63 (12.5) | 106 (11.0) |
| Alefacept | 23 (4.6) | 34 (3.5) |
| Efalizumab | 20 (4.0) | 30 (3.1) |
| Other | 24 (4.8) | 43 (4.5) |
| Laser | 3 (0.6) | 4 (0.4) |
aPatients may have received multiple previous treatments; therefore, the sums may exceed 100 %
Table 3.
Response to prior psoriasis treatment within the previous 12 months
| Prior treatment, n (%) | Placebo group in current analysis | Adalimumab group in current analysis | ||
|---|---|---|---|---|
| Prior response: same or worse | Prior response: better | Prior response: same or worse | Prior response: better | |
| Phototherapy | 49 (55.1) | 40 (44.9) | 79 (44.9) | 97 (55.1) |
| Systemic non-biologic | 47 (38.8) | 74 (61.2) | 121 (50.0) | 121 (50.0) |
| Systemic biologic | 21 (31.8) | 45 (68.2) | 45 (41.7) | 63 (58.3) |
Efficacy Assessments
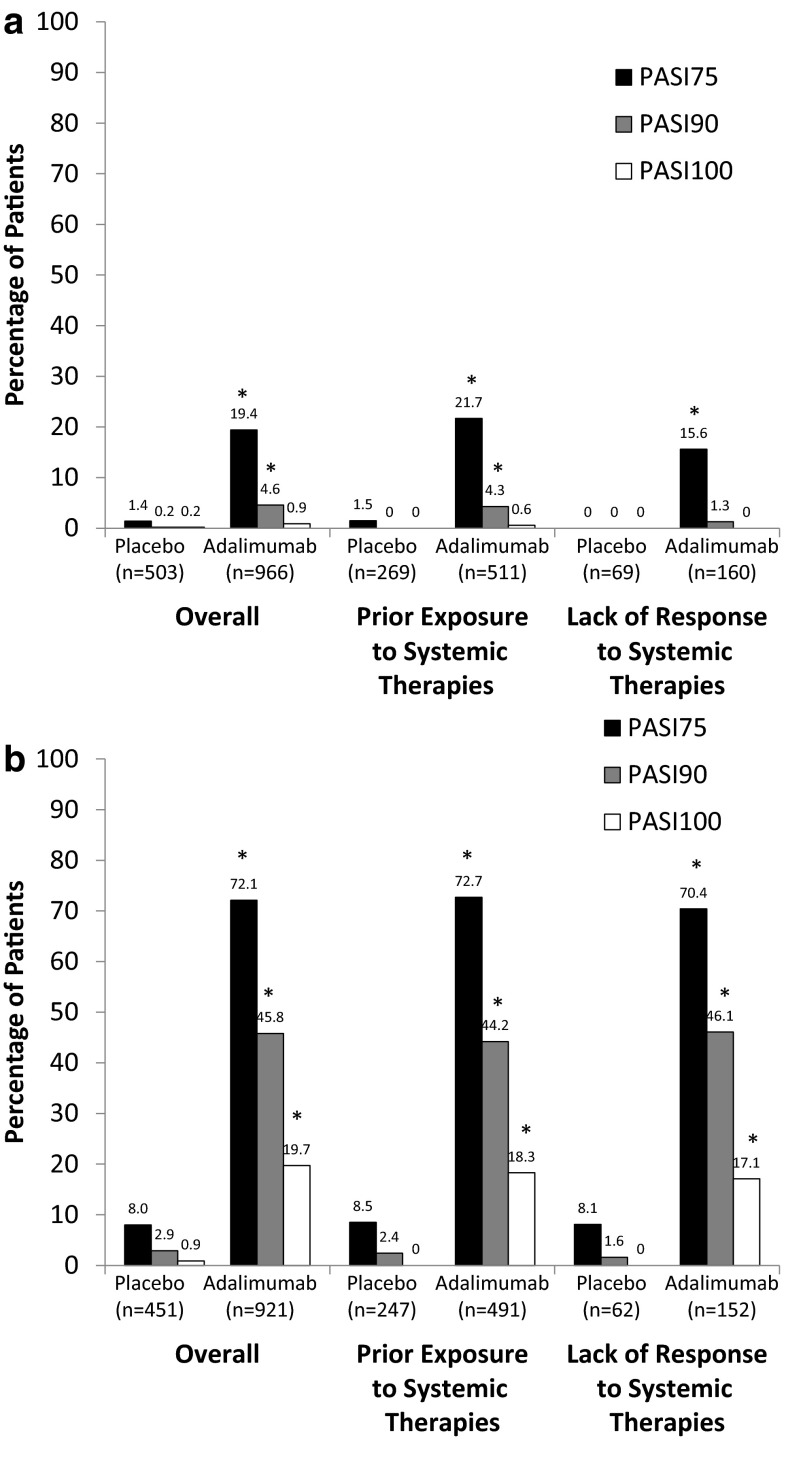
Patients treated with adalimumab achieved significantly higher PASI75 response rates compared with patients receiving placebo, regardless of whether they had previously received systemic treatment or had not responded to previous systemic therapy (Fig. 1). By 4 weeks (first evaluation), PASI75 response rates were significantly greater (p < 0.001) in patients treated with adalimumab compared with patients receiving placebo in all treatment groups (overall, 19.4 % [187/966] vs. 1.4 % [7/503]; prior treatment, 21.7 % [111/511] vs. 1.5 % [4/269]; and prior failures, 15.6 % [25/160] vs. 0 % [0/69]; Fig. 1a). At 16 weeks, a significantly higher (p < 0.001) percentage of patients treated with adalimumab compared with those receiving placebo achieved PASI75 in all treatment groups (overall, 72.1 % [664/921] vs. 8.0 % [36/451]; prior treatment, 72.7 % [357/491] vs. 8.5 % [21/247]; and prior failures, 70.4 % [107/152] vs. 8.1 % [5/62]; Fig. 1b).
Fig. 1.
PASI75, PASI90, and PASI100 response rates overall and by experience with prior systemic therapies at a 4 weeks and b 16 weeks. Patients from Study M02-528 were excluded from the analysis at 16 weeks because it was a 12-week study. PASI75, PASI90, and PASI100 improvement of ≥75, ≥90, and 100 % from baseline, respectively, in the Psoriasis Area and Severity Index score. *p < 0.001 compared with placebo
Similar results between adalimumab and placebo were observed for PASI90 and PASI100 responses. At 4 weeks, PASI90 response rates were significantly greater (p < 0.001) in patients treated with adalimumab compared with patients receiving placebo overall (4.6 % [44/966] vs. 0.2 % [1/503]) and in patients who had previously received systemic therapy (4.3 % [22/511] vs. 0 % [0/269]); improvement was not significantly different with adalimumab vs. placebo for patients who had not responded to prior therapy (1.3 % [2/160] vs. 0 % [0/69]; Fig. 1a). No statistically significant difference in PASI100 response rates was observed between adalimumab and placebo at 4 weeks. At 16 weeks, however, significantly higher (p < 0.001) percentages of patients treated with adalimumab compared with placebo achieved PASI90 and PASI100 in all groups based on prior treatment experience (Fig. 1b).
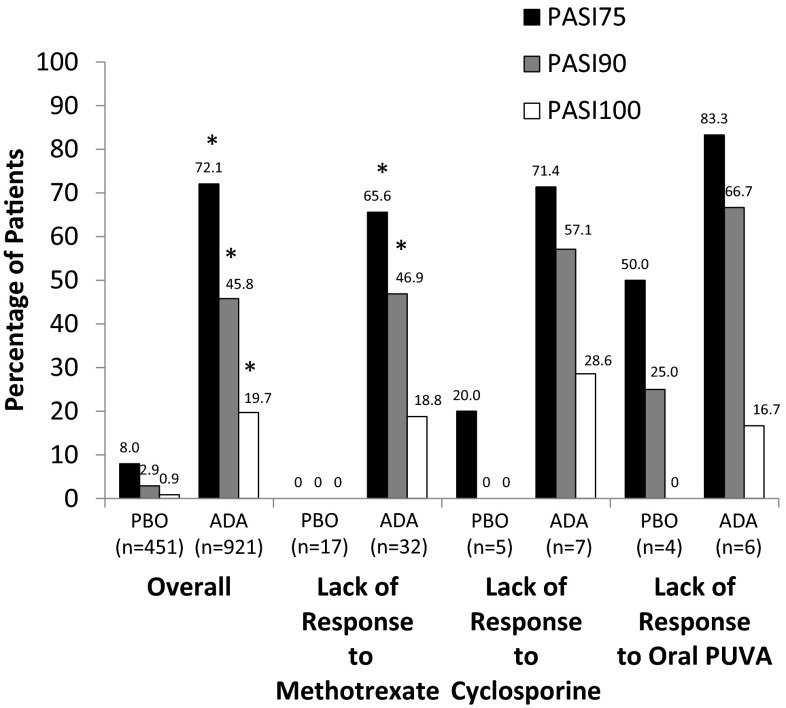
For patients whose previous MTX therapy failed, the percentage of patients achieving PASI75 at week 16 with adalimumab was significantly greater than for patients who received placebo (65.6 % [21/32] vs. 0 % [0/17]; p < 0.001; Fig. 2). PASI90 rates were also higher with adalimumab compared with placebo (46.9 % [15/32] vs. 0 % [0/17]; p = 0.001); however, PASI100 responses with adalimumab compared with placebo (18.8 % [6/32] vs. 0 % [0/17]) were numerically greater but not statistically significantly different. The sample sizes of patients with failure of previous cyclosporine or PUVA therapy were extremely small. At week 16, the percentage of patients achieving PASI75 who did not respond to previous therapy with cyclosporine or PUVA was numerically higher in patients treated with adalimumab (71.4 % [5/7] and 83.3 % [5/6], respectively) compared with patients receiving placebo (20.0 % [1/5] and 50.0 % [2/4], respectively; Fig. 2); similar patterns were observed with PASI90 and PASI100 response rates.
Fig. 2.
PASI75, PASI90, and PASI100 response rates at week 16 overall and by type of prior failed systemic therapy. Patients from Study M02-528 were excluded because it was a 12-week study. ADA adalimumab, PASI75, PASI90, and PASI100 improvement of ≥75, ≥90, and 100 % from baseline, respectively, in the Psoriasis Area and Severity Index score, PBO placebo, PUVA psoralen plus ultraviolet A. *p ≤ 0.001 compared with placebo
Safety
Generally, adverse event (AE) occurrence was similar across groups. Among the patients who reported AEs, most reported typically mild to moderate AEs (Table 4). Serious AEs occurred in ≤3 % of patients in any group; serious infections occurred in ≤1 % of patients in any group. No patients developed tuberculosis during the double-blind randomized periods described in this analysis. One patient receiving adalimumab and one receiving placebo had malignancies other than lymphoma, hepatosplenic T-cell lymphoma, leukemia, nonmelanoma skin cancer, or melanoma.
Table 4.
Adverse events
| Patients, n (%) | Overall | Prior exposure to systemic therapies | Lack of response to systemic therapies | |||
|---|---|---|---|---|---|---|
| Placebo (n = 503) | Adalimumab (n = 966) | Placebo (n = 269) | Adalimumab (n = 511) | Placebo (n = 69) | Adalimumab (n = 160) | |
| AE | 297 (59) | 615 (64) | 162 (60) | 311 (65) | 47 (68) | 96 (60) |
| Serious AE | 8 (2) | 18 (2) | 1 (<1) | 10 (2) | 0 | 4 (3) |
| AE leading to discontinuation | 11 (2) | 18 (2) | 5 (2) | 8 (2) | 5 (7) | 5 (3) |
| Severe AE | 15 (3) | 27 (3) | 5 (2) | 12 (2) | 2 (3) | 6 (4) |
| Drug-related AE | 87 (17) | 221 (23) | 55 (20) | 122 (24) | 14 (20) | 31 (19) |
| AE leading to death | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 118 (23) | 287 (30) | 63 (23) | 154 (30) | 20 (29) | 42 (26) |
| Serious infection | 4 (1) | 5 (1) | 0 | 3 (1) | 0 | 1 (1) |
| Legionella infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Diverticulitis | 0 | 1 (<1) | 0 | 0 | 0 | 0 |
| Opportunistic infection (excluding oral candidiasis and TB) | 0 | 0 | 0 | 0 | 0 | 0 |
| Oral candidiasis | 0 | 0 | 0 | 0 | 0 | 0 |
| TB | 0 | 0 | 0 | 0 | 0 | 0 |
| Parasitic infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Reactivation of hepatitis B | 0 | 0 | 0 | 0 | 0 | 0 |
| PML | 0 | 0 | 0 | 0 | 0 | 0 |
| Malignancy | 2 (<1) | 6 (1) | 0 | 2 (<1) | 0 | 1 (1) |
| Lymphoma | 0 | 0 | 0 | 0 | 0 | 0 |
| HSTCL | 0 | 0 | 0 | 0 | 0 | 0 |
| NMSC | 1 (<1) | 4 (<1) | 0 | 2 (<1) | 0 | 1 (1) |
| Melanoma | 0 | 1 (<1) | 0 | 0 | 0 | 0 |
| Leukemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Malignancy other than lymphoma, HSTCL, leukemia, NMSC, or melanoma | 1 (<1) | 1 (<1) | 0 | 0 | 0 | 0 |
| Allergic reaction (including angioedema and anaphylaxis) | 3 (1) | 13 (1) | 3 (1) | 7 (1) | 0 | 1 (1) |
| Lupus-like reactions and SLE | 0 | 0 | 0 | 0 | 0 | 0 |
| Vasculitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Sarcoidosis | 0 | 1 (<1) | 0 | 1 (<1) | 0 | 1 (1) |
| Autoimmune hepatitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Myocardial infarction | 1 (<1) | 1 (<1) | 1 (<1) | 0 | 0 | 0 |
| Cerebrovascular accident | 0 | 0 | 0 | 0 | 0 | 0 |
| CHF | 0 | 1 (<1) | 0 | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 0 | 0 | 0 | 0 |
| Interstitial lung disease | 0 | 0 | 0 | 0 | 0 | 0 |
| Intestinal perforation | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 2 (<1) | 0 | 1 (<1) | 0 | 0 |
| Stevens–Johnson syndrome | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythema multiforme | 0 | 0 | 0 | 0 | 0 | 0 |
| Psoriasis worsening or new onset | 11 (2) | 10 (1) | 5 (2) | 4 (1) | 3 (4) | 3 (2) |
| Demyelinating disorder | 0 | 0 | 0 | 0 | 0 | 0 |
| Amyotrophic lateral sclerosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Reversible posterior leukoencephalopathy | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematologic disorders (including pancytopenia) | 1 (<1) | 2 (<1) | 1 (<1) | 1 (<1) | 1 (1) | 0 |
| Liver failure/event | 2 (<1) | 2 (<1) | 0 | 1 (<1) | 0 | 0 |
| Administration-related medication error | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection-site reaction | 26 (5) | 69 (7) | 16 (6) | 40 (8) | 1 (1) | 10 (6) |
AE adverse event, CHF congestive heart failure, HSTCL hepatosplenic T-cell lymphoma, NMSC non-melanoma skin cancer, PML progressive multifocal leukoencephalopathy, SLE systemic lupus erythematosus, TB tuberculosis
Discussion
This analysis confirmed that adalimumab is efficacious for the treatment of moderate to severe psoriasis in patients who have received prior systemic therapy, including patients who did not respond to previous treatment. PASI75 response rates for patients treated with adalimumab who had previous exposure to, or lacked a response to, other systemic therapies or phototherapy were similar to the overall population. The effect was evident at the earliest assessment (week 4) and was maintained through the end of the analysis period (week 16). Similar patterns were observed for PASI90 and PASI100 response rates. This finding demonstrates that adalimumab is an efficacious treatment option for patients who received prior systemic therapy regardless of their prior treatment responses. There were no unexpected differences in safety profiles between adalimumab and placebo for the assessed groups that were based on experience with prior psoriasis therapy.
Only a few small studies to date have examined the effectiveness of adalimumab in patients who had an inadequate therapeutic response to other systemic therapies [15–18]. Of these studies, two evaluated the efficacy of adalimumab in patients who changed therapy from another TNF antagonist [15, 16]. A third study included patients who previously did not respond to conventional systemic therapies and up to two TNF antagonists (etanercept and infliximab) and, in some cases, also did not respond to treatment with efalizumab [17]. A fourth study examined patients who began treatment with adalimumab after failure of a variety of systemic therapies, including MTX, cyclosporine, PUVA, retinoids, fumaric acid esters, hydroxycarbamide, and biologics [18]. In an open-label uncontrolled study in 50 patients whose prior etanercept therapy failed, 40 % achieved a PASI75 response at week 12 with adalimumab [16]. In an open-label retrospective study in 13 patients treated with adalimumab after failure of etanercept, PASI75 was achieved by two patients (15 %) at week 12 and by three patients (23 %) at week 24 [15]. In an open-label study of 30 patients whose psoriasis was unresponsive to conventional systemic treatments and did not respond to all other biologics, 87 % achieved a PASI75 response at week 12 with treatment with adalimumab [17]. In a retrospective study of 21 patients whose prior systemic therapy failed, 38 % achieved a PASI75 response at week 16 with treatment with adalimumab [18]. These studies were neither placebo controlled nor randomized; thus, the results should be viewed with caution.
Two larger studies evaluated the efficacy of adalimumab in patients who had previously not responded to other systemic therapies that included biologics [13, 14]. In patients who either never responded, lost response, or were intolerant to prior TNF antagonist treatment (n = 282), PASI75 response was achieved in 53.8, 65.7, and 50.0 % of patients, respectively, following treatment with adalimumab for 16 weeks [13]. The current findings are consistent with data from the PROGRESS trial, a 16-week, open-label phase IIIb trial in which 61, 49, and 48 % of patients (n = 152) with a suboptimal response to MTX, etanercept, or phototherapy, respectively, achieved a PGA of “clear” or “minimal” at week 16 of treatment with adalimumab [14].
Although the current study included a large population from placebo-controlled double-blind studies, the results were obtained by a post hoc analysis of data pooled from three different trials, with the resultant possibility of heterogeneity; additionally, the statistical analyses were not adjusted for multiple comparisons. Although no data were collected after week 16, patients could continue adalimumab for 252 weeks in an open-label extension study (NCT00195676) [20]. Whereas response to adalimumab for this analysis was defined as PASI75, response to prior therapy was based on patient recall, which may be less reliable than data from medical records. It should be kept in mind that the definition of treatment failure or contraindication in clinical trials may not exactly correspond to the definitions in clinical practice, which may be influenced by the specific reimbursement system available to the patient. Data were unavailable for prior reimbursement status, the doses of prior therapies, and whether those dosages were optimized. The analysis did not include representative samples of patients who experienced treatment failure with several therapies that are currently widely used; for example, patients who received prior TNF antagonists were excluded from the trials. The pre-specified washout periods did not reflect clinical practice, in which patients may need treatment immediately following failed therapies. For some prior treatment subgroups (i.e., cyclosporine and PUVA), the number of patients who experienced treatment failure was small and may be responsible for the lack of significance in these groups.
Conclusion
Adalimumab was efficacious for the treatment of moderate to severe psoriasis regardless of prior exposure to or the lack of a response to treatment with systemic therapies. The PASI75, PASI90, and PASI100 responses for each subgroup based on prior treatment were similar to those of the overall population.
Acknowledgments
The authors acknowledge Marty Okun, MD, PhD, formerly of AbbVie Inc., for critical assistance with the analyses.
Compliance with Ethical Standards
Kim A. Papp has received grant/research support from AbbVie, Amgen, Anacor, Astellas, Boehringer Ingelheim, Celgene, Celtic, Cipher, Dow Pharma, Eli Lilly, Galderma, Janssen, Janssen Biotech (Centocor), Genentech, Isotechnika, Kyowa Kirin, MedImmune, Merck (MSD), Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Takeda, and UCB Pharma; he has served as a consultant for 3M, AbbVie, Akesis, Akros, Alza, Amgen, Astellas, Baxter, Boehringer Ingelheim, Celgene, Centocor, Cipher, Eli Lilly, Forward Pharma, Funxional Therapeutics, Galderma, Genentech, GlaxoSmithKline, Isotechnika, Janssen, Janssen Biotech (Centocor), J&J, Kyowa Kirin, Lypanosys, Medical Minds, MedImmune, Meiji Seika Pharma Co., Ltd., Merck (MSD), Merck-Serono, Mitsubishi Pharma, Mylan, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Serono, Stiefel, Takeda, UCB Pharma, and Vertex. April W. Armstrong serves as an investigator and/or consultant to AbbVie, Amgen, Janssen, Merck, Lilly, Celgene, Novartis, Pfizer, and Modernizing Medicine. Kristian Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Covagen, Forward Pharma, GlaxoSmithKline, Janssen-Cilag, Leo, Eli Lilly, Medac, Merck Sharp & Dohme, Novartis, Ocean Pharma, Pfizer, Regeneron, Takeda, UCB, and Xenoport. Mahinda Karunaratne and Wendell Valdecantos are employees of AbbVie and may own AbbVie stock and/or stock options.
Writing Assistance
Editorial and medical writing support was provided by Katherine Groschwitz, PhD, and Patrick Little, PhD, at Complete Publication Solutions, LLC; this support was funded by AbbVie Inc.
Financial Support
AbbVie sponsored the study and contributed to the design and conduct of the study, data management, data analysis, interpretation of the data, and in the preparation and approval of the manuscript.
References
- 1.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Psoriasis Guidelines Committee. Canadian Guidelines for the Management of Plaque Psoriasis, 2009. http://www.dermatology.ca/psoriasisguidelines. Accessed Jan 6, 2015.
- 3.Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1–70. doi: 10.1111/j.1468-3083.2009.03389.x. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald A, Burden AD. Psoriasis: advances in pathophysiology and management. Postgrad Med J. 2007;83(985):690–697. doi: 10.1136/pgmj.2007.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63(3):457–465. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 8.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 9.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis: results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 10.Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 11.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortonne JP, Chimenti S, Reich K, Gniadecki R, Sprogel P, Unnebrink K, et al. Efficacy and safety of adalimumab in patients with psoriasis previously treated with anti-tumour necrosis factor agents: subanalysis of BELIEVE. J Eur Acad Dermatol Venereol. 2011;25(9):1012–1020. doi: 10.1111/j.1468-3083.2010.03944.x. [DOI] [PubMed] [Google Scholar]
- 14.Strober BE, Poulin Y, Kerdel FA, Langley RG, Gu Y, Gupta SR, et al. Switching to adalimumab for psoriasis patients with a suboptimal response to etanercept, methotrexate, or phototherapy: efficacy and safety results from an open-label study. J Am Acad Dermatol. 2011;64(4):671–681. doi: 10.1016/j.jaad.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang T-S, Tsen-Fang T. Safety and effectiveness of adalimumab in patients with moderate-to-severe psoriasis who had inadequate therapeutic response to prior etanercept. Dermatologica Sinica. 2013;31(1):11–18. doi: 10.1016/j.dsi.2012.07.004. [DOI] [Google Scholar]
- 16.Bissonnette R, Bolduc C, Poulin Y, Guenther L, Lynde CW, Maari C. Efficacy and safety of adalimumab in patients with plaque psoriasis who have shown an unsatisfactory response to etanercept. J Am Acad Dermatol. 2010;63(2):228–234. doi: 10.1016/j.jaad.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Papoutsaki M, Chimenti MS, Costanzo A, Talamonti M, Zangrilli A, Giunta A, et al. Adalimumab for severe psoriasis and psoriatic arthritis: an open-label study in 30 patients previously treated with other biologics. J Am Acad Dermatol. 2007;57(2):269–275. doi: 10.1016/j.jaad.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Ryan C, Kirby B, Collins P, Rogers S. Adalimumab treatment for severe recalcitrant chronic plaque psoriasis. Clin Exp Dermatol. 2009;34(7):784–788. doi: 10.1111/j.1365-2230.2008.03161.x. [DOI] [PubMed] [Google Scholar]
- 19.Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi C, Sobell JM, Crowley JJ, Mrowietz U, Bao Y, Mulani PM, et al. Efficacy, safety and medication cost implications of adalimumab 40 mg weekly dosing in patients with psoriasis with suboptimal response to 40 mg every other week dosing: results from an open-label study. Br J Dermatol. 2012;167(3):658–667. doi: 10.1111/j.1365-2133.2012.11041.x. [DOI] [PubMed] [Google Scholar]