Abstract
Objective:
To test whether a silent new ischemic lesion (SNIL) on MRI after stroke predicted future recurrent ischemic stroke or vascular events.
Methods:
In this prospective study, we analyzed data from patients presenting with acute ischemic stroke who underwent MRI <24 hours and 5 and 30 days after symptom onset. The presence of a SNIL at 5 (5D-SNIL) and 30 (30D-SNIL) days was determined on diffusion-weighted and fluid-attenuated inversion recovery images. Patients were contacted every 3–6 months to identify recurrent clinical events. The log-rank test and Cox proportional hazard model were used to estimate the hazard ratio of recurrent ischemic stroke and composites of recurrent ischemic stroke, transient ischemic attack, acute coronary syndrome, and vascular death.
Results:
The 5D- and 30D-SNILs were found in 24.4% (66/270) and 7.4% (19/256) of patients. During the 5-year follow-up, clinical events were observed in 42 patients (15.6%). The 5D- and 30D-SNIL independently predicted recurrent ischemic stroke (hazard ratio [95% confidence interval] 2.9 [1.3–6.4] and 9.6 [4.1–22.1], respectively) and composite vascular events (2.4 [1.3–4.5] and 6.1 [3.1–12.4], respectively).
Conclusions:
Patients with a SNIL within the first few weeks after index stroke have an increased risk of recurrent ischemic stroke or vascular events. The presence of a SNIL on MRI could serve as a surrogate endpoint for clinical recurrence in secondary prevention clinical trials.
Because recurrent ischemic stroke (IS) increases the risk of disability and mortality,1,2 the identification of high-risk patients for recurrent IS and the exploration of effective secondary stroke prevention are of great importance. Moreover, development of a surrogate marker for recurrent IS may be useful because clinical recurrence rate has substantially decreased over the last half-century.3 A surrogate endpoint, which requires smaller sample size and shorter study duration of clinical trials, allows early go/no-go decisions.4
Clinical recurrent IS might be underestimated, because clinically unnoticed acute cerebral infarcts can be seen on diffusion-weighted imaging (DWI).5 It has been reported consistently that silent new ischemic lesions (SNILs) on DWI are common during the early poststroke period.6–12 Serial MRI studies detected a SNIL from approximately 30% of patients up to 90 days after symptom onset.7 These SNILs were associated with subsequent clinical stroke and vascular death.13 Therefore, SNIL has been suggested as a potential surrogate endpoint for recurrent ischemic events. However, previous studies were limited by small sample size, single center, or retrospective design.
In this prospective study, we tested the hypothesis that patients having SNILs on follow-up MRIs at the first week and 30 days after stroke onset would carry high risk of future recurrent clinical IS and vascular events.
METHODS
Participants.
This was a prospective study performed at 2 university hospitals in South Korea. We screened consecutive stroke patients from September 1, 2005, to December 31, 2006, and enrolled patients who were aged ≥20 years and had an acute IS confirmed by initial DWI performed within 24 hours of symptom onset. Patients were excluded if they had severe neurologic disability (modified Rankin Scale score ≥5) at the time of discharge, had concomitant serious medical disorders making clinical follow-up unlikely or impossible (e.g., cancer or hepatic failure), had any contraindication to MRI scan, received thrombolysis (intravenous or intra-arterial), or underwent interventional procedures (e.g., catheter angiography, stenting, or angioplasty) or neurosurgery that potentially cause new brain infarcts.
Demographics, risk factors, baseline NIH Stroke Scale (NIHSS), stroke subtypes,14 laboratory data, and modified Rankin Scale score at discharge were collected using standardized case report forms.
Standard protocol approvals, registrations, and patient consents.
The institutional review board of each medical center approved the study, and each patient or legal guardian gave written informed consent.
MRI measures.
Two participating centers had the same MRI protocol for acute stroke. Acute stroke patients arriving at the emergency department (ED) within 24 hours of symptom onset underwent MRI as soon as possible and received follow-up MRI at 5 days after onset, while patients arriving at the ED beyond 24 hours of onset underwent single time point MRI unless they had symptom recurrence or progression.
Each patient enrolled in this study was scanned in a consistent 1.5-T MRI machine (Signa, GE Medical Systems, Milwaukee, WI, or Philips Medical Systems, Netherlands), as described previously.15 The acute stroke MRI protocol included DWI, fluid-attenuated inversion recovery (FLAIR) imaging, gradient echo T2*-weighted imaging, 3D time-of-flight magnetic resonance angiography (MRA), and contrast-enhanced MRA. In some patients, MRA was replaced with CT angiography. Follow-up angiography was performed in selected patients who had steno-occlusive arterial lesions on baseline angiography. For this study, follow-up DWI and FLAIR imaging were scheduled at 5 (±1) days and 30 (±5) days after symptom onset.
A SNIL at 5 days (5D-SNIL) was defined as a new lesion on 5-day DWI that was outside the region of the acutely symptomatic (index) lesion.6,7 Because some new ischemic lesions in the same vascular territory as the index stroke might represent fragmentation of the initial embolus rather than a recurrent ischemic event, we did not consider new lesions accompanying significant (residual stenosis less than 50% on follow-up MRA) or complete recanalization as SNILs.9,10 In contrast, new ischemic lesions in the same vascular territory as the index stroke that were accompanied by significant residual stenosis (more than 50% stenosis on follow-up MRA) were considered as SNILs because they might represent recurrent embolism from intrinsic atherosclerotic lesions.9 A SNIL at 30 days (30D-SNIL) was defined as a new lesion on 30-day DWI/FLAIR images that was outside the area of the index lesion on baseline and 5-day DWI/FLAIR images.7,13
The presence of SNILs on follow-up images was determined by slice-to-slice comparison with previous images.6,7 Enlargement of previous lesions was not considered as a recurrent lesion. The 5D- and 30D-SNILs on DWI should be accompanied by low signal intensity on apparent diffusion coefficient maps. The location of SNILs was classified as occurring in the same or different vascular territory as the index ischemic lesion. Two stroke neurologists (D.-W.K. and H.S.) independently reviewed DWI and FLAIR images to determine the presence of SNILs blinded to all clinical information. Discrepancy between the 2 readers was reconciled by a third reader (H.-J.K.).
Clinical follow-up and outcome.
Patients were followed up to December 31, 2011. All patients received appropriate therapy for secondary stroke prevention (e.g., antiplatelet agents, anticoagulants, or statins) based on our centers' stroke care pathway. In brief, patients with high risk of cardioembolic sources (e.g., atrial fibrillation, prosthetic valve) or coagulopathies (e.g., protein C, S deficiency or antiphospholipid antibody syndrome) received anticoagulants, while those with large artery atherosclerosis, small vessel disease, or cryptogenic stroke were given antiplatelets.16 The presence of a SNIL did not affect the treatment decision or the choice of antithrombotic therapy.
A certified research coordinator blinded to imaging variables evaluated patients every 3–6 months at an outpatient clinic or by telephone interview depending on patients' circumstances or preferences. A questionnaire for verifying stroke-free status17 was used for the assessment of new clinical symptoms. We also instructed patients to report any new neurologic symptoms to a research coordinator immediately. If clinical events were suspected, patients were brought to medical attention and prompt neuroimaging.
The primary clinical endpoint was the time to occurrence of recurrent IS, which was defined as clinical findings consistent with stroke occurrence lasting ≥24 hours or lasting <24 hours but with imaging evidence of acute infarction.13 During the acute phase, systemic causes of clinical deterioration (e.g., infection) or worsening of initial symptoms (e.g., progression or hemorrhagic transformation) were not classified as a clinical recurrence. The secondary clinical endpoint was the time to occurrence of a composite of vascular events, which included recurrent IS, transient ischemic attack (TIA), acute coronary syndrome (ACS), or vascular death.13 TIA was defined as clinical findings consistent with the occurrence of stroke that lasted <24 hours and without imaging evidence of acute infarction. ACS included any symptoms attributed to obstruction of the coronary arteries (e.g., myocardial infarction and unstable angina). Vascular deaths included sudden death, death within 30 days of a cardiac event, or any sudden death that was not clearly nonvascular causes.13 If documented IS preceded a death, the outcome was classified as IS. All reports of recurrent clinical events were confirmed by investigators (D.-W.K. or M.-K.H.) after a review of medical records and available brain scans.
Statistical analysis.
Baseline clinical and imaging variables were compared between patients with and without a SNIL. Univariate analysis was performed using Pearson χ2 test, Student t test, or the Mann-Whitney U test as appropriate. The Kaplan-Meier method estimated the proportion of patients with clinical endpoints in groups stratified according to all clinical and imaging variables. Survival time was calculated from the onset of index stroke until the date of clinical events or last follow-up without a clinical event.13 Hypothesis testing was performed using the log-rank test. Cox proportional hazards model estimated the independent contributions of variables to the development of clinical endpoints. Variables with a p value <0.10 in univariate analyses were candidates for multivariable models. A backward elimination process (p < 0.05 to retain) was used to develop the final multivariable model. A 2-tailed p < 0.05 was considered significant. All statistical analyses were performed using SPSS for Windows (version 18.0; SPSS, Chicago, IL).
RESULTS
General characteristics.
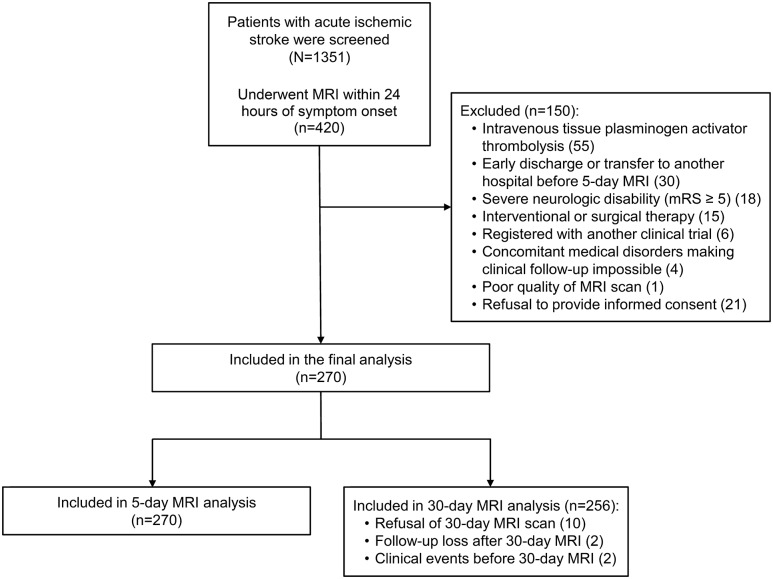
The 1,351 consecutive patients with acute IS were screened. Of those, 931 patients visited the ED beyond 24 hours of onset or could not undergo MRI due to contraindications, and the remaining 420 patients underwent a baseline MRI scan within 24 hours of onset. Of 420 patients, 150 were excluded (figure 1), and 270 patients were included for the final analysis. The mean (SD) age was 62.81 (11.56) years and 170 patients (63%) were male. All patients underwent a baseline and 5-day MRI scan, and 256 patients (94.8%) underwent a 30-day MRI scan (figure 1). There was no difference in baseline clinical, laboratory, and MRI characteristics between patients who underwent the 30-day MRI scan and those who did not.
Figure 1. Flow diagram of the study population.
Inclusion, exclusion, and the final number of patients analyzed. mRS = modified Rankin Scale.
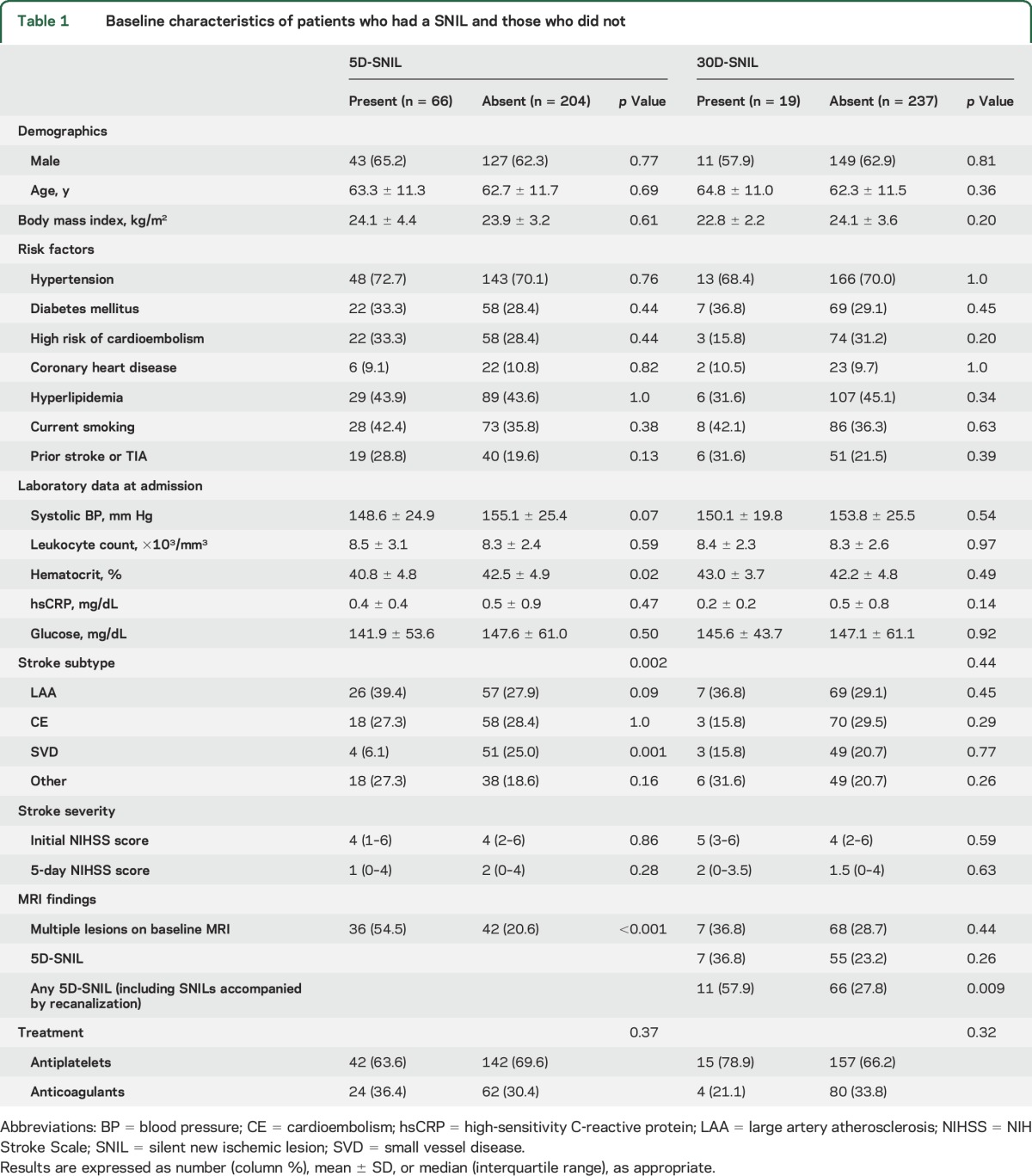
The median (interquartile range) time from symptom onset to MRI scan was 8.78 hours (4.22–14.67) for the baseline MRI, 4.68 days (4.09–5.31) for the 5-day MRI, and 31.76 days (29.97–34.73) for the 30-day MRI scan. Eighty-three patients (30.7%) had large artery atherosclerosis, 76 (28.1%) had cardioembolism, 55 (20.4%) had small vessel disease, and 56 (20.7%) had other or undetermined etiology. Baseline characteristics are described in table 1.
Table 1.
Baseline characteristics of patients who had a SNIL and those who did not
SNIL on MRI.
A 5D-SNIL was observed in 66 (24.4%) of the 270 patients: 84 (31.1%) had 5D-SNIL, but 18 of those had SNILs that were accompanied by significant or complete recanalization. A 30D-SNIL was observed in 19 (7.4%) of the 256 patients who underwent the 30-day MRI scan. Seven patients (2.6%) had both 5D- and 30D-SNILs. Of the 19 patients with a 30D-SNIL, 3 had a SNIL on both DWI and FLAIR imaging, 8 had a SNIL on DWI only, and another 8 had a SNIL on FLAIR imaging only. Regarding the location of the SNILs, 81.8% (54/66) of the patients with a 5D-SNIL and 68.4% (13/19) of the patients with a 30D-SNIL developed the SNIL within the same vascular territory as the index stroke lesion.
Multiple acute ischemic lesions on baseline DWI were associated with 5D-SNILs. Large artery atherosclerosis was more frequent, small vessel disease was less frequent, and hematocrit level was lower in patients who had a 5D-SNIL. There was no difference between patients with and without 30D-SNILs, except that any 5D-SNIL (i.e., including 5D-SNIL accompanied by recanalization) was associated with a 30D-SNIL. Antiplatelet or anticoagulant treatments were not associated with the occurrence of SNILs. These results are shown in table 1.
Clinical outcome.
Patients were followed for a median of 47.9 months (interquartile range 33.0–60.0 months) after the onset of index stroke. During follow-up, clinical recurrent events occurred in 42 patients (15.6%): recurrent IS in 25, TIA in 6, ACS in 5, and vascular death in 6. No patient had recurrent IS before the 5-day MRI scan. Two patients had a clinical event between the 5- and 30-day MRI scans (IS and vascular death, each in one patient). Of the 25 patients with recurrent IS, 11 (44%) had a 5D-SNIL, 9 (36%) had a 30D-SNIL, and 4 (16%) had both a 5D- and a 30D-SNIL. The lesion location of recurrent IS was within the same vascular territory as the index stroke in 10 patients (40%), the same vascular territory as the 5D-SNIL in 4 of the 11 patients (36.4%), and the same vascular territory as the 30D-SNIL in 3 of the 9 patients (33.3%).
SNIL and clinical outcome.
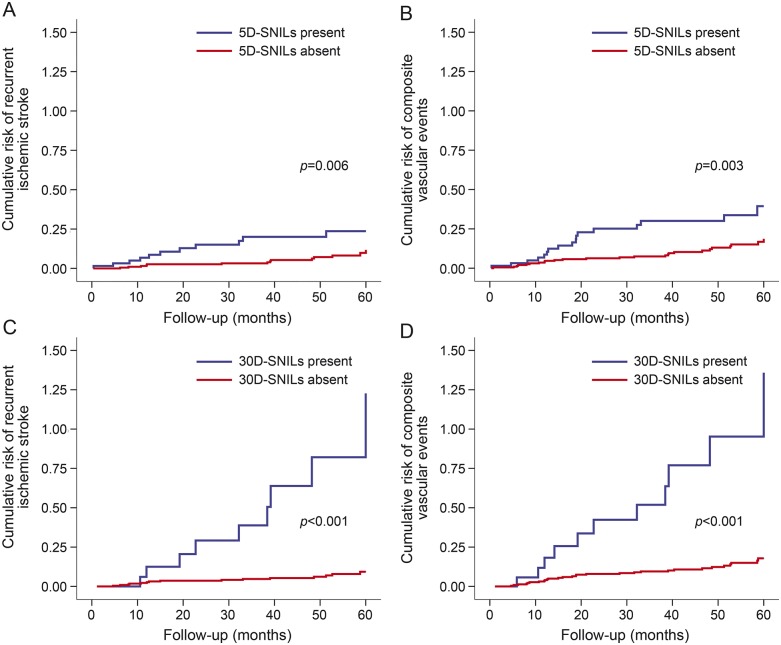
The log-rank test using the Kaplan-Meier method indicated that diabetes mellitus, hyperlipidemia, 5D-SNIL, and 30D-SNIL were associated with recurrent IS. Current smoking, 5D-SNIL, and 30D-SNIL were associated with composite vascular events (table 2, figure 2).
Table 2.
Association of variables with clinical outcomes in univariate analysis
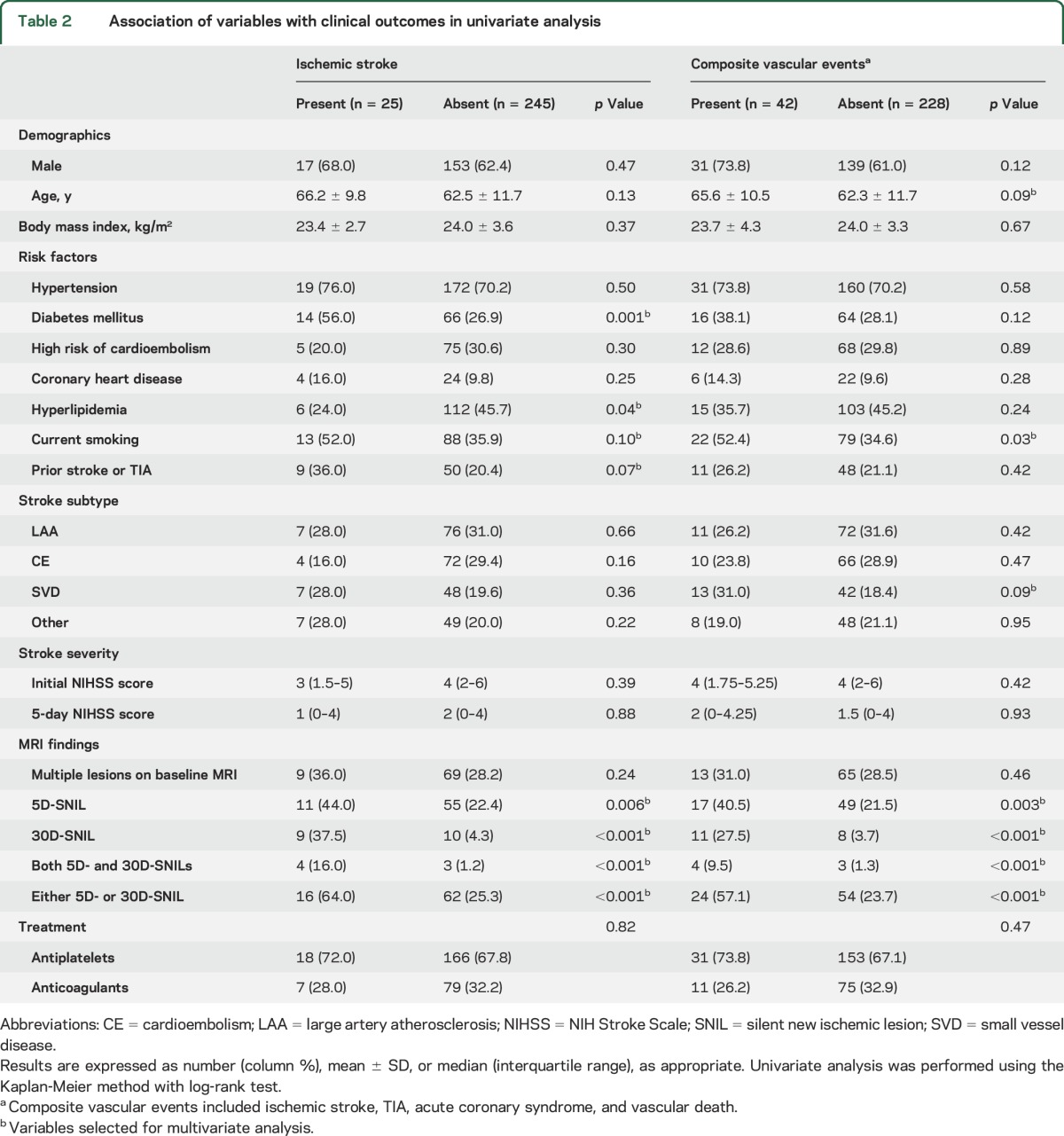
Figure 2. Results of Kaplan-Meier analysis.
Results show the cumulative risk of recurrent ischemic stroke and composite vascular events according to presence of a silent new ischemic lesion at 5 days (5D-SNIL) (A, B) and a 30D-SNIL (C, D). The curves differed according to the log-rank test.
Cox proportional hazards model showed that 5D-SNIL and 30D-SNIL were independent predictors of recurrent IS. For the secondary endpoint, 5D- and 30D-SNIL were associated with a composite of recurrent IS, TIA, ACS, and vascular death (table 3). An example of a patient with SNIL and clinical recurrence is shown in figure 3.
Table 3.
Independent variables associated with clinical outcomes by Cox proportional hazard model
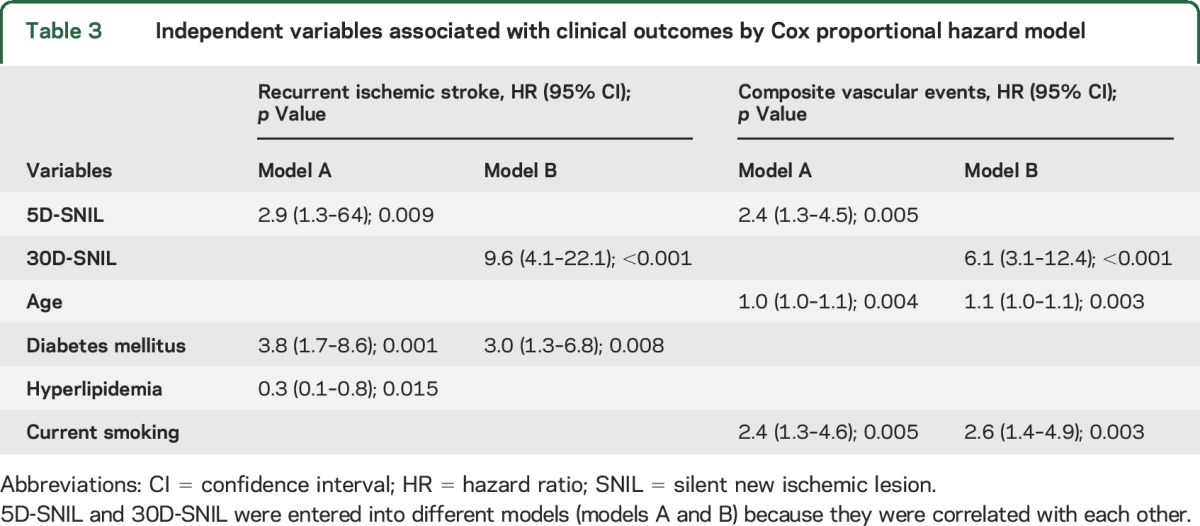
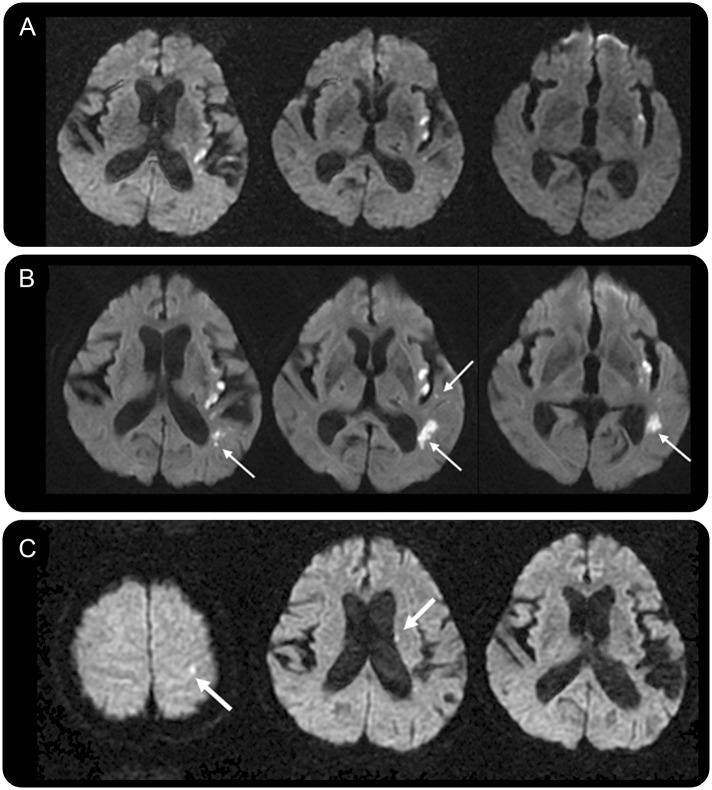
Figure 3. MRI recurrence and clinical recurrent ischemic stroke.
Images from a 69-year-old woman with sensory aphasia and dysarthria. (A) On the baseline diffusion-weighted imaging scan, there were multifocal infarcts in the left insular area. (B) Silent new ischemic lesions at 5 days (5D-SNIL) (thin arrows) were observed. (C) Twenty-three months later, the patient presented with right-sided weakness with new ischemic lesions in the left corona radiate and cortical area (thick arrows).
DISCUSSION
The present study investigated whether SNILs on MRI obtained at 5 and 30 days after stroke onset were associated with subsequent recurrent IS or vascular events. This study showed that 5D- and 30D-SNILs on MRI could independently predict subsequent recurrent IS and vascular events. These results provide confirmative evidence that the presence of a SNIL on MRI during the early poststroke period could identify patients carrying a high risk of clinical recurrence. This study also suggests that SNILs could serve as a surrogate endpoint of clinical recurrence in the early phase of secondary stroke prevention trials.
We found that 30D-SNILs were a stronger predictor of clinical recurrence than 5D-SNILs. One possible explanation for the better predictive power of 30D-SNILs for clinical recurrence is that they have a different pathomechanism than 5D-SNILs. Although we excluded SNILs accompanied by recanalization from 5D-SNILs, it is still possible that 5D-SNILs may include not only true recurrent ischemic events but also distal fragmentation of the initial embolus caused by recanalization.11 In this regard, 30D-SNILs might better reflect true recurrent ischemic events.
Multiple acute ischemic lesions on baseline DWI were associated with 5D-SNILs, which accords with the results of previous studies.6,11,12 However, multiple acute ischemic lesions on baseline DWI, unlike 5D- or 30D-SNILs, were not associated with recurrent clinical events in this study, conflicting with previous data.18,19 This discrepancy might be due partly to the difference in the definition of vascular territories of ischemic lesions. In addition, multiple ischemic lesions on baseline DWI do not necessarily mean stepwise occurrence of ischemic events. Likewise, patients with silent brain infarcts detected on baseline MRI were not at increased risk for recurrent stroke.20 In any case, the present study suggests that serial MRI evaluation may have an advantage over a single time point MRI scan for identifying patients with a high risk of clinical recurrence. Any 5D-SNILs were independently associated with 30D-SNILs, which is consistent with the previous study.7 This result supports the idea that patients with early new ischemic lesions may have a prolonged risk for recurrent ischemia.7
The rate of clinical recurrent IS in this study is lower than previous reports,21–23 which reflects that recurrence of stroke and vascular events has declined substantially over the past 5 decades due to advances in stroke management including antiplatelet therapy, statin, and improved risk factor control.3 On the other hand, silent recurrent ischemic lesions on MRI were much more frequent than clinical recurrence. It largely depends on the size and location of ischemic lesions whether the lesions are symptomatic or silent, because the pathologic processes of silent ischemic lesions on MRI and clinical stroke are same.24 The incidence of 5D- and 30D-SNILs in this study was relatively low compared to previous studies, which have reported the incidence as 23%–50% for early new DWI lesions6,7,10–13,16 and 22%–26% for SNILs at 30 or 90 days.7,13 The difference in incidence of SNILs among studies may be explained by several factors, such as the definition of SNILs (i.e., SNILs outside or inside initial hypoperfusion area, or any SNILs or SNILs without being accompanied by recanalization), time points of MRI taken, and stroke subtypes of the study population. One important reason for the lower incidence of SNILs in this study is that this study included more patients with small vessel disease than previous studies. It is well known that large artery atherosclerosis carries the highest risk of early recurrent ischemic lesions vs other stroke subtypes.6,9,11
A large sample size and long study duration are typically required for stroke prevention clinical trials. Furthermore, the number of patients or a follow-up period will be increasing in future trials because the rate of clinical recurrent stroke is gradually decreasing.3 Thus, a surrogate endpoint, which can be measured earlier and more frequently than the final endpoint, allows substantially fewer patients and a shorter follow-up period to test treatment effects.4 In multiple sclerosis, lesion burden or new lesions on MRI have already been used as an MRI surrogate endpoint for disease recurrence or progression.25 The use of MRI surrogate markers in phase II trials can inform an early decision as to whether or not to proceed with further trials of new drugs or devices.
Several limitations of this study should be noted. First, the patients included in this study were not a representative sample of all screened patients. Only patients who arrived early at hospital and underwent MRI within 24 hours of onset were enrolled. Because MRI scans should be performed at 5 and 30 days after stroke onset, patients who could not undergo serial MRI scans due to severe stroke or early discharge or transfer were excluded. Second, patients receiving IV thrombolysis were excluded. Not only endovascular but IV thrombolysis may affect the occurrence of SNILs and the relationship between SNILs and clinical events. It has been reported that IV thrombolysis was associated with early new DWI lesions, probably due to a short half-life of tissue plasminogen activator and early recanalization.11 Third, although the presence of SNILs was not supposed to affect the choice of treatment, treating physicians could not be blinded to MRI information. However, antiplatelet or anticoagulant treatment was not associated with SNILs or clinical events. Fourth, serial images were not coregistered; therefore small ischemic lesions may have been missed or identified as false-positives. We tried to overcome this limitation by having 2 experienced neurologists independently review the MRI scans. Finally, MRIs were obtained using 1.5-T machines, and more powerful machines may have detected more new ischemic lesions.
GLOSSARY
- ACS
acute coronary syndrome
- DWI
diffusion-weighted imaging
- ED
emergency department
- FLAIR
fluid-attenuated inversion recovery
- IS
ischemic stroke
- MRA
magnetic resonance angiography
- NIHSS
NIH Stroke Scale
- SNIL
silent new ischemic lesion
- TIA
transient ischemic attack
AUTHOR CONTRIBUTIONS
Dr. Kang contributed to the study concept, study design, data interpretation, and drafting and revising the manuscript. Dr. Han contributed to the study design, data interpretation, and drafting and revising the manuscript. Dr. H.J. Kim contributed to the data analysis and manuscript revision. Dr. Sohn contributed to the data analysis and manuscript revision. Dr. B.J. Kim contributed to the data analysis and manuscript revision. Dr. Kwon contributed to the data interpretation and manuscript revision. Dr. J.S. Kim contributed to the data interpretation and manuscript revision. Dr. Warach contributed to the study concept, data interpretation, and manuscript revision.
STUDY FUNDING
Supported by National Research Foundation of Korea grants 2011–0016868 and NRF-2014R1A2A1A11051280 funded by the Korean government and the Korea Health Technology R&D Project, Ministry for Health & Welfare, Republic of Korea, grants HI12C1847 and HI14C1983.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sacco RL, Benjamin EJ, Broderick JP, et al. American Heart Association Prevention Conference: IV: prevention and rehabilitation of stroke: risk factors. Stroke 1997;28:1507–1517. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Stroke recurrence: predictors, severity, and prognosis: The Copenhagen Stroke Study. Neurology 1997;48:891–895. [DOI] [PubMed] [Google Scholar]
- 3.Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation 2011;123:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellenberg S, Hamilton JM. Surrogate endpoints in clinical trials: cancer. Stat Med 1989;8:405–413. [DOI] [PubMed] [Google Scholar]
- 5.Kim BJ, Kang HG, Kim HJ, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke 2014;16:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol 2003;54:66–74. [DOI] [PubMed] [Google Scholar]
- 7.Kang DW, Latour LL, Chalela JA, Dambrosia JA, Warach S. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology 2004;63:2261–2265. [DOI] [PubMed] [Google Scholar]
- 8.Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology 2005;65:513–517. [DOI] [PubMed] [Google Scholar]
- 9.Kang DW, Kwon SU, Yoo SH, et al. Early recurrent ischemic lesions on diffusion-weighted imaging in symptomatic intracranial atherosclerosis. Arch Neurol 2007;64:50–54. [DOI] [PubMed] [Google Scholar]
- 10.Usnich T, Albach FN, Brunecker P, Fiebach JB, Nolte CH. Incidence of new diffusion-weighted imaging lesions outside the area of initial hypoperfusion within 1 week after acute ischemic stroke. Stroke 2012;43:2654–2658. [DOI] [PubMed] [Google Scholar]
- 11.Nolte CH, Albach FN, Heuschmann PU, et al. Silent new DWI lesions within the first week after stroke. Cerebrovasc Dis 2012;33:248–254. [DOI] [PubMed] [Google Scholar]
- 12.Braemswig TB, Usnich T, Albach FN, et al. Early new diffusion-weighted imaging lesions appear more often in stroke patients with a multiple territory lesion pattern. Stroke 2013;44:2200–2204. [DOI] [PubMed] [Google Scholar]
- 13.Kang DW, Lattimore SU, Latour LL, Warach S. Silent ischemic lesion recurrence on magnetic resonance imaging predicts subsequent clinical vascular events. Arch Neurol 2006;63:1730–1733. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 15.Kang DW, Han MK, Kim HJ, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology 2012;79:848–855. [DOI] [PubMed] [Google Scholar]
- 16.Kang DW, Yoo SH, Chun S, et al. Inflammatory and hemostatic biomarkers associated with early recurrent ischemic lesions in acute ischemic stroke. Stroke 2009;40:1653–1658. [DOI] [PubMed] [Google Scholar]
- 17.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke 2001;32:2232–2236. [DOI] [PubMed] [Google Scholar]
- 18.Wen HM, Lam WW, Rainer T, et al. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology 2004;63:1317–1319. [DOI] [PubMed] [Google Scholar]
- 19.Ay H, Gungor L, Arsava EM, et al. A score to predict early risk of recurrence after ischemic stroke. Neurology 2010;74:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber R, Weimar C, Wanke I, et al. Risk of recurrent stroke in patients with silent brain infarction in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) imaging substudy. Stroke 2012;43:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke: The Framingham study. Stroke 1982;13:290–295. [DOI] [PubMed] [Google Scholar]
- 22.Viitanen M, Eriksson S, Asplund K. Risk of recurrent stroke, myocardial infarction and epilepsy during long-term follow-up after stroke. Eur Neurol 1988;28:227–231. [DOI] [PubMed] [Google Scholar]
- 23.Hankey GJ, Jamrozik K, Broadhurst RJ, et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke 1998;29:2491–2500. [DOI] [PubMed] [Google Scholar]
- 24.Kelly PJ, Hedley-Whyte ET, Primavera J, He J, Gonzalez RG. Diffusion MRI in ischemic stroke compared to pathologically verified infarction. Neurology 2001;56:914–920. [DOI] [PubMed] [Google Scholar]
- 25.Radue EW, O'Connor P, Polman CH, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol 2012;69:1259–1269. [DOI] [PubMed] [Google Scholar]