Abstract
Objective:
To assess the relationship between seizure burden on continuous EEG (cEEG) and functional as well as cognitive outcome 3 months after subarachnoid hemorrhage (SAH).
Methods:
The study included all consecutive patients with a spontaneous SAH admitted to the Columbia University Medical Center Neurological Intensive Care Unit and monitored with cEEG between 1996 and 2013. Seizure burden was defined as the duration, in hours, of seizures on cEEG. Cognitive outcomes were measured with the Telephone Interview for Cognitive Status (TICS, ranging from 0 to 51, indicating poor to good global mental status).
Results:
Overall, 402 patients with SAH were included with a median age of 58 years (interquartile range [IQR] 46–68 years). The median duration of cEEG monitoring was 96 hours (IQR 48–155 hours). Seizures were recorded in 50 patients (12%), in whom the median seizure burden was 6 hours (IQR 1–13 hours). At 3 months, in multivariate analysis, seizure burden was associated with unfavorable functional and cognitive outcome. Every hour of seizure on cEEG was associated with an odds ratio of 1.10 (95% confidence interval [CI] 1.01–1.21, p = 0.04) to 3-month disability and mortality, and the TICS-score decreased, on average, by 0.16 points (adjusted coefficient −0.19, 95% CI −0.33 to −0.05, p = 0.01).
Conclusion:
In this study, after adjusting for established predictors, seizure burden was associated with functional outcome and cognitive impairment 3 months after SAH.
Seizures are seen in 10%–30% of critically ill patients undergoing continuous EEG (cEEG) and the vast majority of these are nonconvulsive.1,2 Among patients with subarachnoid hemorrhage (SAH), the presence of nonconvulsive status epilepticus (NCSE) is associated with a higher mortality at 3 months, with more than 90% of patients with SAH and NCSE dying within 3 months from SAH onset.3,4 Seizure prophylaxis is controversial, and most prior studies have not investigated the incremental impact of ongoing seizures—also known as the seizure burden—in patients with SAH.5 The argument for aggressive treatment of established nonconvulsive seizures (NCSZ) must take into account the impact of ongoing seizures on outcome, which is poorly understood and not yet studied in adults. One prior study in critically ill children found that seizure burden is associated with worse neurologic outcome.6
We studied the relationship between seizure burden and functional as well as cognitive outcome 3 months after onset of SAH.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the institutional review board. All patients or their health care proxies provided written informed consent for the collection of data and subsequent analyses.
Participants and study design.
All patients 18 years or older with a spontaneous SAH admitted to the Columbia University Medical Center Neurological Intensive Care Unit from October 1996 through May 2013 were offered enrollment into the Columbia University SAH Outcomes Project (SHOP), a prospectively maintained clinical SAH database. We then cross-matched the SHOP database with the cEEG database, and included patients who underwent continuous EEG monitoring for at least 12 consecutive hours. The study design is retrospective.
cEEG recording.
The primary indication for obtaining cEEG was to screen for NCSZ, and to monitor for changes consistent with delayed cerebral ischemia.4,7 cEEG was recorded digitally using 21 electrodes according to the International 10–20 System. Recordings were reviewed at least twice daily by a board-certified electroencephalographer. Two board-certified neurologists (G.M.D.M. and D.P.) reviewed all the EEG reports for data abstraction.
cEEG features.
Seizures were defined as any rhythmic ictal event, defined as any spikes, sharp waves, or sharp-slow wave complexes lasting for 10 seconds or more at either a frequency of at least 3 per second or, if lower, of at least 1 per second and with clear evolution in frequency, morphology, or location. NCSE was defined as seizure lasting for 5 consecutive minutes or more.3 Seizure burden was defined as the total amount of recorded time spent seizing on cEEG (in hours).
Clinical definitions.
Severity of admission neurologic grade was assessed using the Hunt-Hess and World Federation of Neurological Surgeons SAH grading scales.8,9 By convention, we used the Hunt-Hess scale as the primary instrument for grading neurologic impairment, with grades 1, 2, and 3 classified as good grade and grades 4 and 5 (stupor and coma, respectively) as poor grade. CT scans were evaluated by study neurointensivists for extent of initial bleeding using the modified Fisher Scale,10 Hijdra SAH score,11 intraventricular hemorrhage score,12 and volume of space-occupying subarachnoid or intraparenchymal clot using the ABC/2 method.13 Hydrocephalus was classified as present or absent based on the bicaudate index according to the upper limit of normal for decile of age.14 Medical and surgical management was otherwise performed as described previously.15 Following institutional protocol, prophylactic antiepileptic medication was administered for 1 week after SAH, which was then discontinued unless seizures had been recorded on EEG.16 Phenytoin was the preferred drug until 2005, i.e., until the publication by Naidech et al.17 associating phenytoin with worse cognitive outcomes. Following the publication, levetiracetam became the prophylactic drug of choice. Isolated scalp seizures were initially treated with phenytoin or levetiracetam and status epilepticus with midazolam infusions. Periodic epileptiform discharges (PEDs) were not considered seizures and there was no attempt made to eliminate them with medications; however, patients with PEDs were maintained on anticonvulsants to prevent seizures.
Outcomes assessment.
At 3 months, trained personnel assessed functional outcome with the modified Rankin Scale (mRS) and cognitive outcome with the Telephone Interview for Cognitive Status (TICS) by in-person or telephone interview. The mRS is a validated 7-point scale categorizing outcome as (0) no neurologic deficits, (1) no significant disability, (2) slight disability, (3) moderate disability (requires assistance in activities of daily life, but able to walk unassisted), (4) moderately severe disability (requires assistance in activities of daily life and unable to walk unassisted), (5) severe disability (bedridden, incontinent), (6) dead. The TICS evaluates orientation, attention, language, long-term memory, motor function, and verbal memory and is scored from 0 (worst) to 51 (best).18 All personnel assessing outcome were blinded to the cEEG findings including seizure burden.
Statistical analysis.
Discrete variables were expressed as counts (percentages) and continuous variables as medians (interquartile range [IQR]). Frequency comparisons for categorical variables were performed by Fisher exact test. Two-group comparisons of continuous variables were performed with the Mann-Whitney U test.
Two sets of multivariate models with different outcome measures were run. First, a multivariable logistic regression model was performed assessing the association between seizure or seizure burden and unfavorable functional outcome (mRS 4–6) at 3 months after adjusting for established outcome predictors.15 To take into consideration the effect of time across the 17 years of the study, we also adjusted for the year of study inclusion.
Second, a multivariable linear regression model was run assessing the association between global cognitive outcome (TICS) at 3 months and seizure or seizure burden after adjusting for established predictors of cognitive outcome. To take into consideration the switch of the preferred anticonvulsive agent from phenytoin to levetiracetam, we adjusted for the study inclusion period before vs after 2005. All statistical tests were 2-tailed, and we considered a p value <0.05 as statistically significant. Stata version 12 was used for statistical software.
RESULTS
Patients.
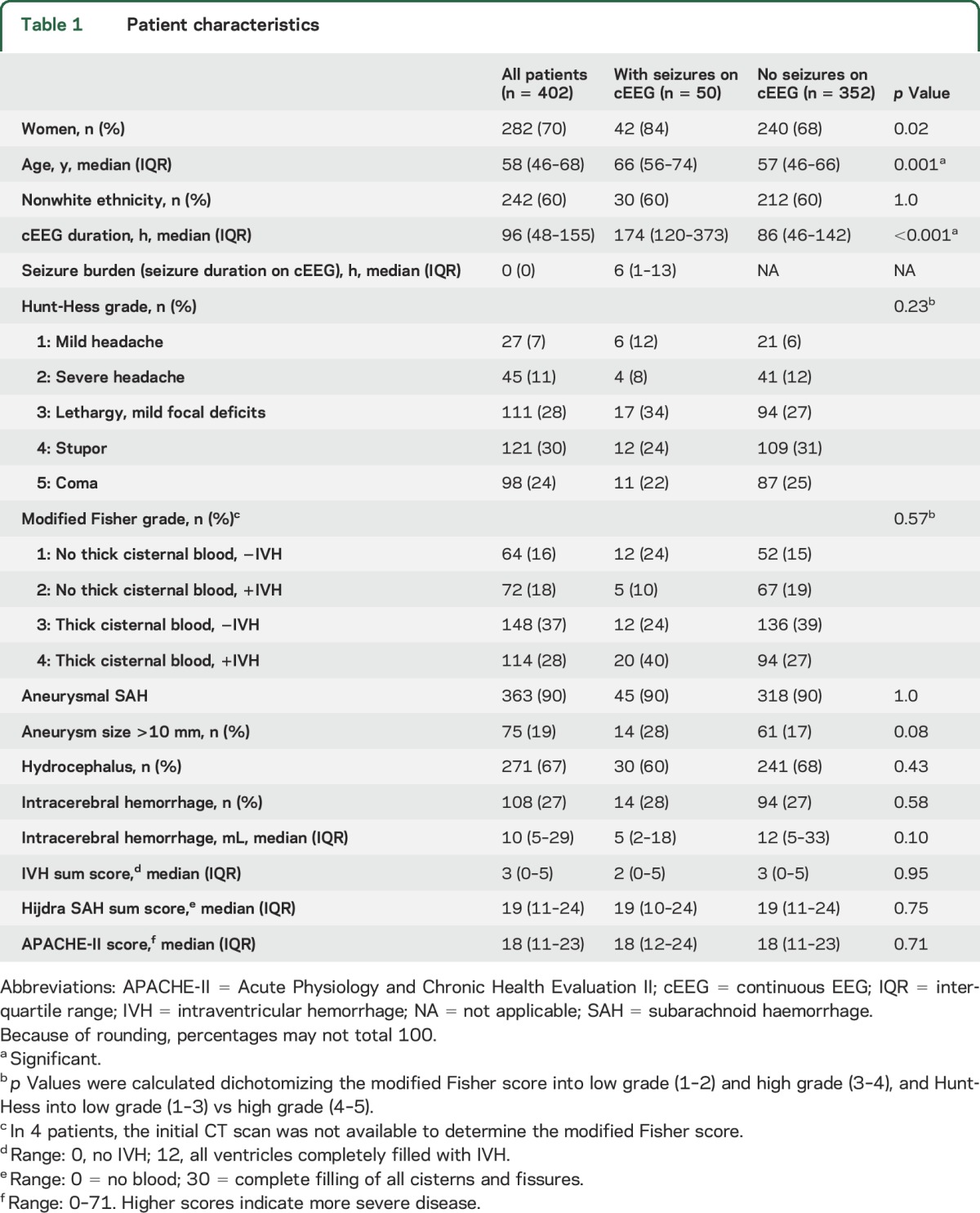
From October 1996 through May 2013, 402 SAH patients underwent cEEG for an average duration of 96 hours (IQR 48–155), totaling 5.75 patient-years of cEEG. Seventy percent of patients were women, 60% were of nonwhite ethnicity, and the median age was 58 years (IQR 46–68). Fifty patients (12%) had seizures on cEEG, 46 of which (92% or 11% of the whole cohort) had NCSE and 4 NCSZ (8% or 1% of the whole cohort). All seizures were nonconvulsive. Patients with seizures were more frequently women (84% vs 68%, p = 0.02), older (66 years [IQR 56–74] vs 57 years [IQR 46–66], p < 0.001), and monitored longer (174 hours [120–373] vs 86 hours [46–142], p < 0.001]) than patients without seizure (table 1). On admission, patients with subsequent seizures on cEEG were not more disabled than patients without seizures (Hunt-Hess 4–5 46% vs 56%, p = 0.23), nor did they show more frequently a thick clot in the basal cisterns (modified Fisher score 3–4 64% vs 66%, p = 0.57) or intracerebral hemorrhage (28% vs 27%, p = 0.58). When present, seizures on cEEG lasted for 6 hours on average (IQR 1–13).
Table 1.
Patient characteristics
Functional outcome at 3 months.
At 3 months, information on functional outcome was available for 308 patients (77%), of whom 178 (58%) were either disabled or dead. Unfavorable outcomes were more frequent among patients with seizures (81% vs 54%, p = 0.001) (table 2). The detection of any seizure on cEEG (NCSZ or NCSE) was associated with more than threefold elevated odds of unfavorable outcome at 3 months (odds ratio [OR] 3.67, 95% confidence interval [CI] 1.34–10.1, p = 0.01) after adjusting for established predictors of outcome (tables 3, e-1 on the Neurology® Web site at Neurology.org). In the same logistic regression model, the magnitude of association between NCSE and unfavorable functional outcome at 3 months was even stronger (OR 4.84, 95% CI 1.53–15.26, p = 0.007). Seizure burden was also associated with worse functional outcome in the multivariate analysis (OR 1.10, 95% CI 1.01–1.21, p = 0.04) after adjusting for established predictors of functional outcome (tables 3, e-2, figure, A).
Table 2.
Univariate analysis for 3-month outcome
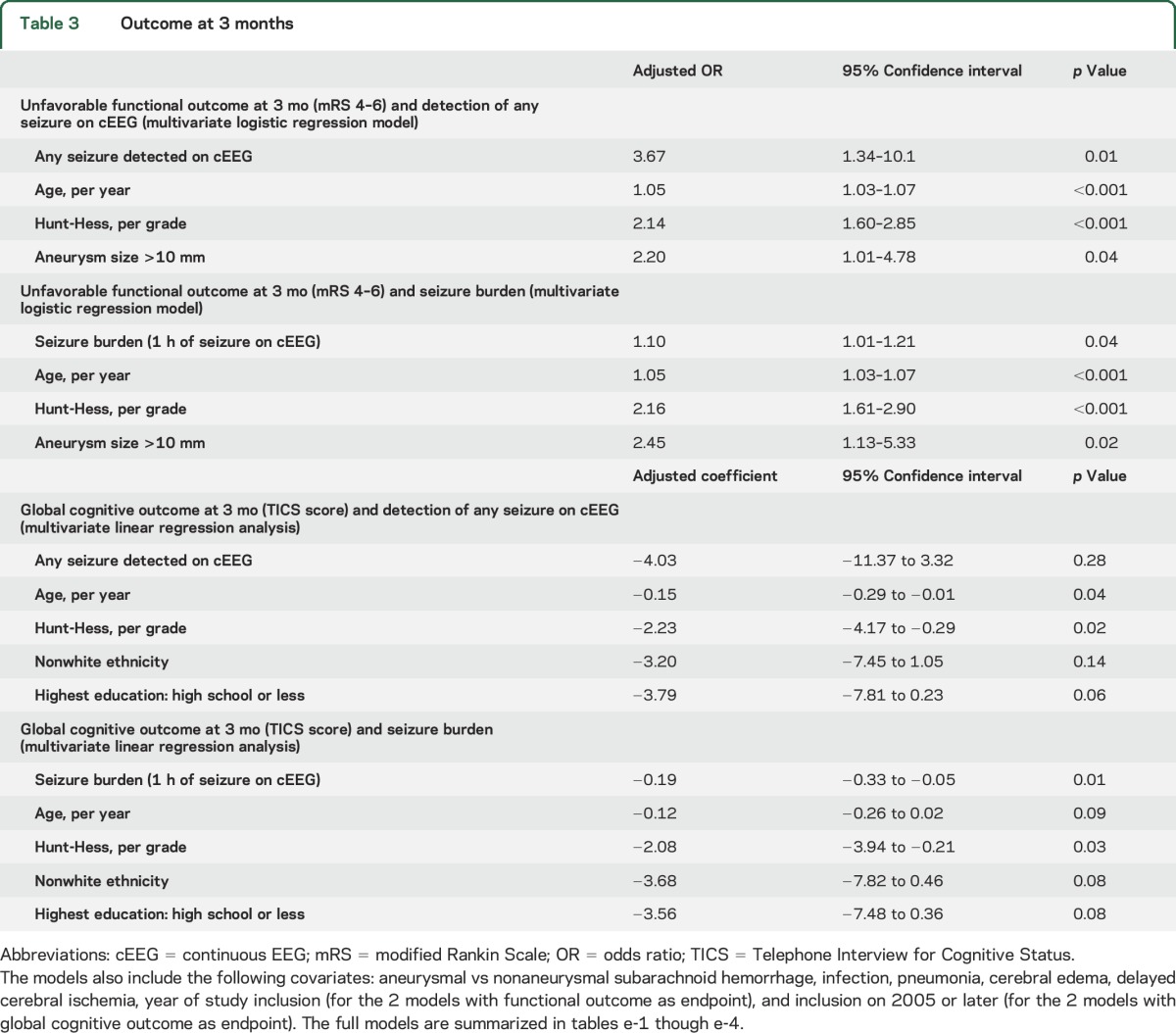
Table 3.
Outcome at 3 months
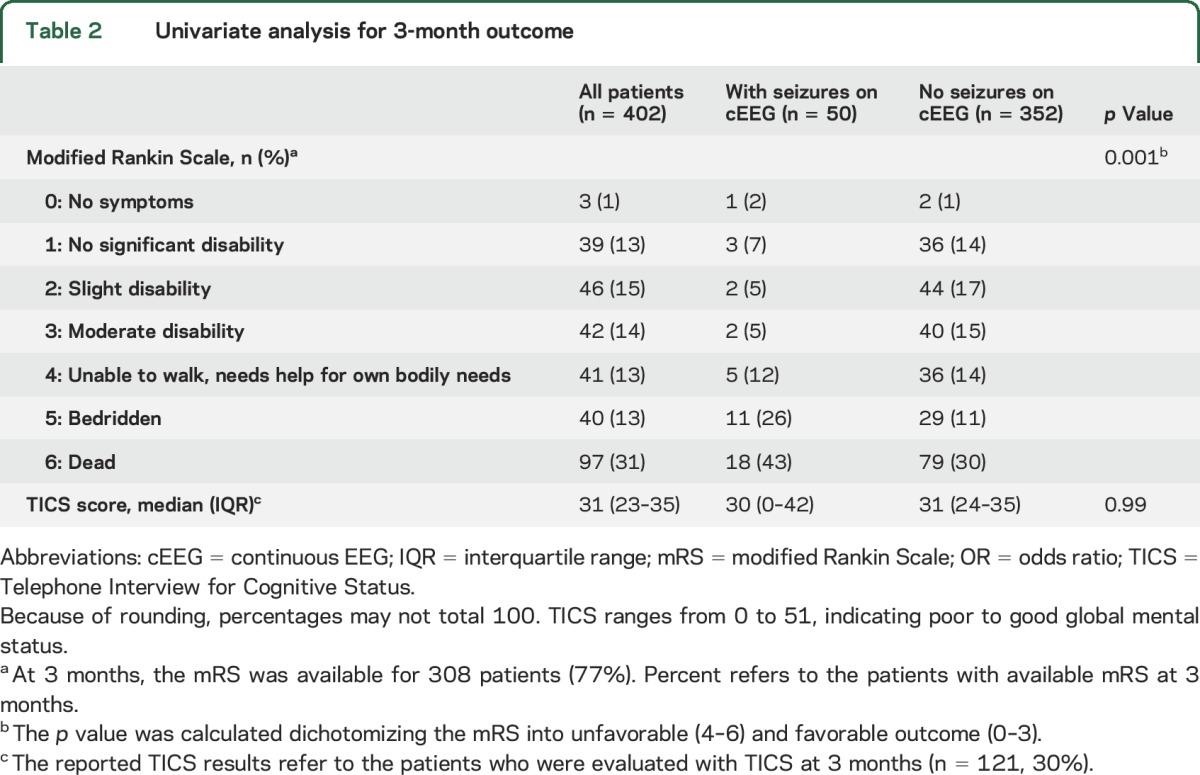
Figure. Predicted outcomes at 3 months relative to seizure burden.
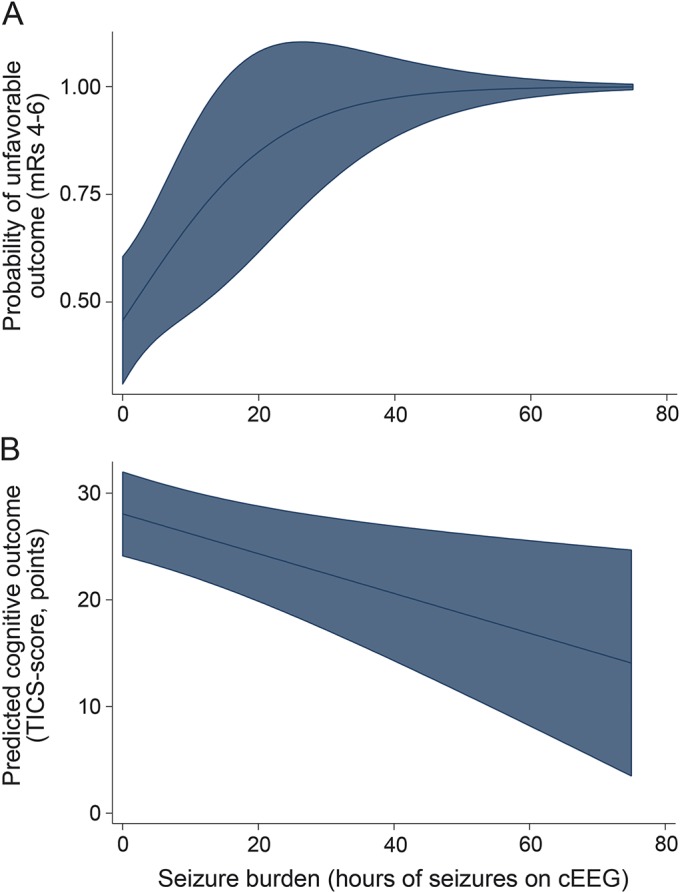
(A) Predicted functional outcome. The plot was generated fitting the logistic regression model (table e-2) with the typical characteristics of subarachnoid hemorrhage (SAH) patients with seizures drawn from table 1 (66 years old, Hunt-Hess grade of 3, aneurysm present, aneurysm size <10 mm, no pneumonia, with global cerebral edema, and no delayed cerebral ischemia). (B) Predicted cognitive outcome. The plot was generated fitting the linear regression model (table e-4) with the typical characteristics of SAH patients with seizures drawn from table 1 (66 years old, nonwhite ethnicity, high school as the highest education, Hunt-Hess grade of 3, included in 2005 or later, aneurysm present, aneurysm size <10 mm, no pneumonia, with global cerebral edema, and no delayed cerebral ischemia). (A, B) The shaded area represents the 95% confidence intervals. cEEG = continuous EEG; mRS = modified Rankin Scale; TICS = Telephone Interview for Cognitive Status.
Refractoriness could not be fully evaluated, as detailed information on whether NCSE responded to standard treatment regimens was not available for the whole cohort. At least 17 patients had refractory NCSE treated with an anesthetic drug, and they all had a mRS score of 5–6 at 3 months.
Cognitive outcome at 3 months.
At 3 months, cognitive evaluation by TICS was available for 121 patients (30.1%). Among these 121 patients, 11 (9.1%) had at least one seizure on cEEG. Detection of at least one seizure on cEEG was not significantly associated with the TICS score at 3 months after adjusting for predictors of cognitive outcome including age, Hunt-Hess, nonwhite ethnicity, education, study inclusion in 2005 or later (study period with levetiracetam preferred over phenytoin), and other predictors of cognitive outcome (tables 3, e-3). Seizure burden, however, remained significantly associated with TICS at 3 months after adjusting for age, Hunt-Hess, and study inclusion in 2005 or later, and other predictors of cognitive outcome. For every hour of seizure on cEEG, the TICS score decreased, on average, by 0.19 points (adjusted coefficient −0.19, 95% CI −0.33 to −0.05, p = 0.01) (tables 3, e-4, figure, B).
DISCUSSION
Seizures occurred in 12% of SAH patients monitored with cEEG, whose main indication was screening for cerebral ischemia or NCSZ. All seizures were nonconvulsive, and would therefore have been undetected without cEEG. Both the presence of NCSZ and seizure burden were significantly associated with higher odds of an unfavorable functional outcome 3 months after SAH. Cognitive outcome at 3 months was, however, associated only with seizure burden and not just presence of NCSZ. The more subtle effect of seizure burden on cognitive outcome suggests that treatment strategies to decrease the seizure burden are warranted independent of the question of seizure prophylaxis.
The frequency of NCSZ observed in the present cohort of SAH patients is in line with the frequency reported in intensive care unit (ICU) populations at large. In 2 previous retrospective studies on unselected comatose ICU patients monitored with EEG, the frequency of NCSZ ranged between 8%2 and 19%,19 with the 2 most common related diagnoses being hypoxic-ischemic encephalopathy2 and epilepsy-related seizures.19 Two studies focused on patients with SAH, whereby the reported frequency of NCSE varied greatly. Among 389 patients with spontaneous SAH, 11 (3%) had evidence of NCSE on cEEG. But since cEEG was not performed in all comatose or poorly responsive patients, the real frequency of NCSE was likely higher.20 In the second study, only SAH patients with a Hunt-Hess IV–V were considered for cEEG, so that the reported frequency of NCSE (31%) likely overestimated the frequency of NCSE among SAH patients at large.21 The diverging findings suggest that the selection of patients for cEEG monitoring is crucial.
SAH patients with seizures were, on average, 9 years older than those without seizures (66 vs 57 years), a finding in line with the previous studies on NCSE in patients with SAH (14 years older [68 years vs 54 years]21 and 15 years older [68 years vs 53 years]20). These findings suggest that the diagnostic yield of cEEG will be higher among older SAH patients. Reasons are speculative on why advanced age emerged as a risk factor for seizures in SAH patients. It is possible that subarachnoid blood may—through contact with an atrophic cortex—uncover a latent susceptibility to seizures. The older age observed among SAH patients with NCSZ differs from the ICU population at large, where younger age emerged as a risk factor for NCSZ.19
The magnitude of association between presence of NCSZ on cEEG and functional outcome at 3 months was clinically relevant, as patients with seizures had more than 3 times higher odds of disability or death after adjusting for established outcome predictors. This finding confirms prior reports associating NCSZ with poor outcome in SAH,1,4 in intracerebral haemorrhage,22 and in cerebral anoxia due to cardiac arrest.23,24 The mechanisms linking NCSZ to functional outcome are unclear, but recent reports contributed to our understanding of this association. Acute brain injury triggers a proinflammatory cascade,25–27 altering blood–brain barrier permeability and resulting in a proconvulsive state. NCSZ increase the brain's metabolic demand, which—if unmet—may result in secondary brain injury, as evidenced by rising markers of metabolic crisis and injury, such as an elevated cerebral lactate-pyruvate ratio and glutamate.3 In turn, NCSZ can contribute to cerebral inflammation, leading to a vicious cycle between inflammation and NCSZ.16 NCSZ may be a surrogate marker for the underlying brain injury that would best be addressed—also considering the recent concerns regarding IV anesthetics as treatment for NCSE28,29—by treatments aimed at the underlying cause of seizures, e.g., in form of anti-inflammatory therapies.
Every hour of NCSZ was associated with a 10% higher odds of disability or death at 3 months. This finding is in line with a recent prospective observational study among critically ill children, whereby seizure burden was associated with short-term neurologic decline, defined as in-hospital mortality and the difference between the Pediatric Cerebral Performance Category score before hospital admission and hospital discharge.6 The probability of neurologic decline rose sharply above a maximum hourly seizure burden of 12 minutes, when a neurologic decline occurred in 98% of children. Beyond obvious differences in the age of the study population, our study differed from this study in several points including underlying etiology, seizure frequency, and outcome measures. The prior study investigated a cohort of children with mixed etiologies with the most common diagnosis being hypoxic ischemic encephalopathy. The frequency of seizures (93/259 children or 36%) in the pediatric study was 3 times higher than observed in the present study, and a lack of 3-month follow-up does not allow us to understand whether the seizure burden was associated with 3-month functional outcome. Another prospective observational study of non-neonatal children admitted to a pediatric ICU showed that NCSZ were not associated with neurologic decline, while NCSE was, underscoring the relevance of seizure burden for short-term functional outcome.30
The observed significant association between seizure burden and cognitive outcome—similar to a dose-effect relationship—suggests that NCSZ injure the brain along a continuum rather than a threshold effect. This is supported by a retrospective cohort study on neonates, where the number of NCSZ was associated with poor neurodevelopmental outcome. In particular, neonates who met at-risk criteria for seizures and who had more than 75 NCSZ had a 3.9 times higher risk of microcephaly and a 6.5 higher risk of cerebral palsy than at-risk neonates with 1–75 NCSZ.31 Similarly, another study on 38 neonates in an ICU showed that NCSZ lasting for longer than 10 minutes per EEG recording hour had higher odds of adverse neurodevelopmental outcome than neonates with shorter seizures.32
The study has several limitations. The retrospective design did not allow us to standardize the selection criteria for cEEG monitoring. Patients with impaired neurologic examination such as Hunt-Hess 4–5 were more likely to be monitored as NCSZ and delayed cerebral ischemia more likely to go clinically undetected if they occurred. EEG recordings were not viewed continuously but were reviewed at least twice daily. Longer seizures may be due to delayed recognition and delayed treatment, resulting in increased seizure refractoriness. In the present study, 54% of patients had Hunt-Hess 4–5 on admission, as compared to 30% of patients with Hunt-Hess 4–5 in the whole SHOP database. This may create a bias and limits the generalizability of our findings. Patients with intracerebral hemorrhages close to the cortex might have an increased risk of seizures. However, information on cortical involvement by intracerebral hemorrhage was not available to test this hypothesis. The spectrum of prescribed antiepileptic drugs changed over the years—in particular since levetiracetam was marketed—and may have influenced outcomes, as phenytoin has been linked to poor cognitive outcome after SAH.17 To address this limitation, we adjusted all the multivariate models for the year of study inclusion. Information on refractoriness was limited, so that a full evaluation of the impact of refractory NCSE on outcome was not possible. Data on neuropsychological outcome were available only for one-third of the cohort, and were missing particularly among patients with unfavorable functional outcomes, as neurologic deficits often prevented a neuropsychological evaluation with the TICS. To measure neuropsychological outcomes in this cohort, we used the TICS since it has been developed and available since 1988, well before the inclusion of the first cohort patient. In the meantime, more refined assessment tools became available (e.g., Montreal Cognitive Assessment), but we kept measuring and reporting the TICS to have a uniform and comparable measure of neuropsychological outcomes across the whole study cohort and years. Missing follow-ups were more frequent by 8% points in the group without than with seizures, and this may limit the generalizability of the study findings.
Among adult SAH patients, after adjusting for established predictors of outcome, the detection of NCSZ on cEEG is linked to functional outcome at 3 months, but not to cognitive outcome. Seizure burden is linked to both functional and cognitive outcome. Future clinical trials on antiepileptic drugs in SAH patients should include both functional and cognitive outcome measures as primary endpoints. The association between NCSZ and functional outcomes may justify preventive antiepileptic treatments in SAH patients but may also suggest that treatments to prevent seizure occurrence should be developed to treat the underlying cause (i.e., inflammation) rather than the symptom (i.e., seizures). The incremental effect of seizure burden on functional and cognitive outcome supports detection of NCSZ using cEEG and treatments aimed at minimizing duration of seizures.
Supplementary Material
GLOSSARY
- cEEG
continuous EEG
- CI
confidence interval
- ICU
intensive care unit
- IQR
interquartile range
- mRS
modified Rankin Scale
- NCSE
nonconvulsive status epilepticus
- NCSZ
nonconvulsive seizures
- OR
odds ratio
- PED
periodic epileptiform discharge
- SAH
subarachnoid hemorrhage
- SHOP
Subarachnoid Hemorrhage Outcomes Project
- TICS
Telephone Interview for Cognitive Status
Footnotes
Supplemental data at Neurology.org
Editorial, page 206
AUTHOR CONTRIBUTIONS
Gian Marco De Marchis: study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content. Deborah Pugin: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Emma Meyers: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Angela Velasquez: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Sureerat Suwatcharangkoon: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Soojin Park: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. M. Cristina Falo: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Sachin Agarwal: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Stephan Mayer: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. J. Michael Schmidt: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. E. Sander Connolly: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Jan Claassen: study concept and design, analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
G. De Marchis was supported by the following grants: Career Development Grant for junior investigators (PBBEP3_139388) by the Swiss National Science Foundation; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr. Ettore Balli (Switzerland); peer reviewed De Quervain research grant for young clinical investigators of the Clinical Trial Unit, University of Bern (Switzerland). D. Pugin, E. Meyers, A. Velazquez Novas, S. Suwatcharangkoon, S. Park, M. Falo, S. Agarwal, S. Mayer, J. Schmidt, E. Connolly, and J. Claassen report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Claassen J, Lokin JK, Fitzsimmons BF, Mendelsohn FA, Mayer SA. Predictors of functional disability and mortality after status epilepticus. Neurology 2002;58:139–142. [DOI] [PubMed] [Google Scholar]
- 2.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 2000;54:340–345. [DOI] [PubMed] [Google Scholar]
- 3.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol 2013;74:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care 2006;4:103–112. [DOI] [PubMed] [Google Scholar]
- 5.Pugin D, Foreman B, De Marchis GM, et al. Is pentobarbital safe and efficacious in the treatment of super-refractory status epilepticus: a cohort study. Crit Care 2014;18:R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004;115:2699–2710. [DOI] [PubMed] [Google Scholar]
- 8.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20. [DOI] [PubMed] [Google Scholar]
- 9.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988;51:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001;32:2012–2020. [DOI] [PubMed] [Google Scholar]
- 11.Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke 1990;21:1156–1161. [DOI] [PubMed] [Google Scholar]
- 12.Brouwers PJ, Dippel DW, Vermeulen M, Lindsay KW, Hasan D, van Gijn J. Amount of blood on computed tomography as an independent predictor after aneurysm rupture. Stroke 1993;24:809–814. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 14.van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg 1985;63:355–362. [DOI] [PubMed] [Google Scholar]
- 15.Komotar RJ, Schmidt JM, Starke RM, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery 2009;64:397–410; discussion 410–411. [DOI] [PubMed] [Google Scholar]
- 16.Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol 2014;75:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005;36:583–587. [DOI] [PubMed] [Google Scholar]
- 18.Mayer SA, Kreiter KT, Copeland D, et al. Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology 2002;59:1750–1758. [DOI] [PubMed] [Google Scholar]
- 19.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 20.Little AS, Kerrigan JF, McDougall CG, et al. Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J Neurosurg 2007;106:805–811. [DOI] [PubMed] [Google Scholar]
- 21.Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002;51:1136–1143; discussion 1144. [DOI] [PubMed] [Google Scholar]
- 22.Vespa PM, O'Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–1446. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 2007;69:255–260. [DOI] [PubMed] [Google Scholar]
- 24.Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care 2012;16:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badjatia N, Carpenter A, Fernandez L, et al. Relationship between C-reactive protein, systemic oxygen consumption, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2011;42:2436–2442. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson PJ, O'Connell MT, Rothwell NJ, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma 2007;24:1545–1557. [DOI] [PubMed] [Google Scholar]
- 27.Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2010;13:339–346. [DOI] [PubMed] [Google Scholar]
- 28.Sutter R, Marsch S, Fuhr P, Kaplan PW, Ruegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology 2014;82:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med 2015;43:1003–1009. [DOI] [PubMed] [Google Scholar]
- 30.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013;41:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55:506–513. [DOI] [PubMed] [Google Scholar]
- 32.Pisani F, Copioli C, Di Gioia C, Turco E, Sisti L. Neonatal seizures: relation of ictal video-electroencephalography (EEG) findings with neurodevelopmental outcome. J Child Neurol 2008;23:394–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


