Abstract
Following analysis of primary cervix, vagina, and first-void female urine specimens for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis via commercial transcription-mediated amplification (TMA), residual material was subjected to Mycoplasma genitalium research-use-only TMA. Representation within a 2,478-specimen retrospective study set was established by comparison to a 6-month audit of clinical C. trachomatis TMA (12,999 specimens) on the basis of the C. trachomatis detection rate, specimen source distribution, clinic location, and age. M. genitalium was detected in 282 (11.4%) patients. This rate was higher than those seen with T. vaginalis (9.0%; P = 0.005), C. trachomatis (6.2%), and N. gonorrhoeae (1.4%). Positive M. genitalium results were confirmed by repeat testing or alternative-target TMA at a rate of 98.7%. The mean age of the M. genitalium-infected females (24.7 years) was lower than that of the T. vaginalis-infected females (mean, 30.1 years; P < 0.0001) and higher than that of the C. trachomatis-infected females (mean, 23.8 years; P = 0.003). Of 566 patient encounters positive for at least one sexually transmitted infection (STI), 35.9% exhibited sole detection of M. genitalium (P ≤ 0.0004 versus sole detection of other STI agents) and 26.1% were solely positive for T. vaginalis (P < 0.0002 versus C. trachomatis). The M. genitalium and T. vaginalis detection rates among 755 patients at urban emergency departments were 14.6% and 13.0%, respectively (P = 0.37). A 10.0% M. genitalium detection rate from other facilities exceeded that of T. vaginalis (7.2%; P = 0.004). Incorporation of M. genitalium TMA into comprehensive testing programs would detect M. genitalium in a significant proportion of females, particularly those in outpatient obstetrics and gynecology (OB/GYN) settings.
INTRODUCTION
The sexually transmitted infection (STI) agent Mycoplasma genitalium has historically had a role of pathogenicity in male nongonococcal urethritis (1). Recent evidence has implicated the bacterium in clinically significant disease in females (2, 3). Additional studies suggest that M. genitalium infection promotes HIV acquisition (4–6) and virus shedding (7, 8). Moreover, in a recent meta-analysis, Lis et al. (9) reported significant associations between M. genitalium infection and cervicitis, pelvic inflammatory disease, preterm birth, and spontaneous abortion.
Until recently, a lack of reliable testing options has curtailed laboratory diagnosis of M. genitalium infection. Culture and serologic modalities have been limited by sensitivity and/or cross-reactivity with other mycoplasmas (1) and are becoming supplanted by molecular diagnostics, largely on a research basis. PCR-based assays have correlated M. genitalium DNA burden with clinical condition (10, 11) and treatment efficacy (12) in males. Quantitative molecular analysis has sought to study progression of genital disease in females (13). In the realm of laboratory diagnosis, initial studies of target capture-based transcription-mediated amplification (TMA) in females reported 87.8% (14) and 96.9% (15) sensitivity values from vaginal specimens. PCR sensitivity values from the same cohorts were 92.9% and 93.8%, respectively.
We recently demonstrated the utility of TMA-based M. genitalium detection in male specimens originating from both STI and community outpatient clinics (16). In contrast, previous studies of M. genitalium TMA in females have largely focused on high-risk demographics (14, 15, 17–19). Assessment of M. genitalium detection rates in a communitywide setting would be beneficial. We now provide findings of the importance of TMA-based M. genitalium evaluation of females in a metropolitan health care system.
(Results of this work were previously presented, in part, at the 115th General Meeting of the American Society for Microbiology, New Orleans, LA, 30 May to 2 June 2015.)
MATERIALS AND METHODS
Setting.
In addition to seven acute care facilities, Wheaton Franciscan Laboratory serves an approximately 125-clinic metropolitan outpatient physician group in a three-county region of southeastern Wisconsin. A portion of this service area extends beyond the Milwaukee-Waukesha-West Allis metropolitan statistical area (MSA). Analyses of data from U.S. MSAs document a long-standing trend of high STI prevalence in the Milwaukee-Waukesha-West Allis MSA. Aggregation of these data revealed an average chlamydia rate of 678.0 per 100,000 population over a 10-year interval (20). This value averaged the second highest in the United States and represented a 77.3% increase over the average national cumulative MSA rate of 382.3 per 100,000 population. The same MSA generated an average annual rank of 2.56 for gonorrhea rate among United States MSAs (264.0 per 100,000 population; 112.6% higher than the national MSA rate of 124.2 per 100,000 population).
Specimen submission for routine screening.
Approximately 2-ml aliquots of first-void female urine were added to Aptima urine specimen transport tubes per the Aptima Combo 2 Assay (Hologic, Incorporated, San Diego, CA) package insert protocol following specimen procurement (21). Aliquots were stored at 2 to 30°C and tested within 30 days of primary collection. Primary genital swab specimens were obtained using an Aptima unisex swab specimen collection kit (21), stored at 2 to 30°C, and tested within 30 days of collection.
The Aptima Combo 2 Assay was utilized for the detection of N. gonorrhoeae-specific 16S rRNA and Chlamydia trachomatis-specific 23S rRNA (22) from all urine aliquots. The samples were additionally subjected to Aptima Trichomonas vaginalis (Hologic) testing for detection of organism-specific 18S rRNA (23).
Retrospective specimen collection for M. genitalium TMA analysis.
A 6-month laboratory information system-based audit of female STI ordering practices was conducted for the purpose of assembling a representative study set for M. genitalium TMA. Assessed parameters included specimen source (urine, cervical specimen, or vaginal specimen), C. trachomatis detection rate, geographic location, and patient age. This study was governed by the Wheaton Franciscan Healthcare Institutional Review Board.
Molecular detection of M. genitalium.
Residual clinical material was subjected to a research-use-only TMA-based assay for detection of M. genitalium-specific 16S rRNA (referred to as M. genitalium TMA; Hologic). Reagents were prepared by using Aptima general-purpose reagents spiked with M. genitalium-specific oligonucleotides. A 50-μl aliquot of target capture oligonucleotide was spiked into Aptima target capture reagent; 50-μl aliquots of T7 and non-T7 oligonucleotides were spiked into reconstituted Aptima reconstitution reagent; and a 50-μl aliquot of acridinium ester-labeled hybridization oligonucleotide was spiked into Aptima hybridization buffer. All assays were performed on an automated TIGRIS DTS platform (Hologic).
Interpretation of results.
M. genitalium TMA relative-light-unit values of ≥50,000 derived from urogenital specimen testing were interpreted as representing positive results (14, 17). A subset of positive results, as each residual specimen allowed, was confirmed by repeat testing. An additional subset of positive specimens was forwarded for a TMA-based alternative-target confirmatory assay (Hologic).
Statistical analysis.
The STI phenotype was defined as a M. genitalium, C. trachomatis, N. gonorrhoeae, and T. vaginalis distribution within a given health care encounter that yielded detection of at least one STI agent. The significance test of proportions determined if differences in either the rates of positive screening results or the STI phenotypes were significant. The t test for independent samples determined if differences in mean patient age associated with positive results were significant in the comparisons between STI etiologies. The alpha level was set at 0.05 before the investigations commenced, and all P values are two tailed.
RESULTS
Collection of specimens for M. genitalium TMA assessment.
Retrospectively collected specimens (n = 2,478) were compared to data gathered from a 6-month audit of routine screening practices for C. trachomatis. Proportional specimen source distributions did not differ between routine C. trachomatis screening practices and specimens gathered for M. genitalium TMA (P ≥ 0.10; Table 1). In addition, C. trachomatis detection rates showed no difference from those seen in the collection for M. genitalium TMA (P = 0.91). Detection rates stratified by specimen source were also similar (P ≥ 0.85; Table 1). Among the urogenital specimens submitted for routine C. trachomatis screening, 18.6% and 48.0% were derived from females aged ≤20 and 21 to 30 years, respectively. Analogous proportions collected for M. genitalium TMA were 19.0% and 47.0% (P = 0.60 and 0.38, respectively; data not illustrated). Comparative data for successive age in decades exhibited no differences (P ≥ 0.07).
TABLE 1.
Distribution of specimen source submissions and C. trachomatis detection rates during a 6-month audit of routine C. trachomatis screening, with comparison to representative distributions of specimens collected for retrospective M. genitalium TMA analysis
| Specimen source | % source distribution within specimens analyzed in: |
P value | % C. trachomatis detection from specimens analyzed in: |
P value | ||
|---|---|---|---|---|---|---|
| C. trachomatis routine screening | M. genitalium TMA assessment | C. trachomatis routine screening | M. genitalium TMA assessment | |||
| Cervix | 77.7 | 76.5 | 0.20 | 6.2 | 6.2 | 0.99 |
| Urine | 17.5 | 17.9 | 0.65 | 6.3 | 6.1 | 0.85 |
| Vagina | 4.8 | 5.6 | 0.10 | 6.9 | 6.5 | 0.87 |
| Total | 6.3 | 6.2 | 0.91 | |||
Twenty health care locations accounted for 89.2% of all routine C. trachomatis submissions (Table 2). No differences in proportional specimen distributions between routine laboratory C. trachomatis screening and the M. genitalium TMA collection (P = 0.11) or stratified by individual health care location (P ≥ 0.28) were noted. These entities were also the basis for arbitrary categorization of health care locations as outpatient obstetrics and gynecology (OB/GYN), inpatient OB/GYN, suburban family care, urban family care, and emergency room (ER)/urgent care. Moreover, 242 (9.8%) specimens in the M. genitalium TMA collection originated from 34 additional health care locations (Table 2).
TABLE 2.
Distribution of specimen submissions among the 20 most frequently visited health care locations during a 6-month audit of routine C. trachomatis screening and comparison to representative distributions of specimens collected for retrospective M. genitalium TMA analysis
| Health care location | % total specimen submissions for C. trachomatis screeninga | % total specimen collections for M. genitalium TMA assessmentb | P value |
|---|---|---|---|
| Outpatient OB/GYN 1 | 16.69 | 16.38 | 0.71 |
| Outpatient OB/GYN 2 | 9.12 | 9.60 | 0.44 |
| Outpatient OB/GYN 3 | 4.14 | 4.08 | 0.89 |
| Outpatient OB/GYN 4 | 3.21 | 3.39 | 0.64 |
| Outpatient OB/GYN 5 | 3.02 | 3.39 | 0.33 |
| Outpatient OB/GYN 6 | 1.96 | 1.86 | 0.73 |
| Outpatient OB/GYN 7 | 1.54 | 1.45 | 0.75 |
| Outpatient OB/GYN 8 | 1.52 | 1.49 | 0.91 |
| Inpatient OB/GYN | 1.46 | 1.69 | 0.38 |
| Suburban family care 1 | 3.62 | 3.55 | 0.86 |
| Suburban family care 2 | 1.58 | 1.53 | 0.87 |
| Urban family care 1 | 5.36 | 5.37 | 0.99 |
| Urban family care 2 | 3.21 | 3.55 | 0.38 |
| Urban family care 3 | 2.93 | 2.70 | 0.54 |
| ER/urgent care 1 | 11.95 | 12.47 | 0.46 |
| ER/urgent care 2 | 4.85 | 4.96 | 0.80 |
| ER/urgent care 3 | 5.12 | 5.00 | 0.82 |
| ER/urgent care 4 | 4.06 | 3.79 | 0.53 |
| ER/urgent care 5 | 2.66 | 2.54 | 0.73 |
| ER/urgent care 6 | 1.15 | 1.41 | 0.28 |
| Top 20 locations | 89.2 | 90.2 | 0.11 |
| 34 other health care locations | 10.8 | 9.8 | 0.11 |
Total submissions, 12,999.
Total collections, 2,478.
Detection of M. genitalium RNA.
The overall detection rate of M. genitalium (11.4%) exceeded those of the other STI agents (P ≤ 0.005). T. vaginalis also demonstrated increased detection (9.0% rate) versus C. trachomatis and N. gonorrhoeae (P ≤ 0.0003). Detection rates for C. trachomatis and N. gonorrhoeae were 6.2% and 1.4%, respectively. A total of 208 specimens with detectable M. genitalium contained enough residual material to allow for repeat analysis; 207 (99.5%) yielded a positive result. In addition, a subset of 242 specimens was subjected to alternative-target TMA analysis (Table 3). A 98.8% concordance of results was observed.
TABLE 3.
Tandem performance of M. genitalium TMA and alternative-target TMA assays on a subset of 242 primary female urogenital specimens
| Alternative-target TMA result | No. of M. genitalium TMA results |
|
|---|---|---|
| Positive | Negative | |
| Positive | 27 | 1 |
| Negative | 2 | 212 |
Female urogenital specimens with detectable M. genitalium RNA.
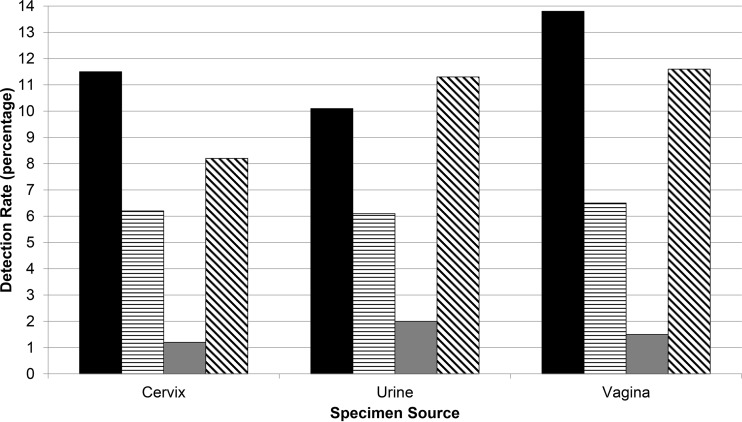
The rates of detection of M. genitalium from 1,896 cervical, 444 urine, and 138 vaginal specimens were 11.5%, 10.1%, and 13.8%, respectively (P ≥ 0.23; Fig. 1). In similar fashion, the C. trachomatis and N. gonorrhoeae detection rates, stratified by specimen source, revealed no differences (P ≥ 0.85 and 0.18, respectively). The T. vaginalis detection rate from urine (11.3%) exceeded the rate from cervical specimens (8.2%; P < 0.04) and was similar to the detection rate (11.6%) from vaginal specimens (P = 0.91).
FIG 1.
Cervical, first-void urine, and vaginal specimen detection rates for M. genitalium (solid bars), C. trachomatis (horizontally hatched bars), N. gonorrhoeae (gray bars), and T. vaginalis (diagonally hatched bars) from 2,478 female patients.
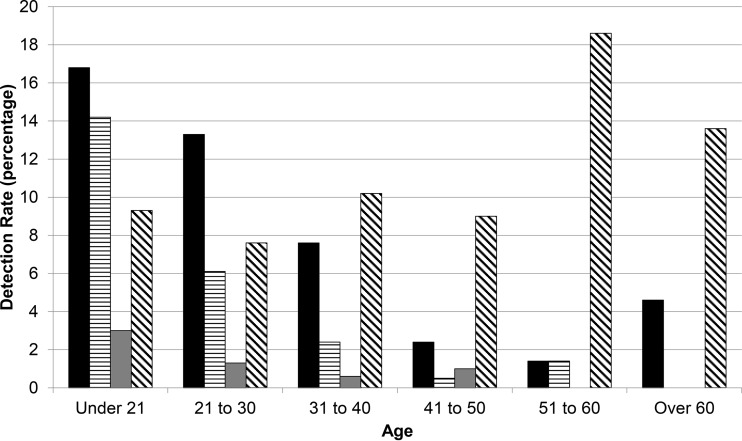
The detection rate of M. genitalium in females aged ≤20 years was 16.8%. This rate decreased for each successive age decade (Fig. 2), leading to a 4.6% value in a subset of 22 females aged >60 years. M. genitalium was detected in females 14 years to 65 years of age, with a mean age of 24.7 years (data not illustrated). This mean was similar to the mean age of N. gonorrhoeae detection (23.5 years; P = 0.30) but different from those for C. trachomatis detection (22.8 years; P = 0.003) and T. vaginalis detection (30.1 years; P < 0.0001).
FIG 2.
M. genitalium (solid bars), C. trachomatis (horizontally hatched bars), N. gonorrhoeae (gray bars), and T. vaginalis (diagonally hatched bars) detection rates, delineated by age, from 2,478 female urogenital specimens.
M. genitalium detection delineated by health care setting.
Other than a 9.0% rate of C. trachomatis detection from the ER/urgent care setting, C. trachomatis and N. gonorrhoeae detection rates did not exceed 5.4% for a given health care setting (data not illustrated). Two health care locations, ER/urgent care and outpatient OB/GYN, accounted for 72.1% of specimens analyzed by M. genitalium TMA. Specimens from outpatient OB/GYN were more likely to contain detectable M. genitalium than T. vaginalis (P = 0.001; Table 4). In contrast, specimens from ER/urgent care had similarly high rates of M. genitalium and T. vaginalis detection (14.6% and 13.0%, respectively; P = 0.37).
TABLE 4.
M. genitalium and T. vaginalis detection rates for females seeking health care in ER/urgent care and non-ER/urgent care settings
| Health care setting | n | No. (%) of specimens with detection of: |
P value | |
|---|---|---|---|---|
| M. genitalium | T. vaginalis | |||
| Outpatient OB/GYN | 1,032 | 106 (10.3) | 66 (6.4) | 0.001 |
| Inpatient OB/GYN | 46 | 7 (15.2) | 5 (10.9) | 0.54 |
| Suburban family care | 261 | 18 (6.9) | 14 (5.4) | 0.47 |
| Urban family care | 384 | 41 (10.7) | 39 (10.2) | 0.81 |
| ER/urgent care | 755 | 110 (14.6) | 98 (13.0) | 0.37 |
Codetection of other STI agents with M. genitalium.
M. genitalium was a component of 49.8% of the female STI phenotypes and the sole component of 35.9% of the phenotypes in this study (Table 5). Sole detection of T. vaginalis was observed with 26.1% of the phenotypes; T. vaginalis was a constituent of 39.2% of the STI phenotypes (P ≤ 0.0004 compared to M. genitalium). These values were greater than the analogous data for C. trachomatis and N. gonorrhoeae.
TABLE 5.
Sexually transmitted infection phenotypes determined by TMA-based assays specific for M. genitalium, C. trachomatis, N. gonorrhoeae, and T. vaginalis, delineated by category of health care setting
| STI phenotypea,b |
No. (%) of specimens with indicated phenotype(s)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| M. genitalium | C. trachomatis | N. gonorrhoeae | T. vaginalis | Outpatient OB/GYN | Inpatient OB/GYN | Suburban family care | Urban family care | ER/urgent care | Totalc |
| + | − | − | − | 85 (41.3) | 5 (41.7) | 12 (29.3) | 26 (33.8) | 75 (32.6) | 203 (35.9) |
| + | + | − | − | 3 (1.5) | 2 (16.7) | 3 (7.3) | 2 (2.6) | 14 (6.1) | 24 (4.2) |
| + | − | − | + | 14 (6.8) | 0 (0.0) | 1 (2.4) | 11 (14.3) | 14 (6.1) | 40 (7.1) |
| − | + | − | − | 43 (20.9) | 0 (0.0) | 8 (19.5) | 9 (11.7) | 30 (13.0) | 90 (15.9) |
| − | + | + | − | 1 (0.5) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 4 (1.7) | 6 (1.1) |
| − | + | − | + | 7 (3.4) | 0 (0.0) | 0 (0.0) | 2 (2.6) | 14 (6.1) | 23 (4.1) |
| − | − | + | − | 4 (1.9) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 8 (3.5) | 13 (2.3) |
| − | − | − | + | 45 (21.8) | 5 (41.7) | 13 (31.7) | 25 (32.5) | 60 (26.1) | 148 (26.1) |
+, positive nucleic acid amplification test result; −, negative nucleic acid amplification test result; STI, sexually transmitted infection.
Phenotypes that were observed in ≥1.5% of patients from at least two health care settings are included. No significant difference was noted within the excluded comparisons.
Phenotypes that were observed in <1.0% of encounters are not included in this category. M. genitalium was a constituent of 49.8% of all phenotypes, C. trachomatis of 27.2%, N. gonorrhoeae of 6.0%, and T. vaginalis of 39.2%.
In general, equal abundances of M. genitalium- and T. vaginalis-based STI phenotypes existed among the inpatient OB/GYN, family care, and ER/urgent care settings (P ≥ 0.26; Table 5). However, 41.3% of the outpatient OB/GYN phenotypes consisted of sole M. genitalium detection, while sole detection of T. vaginalis comprised 21.8% of the phenotypes (P < 0.0002).
Of the 282 specimens with detectable M. genitalium, only 28.0% revealed detection of an additional STI agent (data not illustrated). Between 28.6% and 36.6% of inpatient OB/GYN, family care, and ER/urgent care specimens with detectable M. genitalium involved codetection with another STI agent. In contrast, only 19.8% of the outpatient OB/GYN specimens testing positive for M. genitalium exhibited an additional STI agent.
DISCUSSION
Evidence for the clinical significance of M. genitalium in female reproductive tract disease is increasing. Lis et al. (9) demonstrated a significant association of M. genitalium incidence and increased risk of cervicitis with a pooled odds ratio (OR) of 1.65. A pooled OR of 2.53 (adjusted for coinfection) was calculated for an association with pelvic inflammatory disease. When studies utilizing serodiagnosis were excluded, the OR increased to 2.73. Additional meta-analyses associated M. genitalium infection with increased risk of preterm birth (pooled OR of 2.33, accounting for coinfection) and spontaneous abortion (pooled OR of 1.82). These data point out a clinical need for effective laboratory diagnostics specific to this STI agent.
Our data show elevated rates of M. genitalium detection in females, with significantly greater rates than those for Trichomonas vaginalis—a pathogen previously demonstrated to be present in abundance in the high-prevalence Milwaukee STI community (24, 25). Of 282 detections of M. genitalium, 237 were subjected to confirmation by repeat testing or alternative-target TMA; 98.7% of such tests yielded a positive result. Previous literature has espoused the value of repeat testing in the confirmation of positive TMA results (26). Furthermore, in the course of determining the specificity of M. genitalium TMA, 213 TMA-negative specimens were subjected to alternative-target TMA. Only one specimen (0.5%) yielded a positive result by alternative-target TMA. This rate of discordant results was less than the 1.6% to 4.6% values reported for T. vaginalis alternative-target testing (24, 27, 28). The parasitic species Trichomonas tenax has substantial genetic homology with T. vaginalis (29), which hypothetically could contribute to decreased specificity of T. vaginalis alternative-target TMA, particularly in cases of pharyngeal T. vaginalis carriage (28). The high specificity for M. genitalium alternative-target TMA implies lack of cross-reactivity with other mycoplasmas and clearly verifies the increased M. genitalium detection in this population.
Previous studies of M. genitalium TMA have focused largely on high-risk demographics. Huppert et al. (17) reported a 22.4% detection rate from 331 female attendees of an adolescent health center/emergency department. Furthermore, Gaydos et al. (18) documented an approximate 19% detection rate among female STI clinic patients. A major strength of our large-scale study is the extrapolation of M. genitalium TMA to primary clinical practice in a broad geographic area. Data from Tables 1 and 2 confirm the representative nature of the study set. Past reports from our laboratory have summarized diagnostic assay performance within high-prevalence STI cohorts (16, 20). Subsequent to these reports, our laboratory has extended its testing scope to locales outside the Milwaukee-Waukesha-West Allis MSA. While this has resulted in decreases of STI etiology detection by the laboratory (e.g., the C. trachomatis detection rate at [non-MSA] ER/urgent care 3 was 8.9%, while the analogous detection rate at [MSA] ER/urgent care 1 was 13.3%; data not illustrated), it has also provided a diverse population basis for practical assessment of commercial M. genitalium TMA.
Past surveillance efforts in general European and U.S. female populations using molecular diagnostics (30, 31) have reported M. genitalium detection rates of 1.0% to 2.3%. A meta-analysis published by McGowin and Anderson-Smits (32) calculated a detection rate of 2.0% from low-risk female populations. In a South American OB/GYN cohort of 1,338 women, Hitti et al. (33) documented a 3.1% cervical M. genitalium detection rate by TMA. This report is interesting for a number of reasons. First, it documents M. genitalium detection in an OB/GYN population and associates its presence with preterm birth and younger maternal age. It also reveals a strong association with C. trachomatis detection and a marginal association with T. vaginalis detection. It should be noted that those authors utilized culture for laboratory diagnosis of trichomoniasis, a modality shown to be far less sensitive than molecular diagnostics (34). Our outpatient OB/GYN population was characterized by an increased rate of M. genitalium in general and by a significantly increased rate of M. genitalium detection in comparison to T. vaginalis detection (P = 0.001; Table 4).
STI phenotyping essentially predicts the likelihood of a given etiology for STI diagnosis during a health care encounter. In contrast to past reports showing a strong association between M. genitalium detection and the concomitant presence of C. trachomatis (17, 33), only 4.2% of total STI phenotypes in our study involved codetection of M. genitalium and C. trachomatis (Table 5). Among the STI phenotypes, 7.1% consisted of M. genitalium and T. vaginalis codetection. Moreover, while M. genitalium was a constituent of nearly half of the STI phenotypes, 39.2% and 27.2% of all phenotypes had some component of T. vaginalis and C. trachomatis, respectively. Detection rate and STI phenotype data reveal significant differences with respect to M. genitalium and T. vaginalis in the outpatient OB/GYN setting. These clinics were more likely to produce a significant increase in M. genitalium detection (Table 4) as well as STI phenotypes specific to the organism (Table 5). The other four arbitrary health care classifications did not experience this phenomenon. Napierala et al. (16) previously described a M. genitalium/T. vaginalis dichotomy within a 2,750-male cohort in which the STI phenotypes of STI clinic attendees and patients seeking outpatient clinic care consisted predominately of M. genitalium and T. vaginalis, respectively. The differential distribution of these two STI agents, which apparently may exist to a degree in both genders, warrants further investigation.
With respect to an optimal specimen source for TMA-based detection of M. genitalium on a single-specimen basis, Wroblewski et al. (14) discussed the relative sensitivities of vaginal and cervical specimens, which were 84% and 60%, respectively. The relative sensitivity of first-void urine specimens was 58% and was thought to be the result of increased susceptibility of a cell wall-devoid microbe to lysis in urine. Using an infected-patient standard, Mobley et al. (19) reported TMA-based M. genitalium sensitivities of 72.6% and 58.9% from vaginal and cervical specimens, respectively. Our data reveal equivalent M. genitalium detection rates from cervical specimens (11.5%), vaginal specimens (13.8%), and first-void urine (10.1%; P ≥ 0.23). However, our data are limited by the fact that not all three specimen sources were submitted from a given patient during routine clinical practice. Therefore, comparison of performance characteristics by specimen source could not be accomplished in this retrospective assessment. Of further interest, previous studies in our female patient population demonstrated increased T. vaginalis detection from first-void urine specimens (25, 35). We also show increased T. vaginalis detection from first-void urine compared to cervical specimens (P = 0.04; Fig. 1). These data trended higher than the combined cervical specimen/vaginal specimen data (P = 0.06). Some concern lies in how the increased utility of the first-void urine specimen for T. vaginalis could coexist with the aforementioned M. genitalium data in the context of (single) specimen source recommendations for a four-agent STI screen. Additional large-scale communitywide studies may be necessary to determine the true utility of urine for M. genitalium TMA, particularly when such specimens would be assayed for the organism in routine fashion without prolonged specimen storage.
In summary, increased detection rates of M. genitalium in a communitywide setting over those of other STI agents, including T. vaginalis, suggest that M. genitalium TMA can provide a benefit to multiple demographics and multiple clinical practice specialties. In addition, the setting of the outpatient OB/GYN is itself an important area for M. genitalium screening. M. genitalium TMA has recently become commercially available in an analyte-specific reagent (ASR) format. Prior to FDA clearance in 2011, T. vaginalis TMA was also commercially available as an ASR. Napierala et al. (35) demonstrated progressively increased utilization of that assay over a 3-year interval. Should an analogous pattern be observed with M. genitalium ASR, potential benefits of a more-comprehensive STI screen may be realized in the realm of laboratory diagnosis and public health.
ACKNOWLEDGMENT
K.L.M., M.N., and E.M. have received travel assistance from Hologic/Gen-Probe, Incorporated.
Funding Statement
There were no external funding sources.
REFERENCES
- 1.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manhart LE, Broad JM, Golden MR. 2011. Mycoplasma genitalium: should we treat and how? Clin Infect Dis 53(Suppl 3):S129–S142. doi: 10.1093/cid/cir702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JD. 2005. Is Mycoplasma genitalium a cause of pelvic inflammatory disease? Infect Dis Clin North Am 19:407–413. doi: 10.1016/j.idc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Das K, De la Garza G, Siwak EB, Scofield VL, Dhandayuthapani S. 2014. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis 23:31–38. doi: 10.1016/j.ijid.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavedzenge SN, Van Der Pol B, Weiss HA, Kwok C, Mambo F, Chipato T, Van der Straten A, Salata R, Morrison C. 2012. The association between Mycoplasma genitalium and HIV-1 acquisition in African women. AIDS 26:617–624. doi: 10.1097/QAD.0b013e32834ff690. [DOI] [PubMed] [Google Scholar]
- 6.Vandepitte J, Weiss HA, Bukenya J, Kyakuwa N, Muller E, Buvé A, Van der Stuyft P, Hayes RJ, Grosskurth H. 2014. Association between Mycoplasma genitalium and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect 90:545–549. doi: 10.1136/sextrans-2013-051467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manhart LE, Mostad SB, Baeten JM, Astete SG, Mandaliya K, Totten PA. 2008. High Mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis 197:733–736. doi: 10.1086/526501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napierala Mavedzenge S, Müller EE, Lewis DA, Chipato T, Morrison CS, Weiss HA. 2015. Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis 211:1388–1398. doi: 10.1093/infdis/jiu644. [DOI] [PubMed] [Google Scholar]
- 9.Lis R, Rowhani-Rahbar A, Manhart LE. 2015. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Deguchi T, Ito M, Maeda S-I, Tamaki M, Ishiko H. 2002. Quantitative detection of Mycoplasma genitalium from first-pass urine of men with urethritis and asymptomatic men by real-time PCR. J Clin Microbiol 40:1451–1455. doi: 10.1128/JCM.40.4.1451-1455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupin N, Bijaoui G, Schwarzinger M, Ernault P, Gerhardt P, Jdid R, Hilab S, Pantoja C, Buffet M, Escande JP, Costa JM. 2003. Detection and quantification of Mycoplasma genitalium in male patients with urethritis. Clin Infect Dis 37:602–605. doi: 10.1086/376990. [DOI] [PubMed] [Google Scholar]
- 13.Blaylock MW, Musatovova O, Baseman JG, Baseman JB. 2004. Determination of infectious load of Mycoplasma genitalium in clinical samples of human vaginal cells. J Clin Microbiol 42:746–752. doi: 10.1128/JCM.42.2.746-752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wroblewski JK, Manhart LE, Dickey KA, Hudspeth MK, Totten PA. 2006. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol 44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardick J, Giles J, Hardick A, Hsieh YH, Quinn T, Gaydos C. 2006. Performance of the Gen-Probe transmission-mediated [corrected] amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium infection. J Clin Microbiol 44:1236–1240. doi: 10.1128/JCM.44.4.1236-1240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napierala M, Munson E, Wenten D, Phipps P, Gremminger R, Schuknecht MK, Munson KL, Boyd V, Hamer D, Schell RF, Hryciuk JE. 2015. Detection of Mycoplasma genitalium from male primary urine specimens: an epidemiologic dichotomy with Trichomonas vaginalis. Diagn Microbiol Infect Dis 82:194–198. doi: 10.1016/j.diagmicrobio.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. 2008. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 35:250–254. doi: 10.1097/OLQ.0b013e31815abac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. 2009. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis 36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobley VL, Hobbs MM, Lau K, Weinbaum BS, Getman DK, Seña AC. 2012. Mycoplasma genitalium infection in women attending a sexually transmitted infection clinic: diagnostic specimen type, coinfections, and predictors. Sex Transm Dis 39:706–709. doi: 10.1097/OLQ.0b013e318255de03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munson E, Napierala M, Schell RF. 2013. Insights into trichomoniasis as a result of highly sensitive molecular diagnostics screening in a high-prevalence sexually transmitted infection community. Expert Rev Anti Infect Ther 11:845–863. doi: 10.1586/14787210.2013.814429. [DOI] [PubMed] [Google Scholar]
- 21.APTIMACombo 2® Assay package insert. 2012. Gen-Probe, Incorporated, San Diego, CA. [Google Scholar]
- 22.Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW, Martin DH, Ferrero DV, Schachter J. 2003. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol 41:304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwebke JR, Hobbs MM, Taylor SN, Seña AC, Catania MG, Weinbaum BS, Johnson AD, Getman DK, Gaydos CA. 2011. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 49:4106–4011. doi: 10.1128/JCM.01291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munson E, Napierala M, Olson R, Endes T, Block T, Hryciuk JE, Schell RF. 2008. Impact of Trichomonas vaginalis transcription-mediated amplification-based analyte-specific reagent testing in a metropolitan setting of high sexually transmitted disease prevalence. J Clin Microbiol 46:3368–3374. doi: 10.1128/JCM.00564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munson E, Kramme T, Napierala M, Munson KL, Miller C, Hryciuk JE. 2012. Female epidemiology of transcription-mediated amplification-based Trichomonas vaginalis detection in a metropolitan setting with a high prevalence of sexually transmitted infection. J Clin Microbiol 50:3927–3931. doi: 10.1128/JCM.02078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munson E, Boyd V, Czarnecka J, Griep J, Lund B, Schaal N, Hryciuk JE. 2007. Evaluation of Gen-Probe APTIMA-based Neisseria gonorrhoeae and Chlamydia trachomatis confirmatory testing in a metropolitan setting of high disease prevalence. J Clin Microbiol 45:2793–2797. doi: 10.1128/JCM.00491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munson E, Napierala M, Basile J, Miller C, Burtch J, Hryciuk JE, Schell RF. 2010. Trichomonas vaginalis transcription-mediated amplification-based analyte-specific reagent and alternative target testing of primary clinical vaginal saline suspensions. Diagn Microbiol Infect Dis 68:66–72. doi: 10.1016/j.diagmicrobio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Munson E, Wenten D, Phipps P, Gremminger R, Schuknecht MK, Napierala M, Hamer D, Olson R, Schell RF, Hryciuk JE. 2013. Retrospective assessment of transcription-mediated amplification-based screening for Trichomonas vaginalis in male sexually transmitted infection clinic patients. J Clin Microbiol 51:1855–1860. doi: 10.1128/JCM.00455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucknoor AS, Mundodi V, Alderete JF. 2009. Genetic identity and differential gene expression between Trichomonas vaginalis and Trichomonas tenax. BMC Microbiol 9:58. doi: 10.1186/1471-2180-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. 2007. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health 97:1118–1125. doi: 10.2105/AJPH.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen B, Sokolowski I, Østergaard L, Kjølseth Møller J, Olesen F, Jensen JS. 2007. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex Transm Infect 83:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowin CL, Anderson-Smits C. 2011. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 7:e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitti J, Garcia P, Totten P, Paul K, Astete S, Holmes KK. 2010. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis 37:81–85. doi: 10.1097/OLQ.0b013e3181bf5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nye MB, Schwebke JR, Body BA. 2009. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 200:188.e1–188.e7. doi: 10.1016/j.ajog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Napierala M, Munson E, Munson KL, Kramme T, Miller C, Burtch J, Olson R, Hryciuk JE. 2011. Three-year history of transcription-mediated amplification-based Trichomonas vaginalis analyte-specific reagent testing in a subacute care patient population. J Clin Microbiol 49:4190–4194. doi: 10.1128/JCM.05632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]