Abstract
The cryptococcal antigen lateral flow assay (CrAg LFA) was evaluated for the diagnosis of cryptococcosis in HIV-negative patients. The sensitivity was excellent, suggesting that this assay can replace conventional testing based on latex agglutination (LA). CrAg LFA and LA titers were correlated but were not directly comparable, with implications for conversion between assays.
TEXT
Cryptococcosis is a systemic infection caused by Cryptococcus neoformans and Cryptococcus gattii. The cryptococcal antigen lateral flow assay (CrAg LFA; IMMY Inc., Norman, OK) is a recently developed dipstick sandwich immunochromatographic assay that has shown equivalent or superior overall sensitivity compared to that of enzyme immunoassay (EIA) and latex agglutination (LA) tests (1–5) and has demonstrated good individual sensitivity for the capsular polysaccharide glucuronoxylomannan (GXM) of all four C. neoformans serotypes (6). However, most comparative studies have relied primarily on samples from HIV patients (1, 2, 7–9), so little is known about the relative performance of CrAg LFA in HIV-negative individuals, in whom fungal burden may be lower. The present study aimed to evaluate the sensitivity of CrAg LFA in diagnosing cryptococcosis in HIV-negative adults.
(This work was presented in part at the 115th General Meeting of the American Society for Microbiology, New Orleans, LA, 30 May to 2 June 2015.)
We first conducted a validation of the CrAg LFA assay using 36 frozen archival serum samples (26 positive/10 negative) and 23 cerebrospinal fluid (CSF) specimens (13 positive/10 negative) that were previously tested in the laboratory by LA (CALAS; Meridian Bioscience Inc., Cincinnati, OH). This was performed on archived specimens gathered from patients on an Institutional Review Board (IRB)-approved clinical protocol. Retesting of these specimens by qualitative and semiquantitative CrAg LFA demonstrated 100% agreement with previously tested LA-positive and LA-negative samples in this set. The relative limits of detection of LA and LFA were then tested in two experiments in which serum and CSF specimens were serially diluted to final negative titers for the two assays tested in parallel. The LFA assay remained positive at substantially greater dilutions than the LA assay did for the tested specimens: Final titers (LFA versus LA) were 1:8,192 versus 1:512 for serum and 1:8,192 versus 1:128 for CSF, which is consistent with a lower limit of detection for the LFA assay for the two specimen types.
Following validation, the qualitative and semiquantitative CrAg LFA assays were performed on archived specimens collected from HIV-negative individuals with confirmed diagnoses of cryptococcosis. To challenge the limits of sensitivity of the LFA assay, specimens were selected that had tested negative by EIA, potentially reflecting lower antigen titers. All but four of these samples tested positive by LA. Statistical analysis of LFA results was performed with Prism version 6.0 (GraphPad Software, Inc.), and all values are expressed herein as mean plus or minus standard deviation (SD) or median (minimum, maximum) for continuous variables and as frequencies for categorical variables. LA and LFA titers were log-transformed for analysis. The Spearman rank correlation coefficient (rS) was used to analyze a correlation between the ln LA titer and the ln LFA titer. The Bland-Altman plot was used to analyze the correlation between the ln LA titer and the ln LFA titer (10).
CrAg LFA titers were performed on 51 serum and 9 CSF samples from 31 patients. Seventeen subjects were male, and the mean age was 48 ± 13 years. Coexisting immunodeficiency conditions were idiopathic CD4+ lymphopenia (6/31), anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) antibody (4/31), Good syndrome (1/31), STAT 1 mutation (1/31), T-cell lymphoma (1/31), and myasthenia gravis (1/31). Other underlying diseases (some patients had multiple) were hypertension (9/31), hypothyroidism (2/31), diabetes mellitus (1/31), chronic kidney disease (1/31), and chronic hepatitis B (1/31). Nine patients (29%) had unknown underling diseases. Sites of cryptococcal infection were the central nervous system (20/31), lungs (7/31), and disseminated infection (4/31). C. gattii and C. neoformans was identified in 9 and 5 subjects, respectively, by culture or was documented at outside hospitals. The other 17 subjects had a clinical diagnosis with a positive cryptococcal antigen or pathological diagnosis of cryptococcosis but had negative cultures in our institution. The species of Cryptococcus in these 17 cases was unknown to the investigators in the present study. All samples were positive by CrAg LFA (100% sensitivity; 95% confidence interval (CI), 92% to 100% for serum; 95% CI, 66% to 100% for CSF). CrAg LFA titers and LA titers ranged from <1:2 to 1:5,120 and 1:2 to 1:1,024, respectively (Table 1), and CrAg LFA titers were greater than LA titers in almost all cases. The Spearman rank correlation coefficient (rS) between ln LA and ln LFA titers was 0.79 (95% CI, 0.66 to 0.88; P < 0.0001) (Fig. 1). The Bland-Altman plot showed a bias of 1.9 (SD, 0.98) and 95% limits of agreement (LOA) of 0.01 to 3.8 (Fig. 2).
TABLE 1.
Results of cryptococcal EIA, LA, and LFA among non-HIV-related cryptococcosisa
| Patient No. | Site of infection | Cryptococcus spp.b | Sample typec | EIA | LA titerd | LFA | LFA titere |
|---|---|---|---|---|---|---|---|
| 1 | Lung | C. gattii | BL | NEGf | 64 | POSg | 2,560 |
| BL | NEG | 64 | POS | 1,280 | |||
| 2 | Lung | C. gattii | BL | NEG | 32 | POS | 320 |
| BL | NEG | 32 | POS | 320 | |||
| 3 | Lung | Unknown | BL | NEG | 64 | POS | 640 |
| BL | NEG | 64 | POS | 640 | |||
| BL | NEG | 64 | POS | 320 | |||
| 4 | Disseminated | C. gattii | BL | NEG | 16 | POS | 160 |
| BL | NEG | 16 | POS | 80 | |||
| BL | NEG | 16 | POS | 80 | |||
| BL | NEG | 8 | POS | 80 | |||
| BL | NEG | 4 | POS | 20 | |||
| 5 | CNSh | Unknown | BL | NEG | 16 | POS | 320 |
| 6 | CNS | C. neoformans | BL | NEG | 16 | POS | 160 |
| BL | NEG | 16 | POS | 80 | |||
| 7 | CNS | C gattii | BL | NEG | 32 | POS | 1,280 |
| BL | NEG | 32 | POS | 320 | |||
| 8 | CNS | C. neoformans | BL | NEG | 64 | POS | 10 |
| 9 | CNS | C. gattii | BL | NEG | 128 | POS | 2,560 |
| BL | NEG | 32 | POS | 320 | |||
| 10 | CNS | Unknown | BL | NEG | 8 | POS | 40 |
| BL | NEG | 8 | POS | 40 | |||
| 11 | CNS | Unknown | BL | NEG | 4 | POS | 20 |
| BL | NEG | 4 | POS | 20 | |||
| 12 | CNS | C. neoformans | BL | NEG | 4 | POS | 40 |
| BL | NEG | 4 | POS | 5 | |||
| 13 | CNS | C. gattii | BL | NEG | 32 | POS | 1,280 |
| 14 | CNS | Unknown | BL | NEG | 32 | POS | 160 |
| 15 | Disseminated | Unknown | BL | NEG | 8 | POS | 20 |
| 16 | CNS | Unknown | BL | NEG | 32 | POS | 320 |
| BL | NEG | 32 | POS | 160 | |||
| BL | NEG | 64 | POS | 80 | |||
| BL | NEG | 32 | POS | 80 | |||
| BL | NEG | 32 | POS | 80 | |||
| 17 | Disseminated | C. gattii | BL | NEG | 16 | POS | 160 |
| BL | NEG | 4 | POS | 40 | |||
| 18 | CNS | Unknown | BL | NEG | 256 | POS | 2,560 |
| CSF | NEG | 32 | POS | 320 | |||
| 19 | Lung | Unknown | BL | NEG | 2 | POS | 10 |
| 20 | Lung | Unknown | BL | NEG | 256 | POS | 1,280 |
| BL | NEG | 256 | POS | 1,280 | |||
| BL | NEG | 64 | POS | 640 | |||
| BL | NEG | 32 | POS | 160 | |||
| 21 | CNS | C. neoformans | BL | NEG | 32 | POS | 640 |
| BL | NEG | 8 | POS | 80 | |||
| CSF | NEG | 1,024 | POS | 5,120 | |||
| CSF | NEG | 32 | POS | 80 | |||
| CSF | NEG | 32 | POS | 80 | |||
| CSF | NEG | 4 | POS | 20 | |||
| 22 | Lung | Unknown | BL | NEG | NAi | POS | 5 |
| 23 | CNS | C. gattii | BL | NEG | 16 | POS | 80 |
| 24 | CNS | Unknown | BL | NEG | NEG | POS | 2j |
| 25 | Disseminated | C. gattii | CSF | NEG | 8 | POS | 160 |
| CSF | NEG | 2 | POS | 40 | |||
| 26 | CNS | Unknown | CSF | NEG | 2 | POS | 80 |
| 27 | CNS | Unknown | BL | NEG | 2 | POS | 5 |
| 28 | Lung | Unknown | BL | NEG | NEG | POS | 2 |
| 29 | CNS | Unknown | BL | NEG | NEG | POS | 10 |
| 30 | CNS | Unknown | BL | NEG | 2 | POS | <1:2 |
| 31 | CNS | C. neoformans | CSF | NEG | NEG | POS | 2 |
A total of 60 samples (51 serum samples and 9 CSF samples) are included.
Sample was culture positive or was documented cryptococcal infection at an outside hospital.
BL, serum; CSF, cerebrospinal fluid.
LA titer (1:2 serial dilution).
LFA titer (initial 1:5 and then 1:2 serial dilution).
NEG, negative.
POS, positive.
CNS, central nervous system.
NA, not available.
LFA titer (initial 1:2 dilution).
FIG 1.
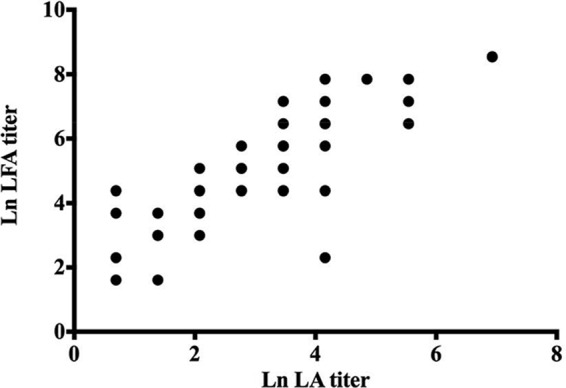
Spearman rank correlation coefficient (rS) between cryptococcal ln LA and ln LFA titers among non-HIV-related cryptococcosis (rS, 0.79; 95% CI, 0.66 to 0.88; P < 0.0001).
FIG 2.

The Bland-Altman plot between cryptococcal ln LA and ln LFA titers among non-HIV-related cryptococcosis (Bias, 1.9; SD, 0.98; and 95% LOA, 0.01 to 3.8).
In summary, we found that the LFA demonstrated 100% sensitivity for identifying cryptococcal antigen in serum and CSF specimens from a set of patients with confirmed cryptococcal infection that had tested negative by EIA and in four specimens that had tested negative by EIA and LA. The correlation between LFA and LA titers of 0.79 in the present study on HIV-negative patients corresponds to values found in previous studies that included HIV-positive patients. Boulware et al. reported correlations of 0.87 (for serum) and 0.82 (for CSF) in a study of HIV-positive patients (7), and McMullan et al. reported a value of 0.84 (for serum and CSF) in a study that included both HIV-positive and HIV-negative patients (4).
Given the apparently greater sensitivity of LFA compared with that of EIA and LA, LFA can reasonably be employed in the diagnosis of cryptococcal infection when conventional immunologic assays give equivocal or discrepant (EIA-negative/LA-positive) results. However, a further evaluation of LFA to diagnose cryptococcal infection in LA-negative patients is warranted to confirm its sensitivity in LA-negative cases. Clinicians should also be aware of cross-reactions of LFA in the serum samples of patients with invasive Trichosporon infection, as reported for other cryptococcal assays (11). In our Institution, we use a combination of screening EIA and confirmatory LA testing for cryptococcosis. Given the poorer sensitivity of the EIA (12) and the significant manual labor and operator dependence of the LA test, LFA represents a viable alternative to this testing algorithm. However, LFA titers should not be interpreted as equivalent to LA titers for the purpose of monitoring post-treatment, as demonstrated by the correlation values reported in this study and elsewhere (4, 7).
In conclusion, CrAg LFA demonstrated excellent sensitivity to detect CrAg in serum and CSF samples from HIV-negative patients with known cryptococcal infections. CrAg LFA and LA titers were correlated, but clinicians should be aware that titers are not directly comparable between the two assays.
ACKNOWLEDGMENTS
We thank the staff of the Microbiology Service, Department of Laboratory Medicine, National Institutes of Health for processing patients' samples for the analysis. We also thank IMMY Inc., USA for providing part of the materials used in the study.
The views expressed in the work are those of the authors and do not necessarily represent those of the NIH or HHS.
Funding Statement
The Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID) and the Clinical Center, National Institutes of Health (NIH) provided funding.
REFERENCES
- 1.Lindsley MD, Mekha N, Baggett HC, Surinthong Y, Autthateinchai R, Sawatwong P, Harris JR, Park BJ, Chiller T, Balajee SA, Poonwan N. 2011. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis 53:321–325. doi: 10.1093/cid/cir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN, Longley N, Harrison TS, Kozel TR. 2011. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis 53:1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binnicker MJ, Jespersen DJ, Bestrom JE, Rollins LO. 2012. Comparison of four assays for the detection of cryptococcal antigen. Clin Vaccine Immunol 19:1988–1990. doi: 10.1128/CVI.00446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMullan BJ, Halliday C, Sorrell TC, Judd D, Sleiman S, Marriott D, Olma T, Chen SC. 2012. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS One 7:e49541. doi: 10.1371/journal.pone.0049541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen J, Slechta ES, Gates-Hollingsworth MA, Neary B, Barker AP, Bauman S, Kozel TR, Hanson KE. 2013. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin Vaccine Immunol 20:52–55. doi: 10.1128/CVI.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gates-Hollingsworth MA, Kozel TR. 2013. Serotype sensitivity of a lateral flow immunoassay for cryptococcal antigen. Clin Vaccine Immunol 20:634–635. doi: 10.1128/CVI.00732-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulware DR, Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, Schutz C, Kwizera R, Butler EK, Meintjes G, Muzoora C, Bischof JC, Meya DB. 2014. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 20:45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. 2014. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis 58:113–116. doi: 10.1093/cid/cit641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escandón P, Lizarazo J, Agudelo CI, Chiller T, Castañeda E. 2013. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Med Mycol 51:765–768. doi: 10.3109/13693786.2013.781692. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307–310. [PubMed] [Google Scholar]
- 11.Rivet-Dañon D, Guitard J, Grenouillet F, Gay F, Ait-Ammar N, Angoulvant A, Marinach C, Hennequin C. 2015. Rapid diagnosis of cryptococcosis using an antigen detection immunochromatographic test. J Infect 70:499–503. doi: 10.1016/j.jinf.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Panackal AA, Dekker JP, Proschan M, Beri A, Williamson PR. 2014. Enzyme immunoassay versus latex agglutination cryptococcal antigen assays in adults with non-HIV-related cryptococcosis. J Clin Microbiol 52:4356–4358. doi: 10.1128/JCM.02017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


