Abstract
Although numerous perioperative samples and culture media are required to diagnose prosthetic joint infection (PJI), their exact number and types have not yet been definitely determined with a high level of proof. We conducted a prospective multicenter study to determine the minimal number of samples and culture media required for accurate diagnosis of PJI. Over a 2-year period, consecutive patients with clinical signs suggesting PJI were included, with five perioperative samples per patient. The bacteriological and PJI diagnosis criteria were assessed using a random selection of two, three, or four samples and compared with those obtained using the recommended five samples (references guidelines). The results obtained with two or three culture media were then compared with those obtained with five culture media for both criteria. The times-to-positivity of the different culture media were calculated. PJI was confirmed in 215/264 suspected cases, with a bacteriological criterion in 192 (89%). The PJI was monomicrobial (85%) or polymicrobial (15%). Percentages of agreement of 98.1% and 99.7%, respectively, for the bacteriological criterion and confirmed PJI diagnosis were obtained when four perioperative samples were considered. The highest percentages of agreement were obtained with the association of three culture media, a blood culture bottle, a chocolate agar plate, and Schaedler broth, incubated for 5, 7, and 14 days, respectively. This new procedure leads to significant cost saving. Our prospective multicenter study showed that four samples seeded on three culture media are sufficient for diagnosing PJI.
INTRODUCTION
There is still no standard definition of prosthetic joint infection (PJI). Although numerous perioperative samples are needed to diagnose PJI, their optimal number is not yet definitively known. In 1998, Atkins et al., using a mathematical model, demonstrated that five or six perioperative samples, with a cutoff of three or more culture-positive samples, are needed to diagnose PJI with high sensitivity and specificity (1). Recent guidelines still recommend that about five samples should be cultured for optimal diagnosis of PJI (2, 3). Since each tissue specimen is cultured on four to five culture media, a total of 20 to 25 culture media have to be analyzed per patient. The optimal duration of incubation for periprosthetic cultures was extended to 13 or 14 days, especially for Propionibacterium acnes recovery (4, 5). The implementation of 14-day culture incubation in combination with the use of multiple solid and liquid aerobic and anaerobic culture media makes the daily monitoring of PJI by the laboratory very complex, costly, and time-consuming. Moreover, the number of cases of PJI will continue to increase in the coming decades. Forecasts predict that by 2030, the demand for total hip and knee arthroplasties will grow to 4 million in the United States (6). A major economic effect is that device-associated costs are significantly higher than native bone and joint infections, as recently reported (7). This will inevitably lead to a dramatic increase in the number of tissue specimens to be processed by bacteriology laboratories, with a major economic impact on the cost of microbiological diagnosis.
Furthermore, the sensitivity of microbiological diagnosis was recently improved by the use of new techniques for preparing tissue specimens and new culture media, allowing us to redefine the diagnostic modalities of PJI.
Sonication of explanted prosthesis has been used to dislodge the bacteria from the surface. Prosthesis sonication culture has been shown to increase culture sensitivity compared with that of periprosthetic tissue cultures (8).
Bacterial extraction using beadmill specimen processing was shown to improve bacteriological diagnosis of PJI in a single-center study (9). Among the clinical questions recently identified by the authors of the Infectious Diseases Society of America (IDSA) guidelines, the role of beadmill processing in the diagnosis of PJI was raised along with a recommendation for future study (2).
Another method to increase the diagnostic yield of microbiological diagnosis is to inoculate samples directly into blood culture bottles. A diagnostic gain was reported not only for joint aspirate fluids but also for periprosthetic tissues from several single-center studies without preliminary beadmill processing (10–16).
Finally, the wide range of culture sensitivity levels observed in the different single-center studies published underlines the need for multicenter studies to accurately evaluate microbiological methods (17). Moreover, the most cost-effective algorithms for microbiological diagnosis in the light of clinical effectiveness still have to be defined.
Our Great French West network organization for the multidisciplinary diagnosis and treatment of bone and joint infections in seven referral centers allowed us to carry out the first prospective multicenter study related to the microbiological diagnosis of PJI (18).
The main issues of the current study were the following. (i) How many samples and which samples are needed to obtain an accurate PJI diagnosis, and can we reduce the number of intraoperative samples? (ii) How many culture media would we need, and how long should we keep them incubated to obtain an accurate PJI diagnosis? (iii) Can we confirm the improvement obtained by using blood culture bottles in a multicenter study? (iv) What is the cost impact of sample care by the microbiology laboratory?
MATERIALS AND METHODS
Study design.
This study was designed as a multicenter, prospective, observational, cross-sectional study of adult patients suspected of having PJI. The study protocol (Programme Hospitalier de Recherche Clinique Interrégional API/N/041) was approved by the institutional review board and ethics committee. Informed consent was obtained from each patient before inclusion.
Study population.
Consecutive patients with clinical signs suggesting acute or chronic PJI were included in seven French university hospitals from December 2010 through March 2012. Six tissue samples were collected during surgery, five samples for the culture and 16S rRNA gene PCR and one periprosthetic membrane sample for histological analysis. An electronic case report form was created to collect the following data for each patient: patient demographic characteristics, arthroplasty localization, presentation of infection, and antibiotic treatment over the 15 days before surgery.
Definition of a PJI.
Acute PJI was suspected in patients with pain, disunion, necrosis, or inflammation of the scar in the 3 months following prosthesis implantation. Chronic infection was suspected in the presence of chronic pain without systemic symptoms, as well as a loosened prosthesis. According to recent IDSA proposals, PJI was diagnosed when at least one of the following criteria was positive (2): (i) a clinical criterion (a sinus tract communicating with the prosthesis and/or purulence around the prosthesis), (ii) histology positive for infection (as specified below), (iii) a bacteriological infection criterion (as specified below).
Microbiological methods.
Periprosthetic tissue cultures were produced in each center following a standard protocol. For each patient, five perioperative specimens (tissue, bone, or joint fluid specimens) were collected in sterile vials with different surgical instruments. After the addition of 10 ml of sterile water and stainless steel beads, the vials were shaken on a Retsch MM401 beadmill. One milliliter was inoculated into a pediatric blood culture bottle and into Schaedler broth, as recommended, and both cultures were incubated for 14 days. Three further 50-μl volumes were each spread onto a blood agar plate and a PolyViteX chocolate agar plate and incubated under a CO2-enriched atmosphere for 7 days and onto a blood agar plate incubated in an anaerobic atmosphere for 7 days. Synovial fluids were seeded directly onto the three solid and the two liquid culture media without beadmill processing. The Schaedler broth was inspected weekly for cloudiness and subcultured on a Schaedler blood agar plate or a blood agar plate incubated in an anaerobic atmosphere for 48 h as soon as it became cloudy or systematically on the 14th day.
Time-to-positivity of blood culture bottles was defined as positive detection by an automatic blood culture system. Isolated bacteria were identified using standard laboratory procedures. Antibiotic susceptibility testing was determined as recommended (19). The bacteriological criterion was considered positive when at least one culture yielded a strict pathogen (Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacteriaceae, and anaerobes) or when two cultures yielded a strain that was a skin commensal (such as coagulase-negative staphylococci [CoNS] or P. acnes) (2, 3).
Histological analysis.
The periprosthetic membrane samples were fixed in buffered formalin, and paraffin block sections were stained with hematoxylin and eosin. Following Feldman's criteria (adapted from Mirra's criteria), the histology was considered positive for infection when at least 5 neutrophils per high-power field (×400 magnification) were found after examination of at least five separate microscopic fields (20, 21). The specimens were examined by pathologists blinded to the suspicion of PJI and to the results of the cultures.
Statistical analysis strategy.
Statistical analyses were performed with SAS software, version 9.3 (SAS Institute, Cary, NC, USA) and R, version 3.1.3 (22).
Main issues. (i) Number of samples required for PJI diagnosis.
Our first study objective was to estimate the impact of processing two, three, or four samples, instead of the five samples usually recommended, on bacteriological and PJI diagnostic criteria. For each patient, we randomly selected four samples and reassessed the bacteriological and the PJI diagnosis criteria. We then checked whether these reassessed bacteriological and PJI diagnosis criteria agreed with those obtained using the full microbiological result data set. This procedure was rerun 1,000 times to estimate an average agreement rate (proportion of correct results, whether PJI or non-PJI correctly classified), with its associated 95% confidence interval. The same operation was also conducted using subsamples of three and two samples.
(ii) Number and type of culture media required for PJI diagnosis.
The Cochran Q test was used to evaluate the overall effect of the culture medium on the proportion of samples that tested positive for aerobic and anaerobic bacteria. The positivity rates of samples from paired culture media were then compared using McNemar's test.
A secondary study objective was to estimate the impact of eliminating two or three culture media on the bacteriological criterion and PJI diagnosis. The results obtained with two or three culture media were compared with those obtained with five culture media for both bacteriological and PJI diagnostic criteria. The results of the different analyses were presented with the percentage of agreement for each criterion.
Secondary issues. (i) Incubation period of culture media required for PJI diagnosis.
As the times-to-positivity in liquid media cannot be determined for polymicrobial infection, they were determined for monomicrobial infections only. The times-to-positivity of the different culture media were determined separately for aerobic and anaerobic bacteria.
(ii) Considerations on cost management of PJI samples for the bacteriology laboratory and possible cost reduction.
A cost per patient was evaluated, including that for five perioperative samples, each seeded in five different culture media, and for part of the technical time spent on the culture and processing of positive cultures. This cost was then compared to that estimated for the optimal number of samples and culture media as determined from our statistical analysis.
RESULTS
Diagnosis of infection.
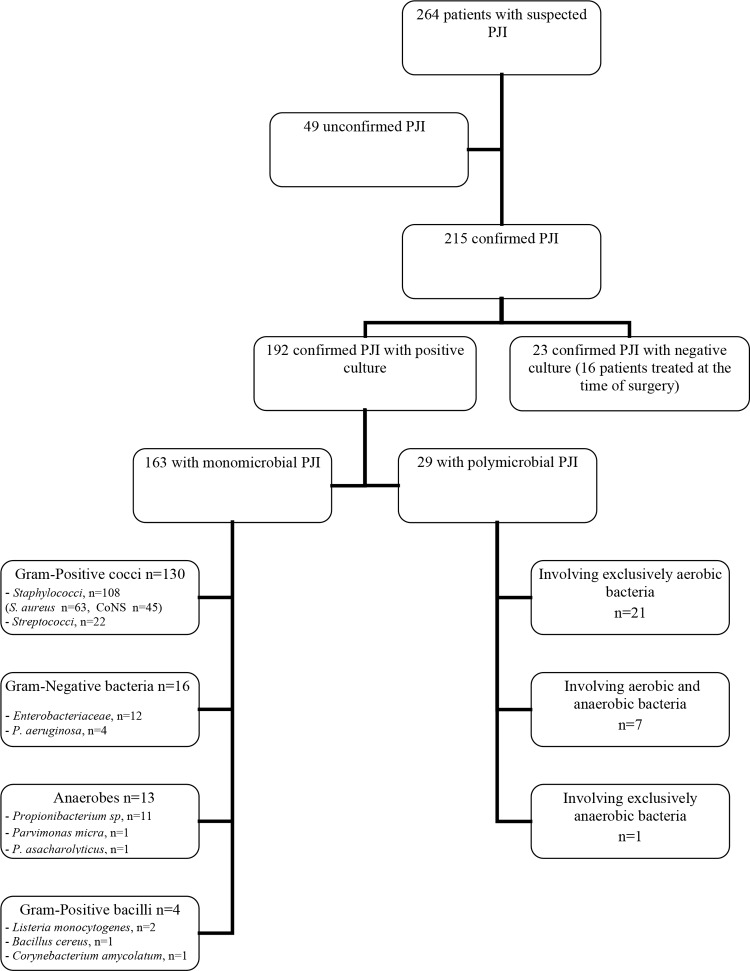
After analysis of clinical, bacteriological, and histological criteria, a definitive diagnosis of infection was confirmed in 215 out of 264 suspected cases of PJI (Fig. 1). The PJIs were chronic for 168/215 (78.1%) and acute for 47/215 (21.9%) of them. For patient characteristics, see Bémer et al. (18).
FIG 1.
Microbiological results. PJI, prosthetic joint infection. For unconfirmed cases, see Bémer et al. (18). P. asaccharolyticus, Peptostreptococcus asaccharolyticus.
Of the 215 patients with confirmed PJI, 192 (89.3%) had a positive bacteriological criterion, with monomicrobial infection in 163 (84.9%) cases and polymicrobial infection in 29 (15.1%). Of the monomicrobial infections, staphylococci were isolated in 66.3%, streptococci and enterococci were isolated in 13.5%, Gram-negative bacilli were isolated in 9.8%, anaerobes were isolated in 8.0%, and Gram-positive bacilli were isolated in 2.5% of cases (Fig. 1).
Of the 29 polymicrobial infections, 21 involved two or more different aerobic bacteria, 7 involved both aerobic and anaerobic bacteria, and 1 involved exclusively anaerobes (Fig. 1).
Among 70 confirmed cases of PJI in patients treated with antibiotics at the time of surgery, 54 (77%) were positive in culture (44 monomicrobial and 10 polymicrobial PJIs), and 16 (23%) remained negative in culture.
Minimal number and best types of samples required for an accurate PJI diagnosis.
Of the 192 patients with a positive bacteriological criterion, a total of 849 (88.4%) out of 960 samples were positive in culture, i.e., an average number of 4.4 per patient. Tissues in contact with material and joint fluids accounted for 45.6% of the collected samples. The rate of positivity ranged from 76.6% to 91.7% depending on the nature of the sample: cancellous bone, 76.6% (n = 36/47); bone in contact with cement, 78.8% (n = 41/52); capsule, 84.8% (n = 67/79); cortical bone, 87.1% (n = 81/93); synovial tissue, 88.2% (n = 90/102); subfascial tissue, 89.3% (n = 133/149); tissue in contact with material, 91.5% (n = 247/270); and joint fluid, 91.7% (n = 154/168).
From the 1,000 generated data sets, the bacteriological criterion and diagnosis of confirmed PJI had a mean percentage of agreement of 85.2% and 98.0%, respectively, when two samples were considered and agreement of 93.9% and 99.2%, respectively, when three samples were considered. Percentages of agreement of 98.1% and 99.7%, respectively, for both criteria were obtained when four perioperative samples were considered (Fig. 2).
FIG 2.
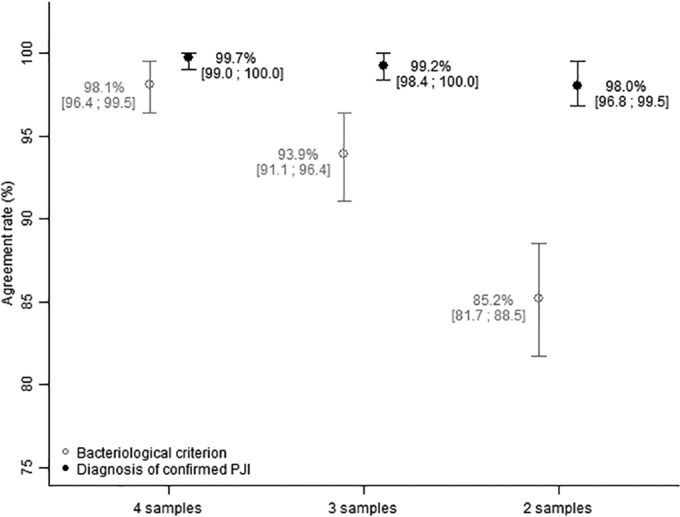
Mean percentage of agreement and 95% confidence interval (97.5th and 2.5th percentiles) obtained from 1,000 data sets generated according to the number of samples obtained from the simulation analysis.
Number and type of culture media required for an accurate PJI diagnosis.
Preliminary comparison of anaerobic culture media showed the superiority of the broth medium as 75.2% of anaerobic bacteria grew on Schaedler broth versus 56.2% on anaerobic blood agar. Thus, for simplicity of analysis, Schaedler broth was the only anaerobic medium considered in the statistical analysis.
Of the 171 cases of PJI involving aerobes (150 monomicrobial and 21 polymicrobial), the rates of positive samples were 70.1% on blood agar plate, 69.0% on chocolate agar plate, 68.8% on Schaedler broth, and 83.0% in the blood culture bottle (Table 1). Of the 21 cases of PJI involving anaerobes (13 monomicrobial and 8 polymicrobial), the rates of positive samples were 31.4% on blood agar plate, 53.3% on chocolate agar plate, 75.2% on Schaedler broth, and 54.3% in the blood culture bottle (Table 2).
TABLE 1.
Paired comparison of positivity rates between culture media from 171 cases of PJI with aerobic bacteria
| Culture medium and no. (%) of positive samples |
P value with indicated culture medium and no. (%) of positive samplesa |
||
|---|---|---|---|
| Blood agar plate, 599 (70.1) | Chocolate agar plate, 590 (69.0) | Anaerobic broth medium, 588 (68.8) | |
| Blood agar plate, 599 (70.1) | |||
| Chocolate agar plate, 590 (69.0) | 0.3507 | ||
| Anaerobic broth medium, 588 (68.8) | 0.4510 | 0.8892 | |
| Blood culture bottle, 710 (83.0) | <0.0001 | <0.0001 | <0.0001 |
Total number of samples, 855.
TABLE 2.
Paired comparison of positivity rates between culture media from the 21 cases of PJI with anaerobic bacteriaa
| Culture medium and no. (%) of positive samples |
P value with indicated culture medium and no. (%) of positive samplesb |
||
|---|---|---|---|
| Blood agar plate, 33 (31.4) | Chocolate agar plate, 56 (53.3) | Anaerobic broth medium, 79 (75.2) | |
| Blood agar plate, 33 (31.4) | |||
| Chocolate agar plate, 56 (53.3) | <0.0001 | ||
| Anaerobic broth medium, 79 (75.2) | <0.0001 | <0.0001 | |
| Blood culture bottle, 57 (54.3) | <0.0001 | 0.8759 | 0.0002 |
Thirteen cases of monomicrobial anaerobic PJI and eight cases of polymicrobial PJI with at least one anaerobe.
Total number of samples, 105.
As the Cochran Q test showed that at least one of the four culture media differed significantly from another, whether for aerobes (P < 0.0001) or anaerobes (P < 0.0001), paired comparisons were performed. These comparisons using the McNemar test showed that the rate of positive samples was significantly higher in the blood culture bottle for aerobes (Table 1) and in Schaedler broth for anaerobes (Table 2) than in other media. The rate of positive cultures on the chocolate agar plate was similar for aerobic bacteria to that observed on the blood agar plate and much higher for anaerobic bacteria due to the predominance of P. acnes. There were four chronic cases of PJI with bacteria detected using blood cultures only, three of which were due to S. aureus (two susceptible and one methicillin resistant) and one due to methicillin-susceptible Staphylococcus epidermidis. Two of the four patients were being treated with antibiotics at the time of surgery.
Finally, a combination of three culture media gave results similar to those obtained with four or five culture media. The highest percentages of agreement of the bacteriological criterion (98.3% for aerobes and 85.7% for anaerobes) and the confirmed diagnosis of PJI (100% for both aerobes and anaerobes) were obtained with a combination of a blood culture bottle, a chocolate agar plate, and Schaedler broth (Table 3).
TABLE 3.
Percentages of agreement according to the combination of culture media for the 192 cases of PJIa
| Culture media | % agreement of bacteriological criterion |
% agreement of confirmed PJI diagnosis |
||
|---|---|---|---|---|
| Aerobes | Anaerobes | Aerobes | Anaerobes | |
| Blood culture bottle, chocolate agar plate, and anaerobic broth medium | 98.3 | 85.7 | 100.0 | 100.0 |
| Blood culture bottle, blood agar plate, and anaerobic broth medium | 97.7 | 85.7 | 100.0 | 100.0 |
| Chocolate agar plate and anaerobic broth medium | 92.4 | 81.0 | 98.3 | 100.0 |
| Blood agar plate and anaerobic broth medium | 92.4 | 76.2 | 99.4 | 100.0 |
| Blood culture bottle and chocolate agar plate | 97.7 | 66.7 | 100.0 | 95.2 |
| Blood culture bottle and blood agar plate | 96.5 | 47.6 | 100.0 | 90.5 |
Of the total number of prosthetic joint infection (PJI) cases, 171 represent infection with aerobes, and 21 represent infection with anaerobes.
Times-to-positivity according to culture media.
All the aerobic bacteria were detected from positive pediatric blood culture bottles in 5 days (except for one sample which was positive in 10 days) and grew in 7 days on chocolate or blood agar plates. Of the aerobic bacteria, 84.1% grew in less than 10 h in blood culture bottles while 71.4% grew in 18 to 24 h on agar media.
Ninety-three percentage of aerobes were isolated during the first 2 days of observation, and 7% were isolated between the third and the seventh day, indicating that 7 days of incubation was still needed for solid media.
Most of the P. acnes strains were isolated from Schaedler broth (14.0% [n = 7] from 2 to 4 days and 86% [n = 36] between 5 and 14 days), against 34 (68%) in 7 days from chocolate agar plates in a CO2 atmosphere or blood agar plates in an anaerobic atmosphere and 26 (52%) in 6 days from pediatric blood cultures. Twenty-eight percent (12/43) of the Schaedler broths, not cloudy after 14 days of incubation, were systematically subcultured on the 14th day.
Cost impact of sample management by the microbiology laboratory.
Cost assessment was based on five samples grown on five culture media per patient as in our multicenter protocol. The total cost was 50.5 euros ($53.5) per patient (23.0 euros for the culture media, including cost of vials with beadmills and 27.5 euros for the technical manipulation time), against 37.4 euros ($39.6) for four samples grown on three culture media, i.e., a difference of 13.1 euros ($14) per patient.
DISCUSSION
Our study is the first prospective multicenter study to have performed multicenter standardization of culture techniques as recently published (18). A uniform number of five samples was collected per patient, with many at the bone-prosthesis interface and the interface membrane. The suspensions processed by beadmill were used to seed solid and liquid culture media, including a blood culture bottle (18). This methodology enabled us to bacteriologically document PJI in 89.3% of cases, compared with 61% or 70% in other studies (8, 23). Furthermore, we were able to document PJI for 95.1% of patients who were not receiving antibiotics at the time of surgery.
The first objective of the present study was to analyze the impact that reduction of one, two, or three samples would have on the microbiological criteria and PJI diagnosis using a random-sampling method repeated 1,000 times. We showed that bacterial analysis of four samples instead of five had no impact on the clinical effectiveness of the microbiological diagnosis for PJI. With respect to the most adequate samples, our study did not confirm the superiority of interface membranes over capsule and synovial fluid specimens, as reported by Bjerkan et al. (24). In fact, the positivity rate varied little according to the various types of samples. However, it seems preferable to privilege tissue in contact with the material and the joint fluid. Bone samples may be more difficult to grind. Joint fluid should be seeded directly into a blood culture bottle by the surgeon in the operating room in a daily routine.
The second objective of our study was to reduce the number and incubation time of culture media while maintaining diagnosis effectiveness. We showed that seeding three culture media instead of five had no impact on the microbiological diagnosis for PJI when a chocolate agar plate incubated in a CO2 atmosphere for 7 days, Schaedler broth, and a blood culture bottle were used. Using Schaedler broth means that we can isolate more anaerobic bacteria than with anaerobic blood agar. In addition, we found that the chocolate agar plate was more sensitive than the anaerobic agar plate, particularly for the anaerobe P. acnes, which can grow in a CO2-enriched atmosphere.
The decision to inoculate a blood culture bottle and the choice of bottle, i.e., aerobic, anaerobic, or pediatric, are important. Blood culture bottles have been previously shown to improve sensitivity in detecting microorganisms from synovial fluids for the diagnosis of infectious arthritis or prosthetic joint infection (10–13). This improvement can easily be explained by optimal culture conditions such as shaking, inactivating antimicrobial agents with resins, and inoculating larger volumes of samples. Other studies have investigated the benefit of using blood culture bottles for culturing tissue samples pretreated by manual dissection or vigorous shaking (14–16). A prospective single-center study comparing eight culture media (four liquid media and four solid ones) showed the high sensitivity of blood culture bottles for the diagnosis of 23 cases of PJI (14). In another recent single-center prospective study with 79 cases of PJI, the sensitivity of blood cultures was 82.3% after 3 days of incubation (16). The authors recommended seeding two blood culture vials, one aerobic and one anaerobic, for each sample, representing 10 vials per patient to be inserted into the automatic blood culturing system (16). Because of the high number of cases of PJI, present and future, it seems important to propose an alternative solution.
In our study, pediatric blood culture bottles proved less efficient than Schaedler broth for cultivating anaerobes, as shown previously (15). However, the pediatric bottle, seeded with a small amount of suspension processed by beadmill, led to the automatic detection of 83% of aerobes in less than 18 h in our study. The bacteria could be identified within 24 h of collection, allowing bacteriological documentation of 85% of the cases of PJI. This represents a remarkable advance for the choice of antibiotics.
Our study showed that Schaedler broth could be used to isolate most anaerobes and was particularly useful in polymicrobial PJI. The optimal duration of culture could not be determined from our study as the majority of broths were subcultured on the 14th day. Nevertheless, one might suggest subculturing once at day 7 and then at day 14 in the event of a negative subculture, according to a recent study (25).
This new procedure would lead to significant cost savings when the expected increase in the near future in the number of cases of PJI is considered (6). This is particularly important as the cost of microbiological diagnosis is not yet taken into account in the overall cost assessment of PJI. No impact was found on the clinical efficacy of PJI diagnosis.
In conclusion, our prospective multicenter study showed that the minimal number of samples required to confirm the diagnosis of PJI can be decreased to four per patient instead of five as recommended by current consensus guidelines. Three culture media, including a blood culture bottle incubated for only 5 days, could be proposed for an accurate microbiological diagnosis of PJI.
ACKNOWLEDGMENTS
This study was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Interrégional API/N/041) and a grant from the Centre de Référence des Infections Ostéo-articulaires du Grand Ouest (CRIOGO).
We thank Louis Bernard for his clinical and methodological assistance. We thank Karine Fèvre and Line Happi for their help with the study and technical assistance. We are indebted to Jane Cottin for her enthusiasm until the end. We are also very grateful to all the members of the CRIOGO for their continuing support.
The CRIOGO Study Team members included the following: J. Cottin (deceased), P. Bizot, and P. Abgueguen (CHU Angers); R. Gérard, E. Stindel, and S. Ansart (CHU Brest); S. Touchais, F. Gouin, D. Boutoille, and N. Asseray (CHU Nantes); A. Guigon, J. Guinard, F. Razanabola, and C. Mille (CH Orléans); A. S. Cognée, L. E. Gayet, F. Roblot, and G. Le Moal (CHU Poitiers); J. Guinard, J. L. Polard, and C. Arvieux (CHU Rennes); L. Bernard, P. Rosset, and G. Gras (CHU Tours).
We declare that we have no conflicts of interest.
P.B., D.T., C.P., L.B., and C.B. conceived and designed the study. P.B., D.T., C.P., A.S.V., A.J.-G., C.L., M.K., G.H.-A., L.B., M.E.J., S.C., and C.B. were site investigators. J.L. and B.G. were study statisticians. P.B., J.L., C.B., C.P., D.T., A.S.V., A.J.-G., S.C., C.L., and M.K. wrote the paper. P.B. was the principal investigator.
REFERENCES
- 1.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR, the Osiris Collaborative Study Group . 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol 36:2932–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 3.Dupon M, Dutronc H, Perpoint T, Berthelot P, Bouscarra J, Chidiac C, Drape JL, Eyrolle L, Grimprel E, Morelec I, Cyteval C, Dubost JJ, Gaudias J, Jenny JY, Lebtahi R, Rogues AM, Senneville E, Arvieux C, Baron R, Bobieux A, Claverie JP, Codine P, Daquet V, Devillers A, Epifanie JL, Fajon O, Fernandez P, Fessy MH, Gromb S, Guggenbuhl P, Hajjar J, Huglo D, Lesens O, Lucht F, Lusting S, Macouillard G, Morand P, Petiot S, Railhac J, Rambaud C, Riegel P, Salmon D, Sarlangue J, Tavernier T, Bernard L, Besnier JM, Boeri C, Bonnet E, Bouscarra J, Claudot F, et al. . 2010. Recommendations for bone and joint prosthetic device infections in clinical practice (prosthesis, implants, osteosynthesis). Société de Pathologie Infectieuse de Langue Française (SPILF). Med Mal Infect 40:185–211. doi: 10.1016/j.medmal.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. 2008. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis 47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 5.Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA III, Cookson BT. 2011. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol 49:2490–2495. doi: 10.1128/JCM.00450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. 2007. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 7.Grammatico-Guillon L, Baron S, Gettner S, Lecuyer AL, Gaborit C, Rosset P, Rusch E, Bernard L. 2012. Bone and joint infections in hospitalized patients in France, 2008: clinical and economic outcomes. J Hosp Infect 82:40–48. doi: 10.1016/j.jhin.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greeleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 9.Roux AL, Sivadon-Tardy Bauer T, Lortat-Jacb A, Herrmann JL, Gaillard JL, Rottman M. 2011. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin Microbiol Infect 17:447–450. doi: 10.1111/j.1469-0691.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- 10.von Essen R, Hölttä A. 1986. Improved method of isolating bacteria from joint fluids by the use of blood culture bottles. Ann Rheum Dis 45:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine BR, Evans BG. 2001. Use of blood culture vial specimens in intraoperative detection of infection. Clin Orthop Relat Res 382:222–231. doi: 10.1097/00003086-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Hughes JG, Vetter EA, Patel R, Schleck CD, Harmsen S, Turgeant LT, Cockerill FR III. 2001. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol 39:4468–4471. doi: 10.1128/JCM.39.12.4468-4471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Font-Vizcarra L, Garcia S, Martinez-Pastor JC, Sierra JM, Soriano A. 2010. Blood culture flasks for culturing synovial fluid in prosthetic joint infections. Clin Orthop Relat Res 468:2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes HC, Newnham R, Athanasou N, Atkins BL, Bejon P, Bowler IC. 2011. Microbiological diagnosis of prosthetic joint infections: a prospective evaluation of four bacterial culture media in the routine laboratory. Clin Microbiol Infect 17:1528–1530. doi: 10.1111/j.1469-0691.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 15.Velay A, Schramm F, Gaudias J, Jaulhac B, Riegel P. 2010. Culture with BACTEC Peds Plus bottle compared with conventional media for the detection of bacteria in tissue samples from orthopaedic surgery. Diagn Microbiol Infect Dis 68:83–85. doi: 10.1016/j.diagmicrobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC. 2014. Use of an automated blood culture system (BD BACTEC™) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bémer P, Plouzeau C, Tande D, Léger J, Giraudeau B, Valentin AS, Jolivet-Gougeon A, Vincent P, Corvec S, Gibaud S, Juvin ME, Héry-Arnaud G, Lemarié C, Kempf M, Bret L, Quentin R, Coffre C, de Pinieux G, Bernard L, Burucoa C, Centre de Référence des Infections Ostéo-articulaires du Grand Ouest (CRIOGO) . 2014. Evaluation of 16S rRNA gene PCR sensitivity and specificity for diagnosis of prosthetic joint infection: a prospective multicenter cross-sectional study. J Clin Microbiol 52:3583–3589. doi: 10.1128/JCM.01459-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing. 2012. Breakpoint tables for interpretation of MICs and zone diameters, version 2.0, valid from 2012-01-01. European Committee on Antimicrobial Susceptibility Testing, Vaxjo, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf. [Google Scholar]
- 20.Feldman DS, Lonner JH, Desai P, Zuckerman JD. 1995. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am 77:1807–1813. [DOI] [PubMed] [Google Scholar]
- 21.Mirra JM, Marder RA, Amstutz HC. 1982. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res 170:175–183. [PubMed] [Google Scholar]
- 22.R Development Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 23.Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. 2013. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol 51:2280–2287. doi: 10.1128/JCM.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjerkan G, Witso E, Nor A, Viset T, Loseth K, Lydersen S, Persen L, Bergh K. 2012. A comprehensive microbiological evaluation of fifty-four patients undergoing revision surgery due to prosthetic joint loosening. J Med Microbiol 61:572–581. doi: 10.1099/jmm.0.036087-0. [DOI] [PubMed] [Google Scholar]
- 25.Shannon SK, Mandrekar J, Gustafson DR, Rucinski SL, Dailey AL, Segner RE, Burman MK, Boelman KJ, Lynch DT, Rosenblatt JE, Patel R. 2013. Anaerobic thioglycolate broth culture for recovery of Propionibacterium acnes from shoulder tissue and fluid specimens. J Clin Microbiol 51:731–732. doi: 10.1128/JCM.02695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]