LETTER
The use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been widely implemented for microbial identification in clinical microbiology in recent years. This development can be attributed to its low operating cost and to its speed and reliability of identification for many bacteria and fungi. However, the use of MALDI-TOF MS for mycobacteriology has been less satisfactory, mainly owing to nonstandardized preparatory methods, poor reproducibility, and comparatively inferior results (1). The accurate and rapid identification of mycobacteria to the species level has implications for effective patient treatment and implementation of timely infection control measures.
Our laboratory set about validating the Bruker Biotyper for the identification of mycobacteria using wild-type Mycobacterium spp. previously identified using genotype CM/AS and mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing by the Irish Mycobacterial Reference Laboratory, St. James's Hospital, Dublin, Ireland, and Bruker's Mycobacteria Extract Method (MycoEX) version 3 (Fig. 1). Heat inactivation coupled with ethanol treatment (as detailed in Fig. 1) proved efficacious in the inactivation of mycobacteria; following extended incubation, there was no growth. Preliminary procedures using Bruker's recommended protocol yielded poor results. Therefore, we set about improving the results attained.
FIG 1.
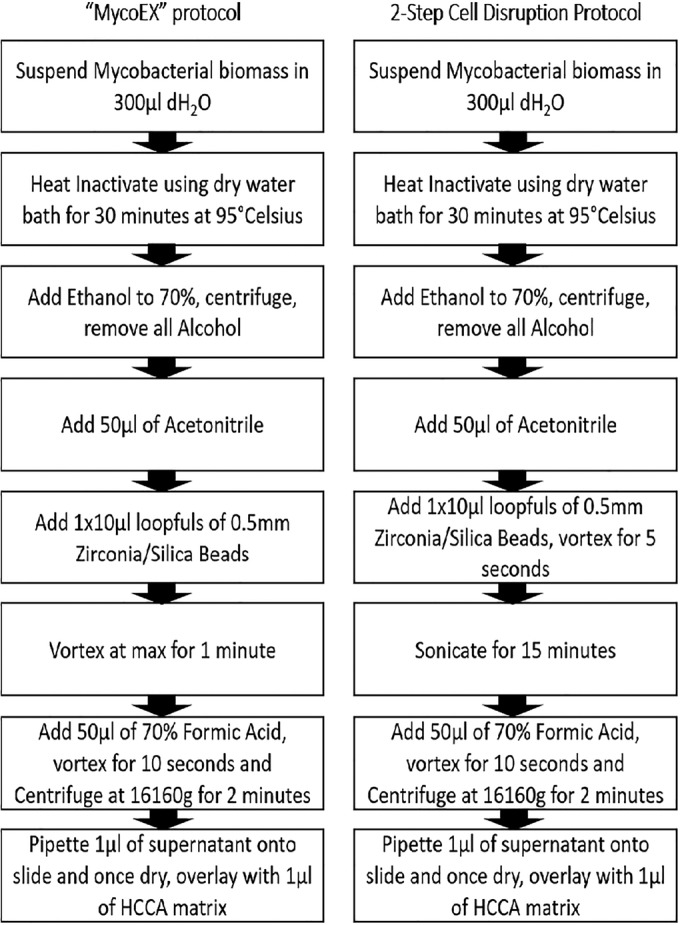
MycoEX protocol and two-step cell disruption protocol. dH2O, distilled water; HCCA, α-cyano-4-hydroxycinnamic acid.
Cell disruption has previously been shown to be a key step in liberating mycobacterial protein for analysis (2). We designed a two-step cell disruption protocol (Fig. 1) and found that the combined use of 0.5-mm-diameter silica/zirconia beads and sonication for 15 min produced greatly improved results (Table 1). A direct comparison between our method and MycoEX showed a marked improvement for Mycobacterium tuberculosis complex isolates using our method; 100% achieved successful identification to the genus level, with 85% achieving a reliable identification to the species level (log score of greater than 2.0). MycoEX resulted in successful identification to the genus level of 79% of isolates, with 62% achieving reliable identification to the species level. The number of results showing no identifications was reduced from 7 (22%) to 0 using the two-step cell disruption protocol. Similar improvements were shown for mycobacteria other than tuberculosis isolates, using the two-step cell disruption protocol; 87.5% achieved a log score of 1.7 or greater and 62.5% achieved a log score of greater than 2 compared to MycoEX, and 67% of isolates achieved a log score of 1.7 or greater, with 29% achieving a log score of greater than 2.0.
TABLE 1.
Two-step cell disruption and MycoEX protocol results
| Organism | Total no. of isolates | No. (%) of isolates after pretreatment resulting in the indicated log score attained using: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Two-step cell disruption protocol |
MycoEX protocol |
||||||||
| >2.0 | 1.7–1.999 | <1.7 | Incorrect identification | >2.0 | 1.7–1.999 | <1.7 | Incorrect identification | ||
| M. tuberculosis | 32 | 27 | 5 | 0 | 0 | 20 | 5 | 7 | 0 |
| M. avium | 10 | 5 | 5 | 0 | 0 | 3 | 4 | 3 | 0 |
| M. intracellulare | 3 | 3 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| M. abscessus | 3 | 3 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| M. fortuitum | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| M. scrofulaceum | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| M. africanum | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. xenopi | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| M. kumamotonense | 1 | 0 | 0 | 0 | 1a | 0 | 0 | 1 | 0 |
| M. bovis | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total | 56 | 42 (75) | 11 (20) | 2 (3) | 1 (2) | 27 (48) | 14 (25) | 15 (27) | 0 (0) |
One M. kumamotonense isolate was misidentified as M. tuberculosis complex, which is most closely related to the M. terrae complex (1).
In conclusion, using the described two-step cell disruption protocol, there was a 9-fold decrease in failures to achieve correct genus identification across all isolates tested, coupled with a 50% increase in the number of isolates achieving a log score of greater than 2.0. Therefore, the described two-step cell disruption method combines complete inactivation of mycobacteria with improved MALDI-TOF MS identification, thereby improving the efficacy of MALDI-TOF MS for mycobacterial identification over that of previous methods. On the basis of these results, we would strongly recommend the utilization of a two-step cell disruption protocol for MALDI-TOF MS identification of mycobacteria.
ACKNOWLEDGMENTS
Thank you to all the staff of the Microbiology Department, Cork University Hospital.
REFERENCES
- 1.Tortoli E. 2014. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev 27:727–752. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machen A, Kobayashi M, Connelly MR, Wang YF. 2013. Comparison of heat inactivation and cell disruption protocols for identification of mycobacteria from solid culture media by use of Vitek matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 51:4226–4229. doi: 10.1128/JCM.02612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


