Abstract
The front-line assay for the presumptive serodiagnosis of acute Japanese encephalitis virus (JEV) and West Nile virus (WNV) infections is the premembrane/envelope (prM/E)-specific IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA). Due to antibody cross-reactivity, MAC-ELISA-positive samples may be confirmed with a time-consuming plaque reduction neutralization test (PRNT). In the present study, we applied a previously developed anti-nonstructural protein 1 (NS1)-specific MAC-ELISA (NS1-MAC-ELISA) on archived acute-phase serum specimens from patients with confirmed JEV and WNV infections and compared the results with prM/E containing virus-like particle-specific MAC-ELISA (VLP-MAC-ELISA). Paired-receiver operating characteristic (ROC) curve analyses revealed no statistical differences in the overall assay performances of the VLP- and NS1-MAC-ELISAs. The two methods had high sensitivities of 100% but slightly lower specificities that ranged between 80% and 100%. When the NS1-MAC-ELISA was used to confirm positive results in the VLP-MAC-ELISA, the specificity of serodiagnosis, especially for JEV infection, was increased to 90% when applied in areas where JEV cocirculates with WNV, or to 100% when applied in areas that were endemic for JEV. The results also showed that using multiple antigens could resolve the cross-reactivity in the assays. Significantly higher positive-to-negative (P/N) values were consistently obtained with the homologous antigens than those with the heterologous antigens. JEV or WNV was reliably identified as the currently infecting flavivirus by a higher ratio of JEV-to-WNV P/N values or vice versa. In summary of the above-described results, the diagnostic algorithm combining the use of multiantigen VLP- and NS1-MAC-ELISAs was developed and can be practically applied to obtain a more specific and reliable result for the serodiagnosis of JEV and WNV infections without the need for PRNT. The developed algorithm should provide great utility in diagnostic and surveillance activities in which test accuracy is of utmost importance for effective disease intervention.
INTRODUCTION
Mosquito-borne flaviviruses in the family Flaviviridae are responsible for a number of globally significant diseases and are serologically divided into several complexes, including the Japanese encephalitis virus (JEV), dengue virus (DENV), and yellow fever virus (YFV) serocomplexes (1). JEV and West Nile virus (WNV) are two of the most important members of the JEV serocomplex that have emerged into new geographic ranges in the past years (2, 3). JEV occurs in East, South, and Southeast Asia, where DENV is also commonly distributed, but it has spread from the Indonesian archipelago to Papua New Guinea and the Torres Strait islands of northern Australia, and to new areas in western India and Pakistan (4). WNV is originally endemic in parts of Africa, Europe, the Middle East, West Asia, India, and Australia; it then unexpectedly emerged in New York City in 1999 and rapidly expanded over North America to Central America and finally to South America (5, 6). It is believed that the introduction of these flaviviruses into new areas is facilitated by mosquitoes blown by strong winds, bird migration, the movement of infected people and animals, and the increase in vector distribution and transmission dynamics brought about by climate change (7, 8). These factors raise a significant public health concern that these emerging flaviviruses may continue to expand globally, thus underscoring the need for the development of rapid and simple diagnostic tools for early infection, which is crucial in the implementation of effective control and intervention programs to reduce human risk.
JEV and WNV can cause similar disease manifestations in humans, ranging from an asymptomatic infection or self-limiting febrile illness to severe meningitis or encephalitis (9). Diagnosis based on clinical manifestations is difficult and necessitates laboratory methods to differentiate the diseases caused by these two viruses. A specific diagnosis can be attained by virus isolation or viral RNA detection in serum samples, but the short duration of viremia and low virus titers during JEV and WNV infections preclude their use as screening methods (10, 11). Although the cross-reactive nature of antibodies elicited during flavivirus infections can complicate the interpretation of the results, serological testing remains the primary method for the diagnosis of JEV and WNV infections. Traditional approaches, which measure antibodies to the viral surface premembrane (prM) and envelope (E) proteins, include the gold standard plaque reduction neutralization test (PRNT), hemagglutination inhibition (HI) test, indirect immunofluorescence assay (IFA), and IgM and IgG antibody-capture enzyme-linked immunosorbent assays (MAC- and GAC-ELISAs, respectively) (12). Among these, the front-line screening assay widely recommended by the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) for the serodiagnosis of acute JEV and WNV infections is the MAC-ELISA (13, 14). An ELISA-positive sample may be confirmed with a 4-fold rise in PRNT titer against a battery of flaviviruses endemic to a given area, in a comparison of paired acute- and convalescent-phase serum specimens. However, PRNT is labor-intensive, time-consuming, and requires skilled personnel and the handling of live virus, which needs a biosafety level (BSL)-3 facility that is not available in most clinical settings.
An alternative rapid method is to detect antibodies targeting the nonstructural protein 1 (NS1), which is secreted extracellularly as a soluble form during an active flavivirus infection (15). NS1-based indirect and epitope-blocking ELISAs have successfully been used to detect anti-NS1 antibodies as surrogate serological biomarkers of natural infection in populations vaccinated with inactivated JEV or WNV vaccines (16–18). Previous reports also described the application of these NS1 ELISAs in discriminating between infections caused by closely related flaviviruses (19–22). However, these methods intrinsically do not have sufficient sensitivity to detect low levels of anti-NS1 antibodies in human serum, particularly in subclinical infections (17, 21, 23). Recently, we developed modified IgM and IgG antibody-capture ELISAs (MAC- and GAC-ELISAs, respectively) that were highly sensitive for detecting anti-NS1 antibodies during the acute and convalescent phases of DENV infection (24). In the present study, we further expanded the applicability of these novel NS1-specific ELISAs to human serum specimens collected from JEV- and WNV-infected patients and sought to demonstrate the mechanism behind the enhanced detection of low-level anti-NS1 antibodies, compare the performance and determine the agreement between the standard prM/E-specific MAC-ELISA and the newly developed NS1-specific MAC-ELISA in the diagnosis of acute JEV and WNV infections, and establish a diagnostic algorithm that employs the tandem analyses of both prM/E- and NS1-specific MAC-ELISAs for a highly specific and accurate serodiagnosis of current JEV and WNV infections without resorting to PRNT.
MATERIALS AND METHODS
Recombinant plasmids and antigen production.
Previously described eukaryotic cell expression plasmids (25, 26) were used as vectors to express prM/E-containing virus-like particles (VLPs) or soluble NS1 proteins of West Nile virus (WNV) strain NY99 and Japanese encephalitis virus (JEV) strain SA14-14-2 for prM/E and strain CH1392 for NS1. The VLP and NS1 protein antigens were transiently expressed in COS-1 cells electroporated with recombinant expression plasmids carrying the prM/E and NS1 genes, respectively, according to the protocol described previously (27, 28).
Antigen standardization and VLP- and NS1-MAC-ELISAs.
Antigens secreted from plasmid-transformed COS-1 cells were quantified by antigen-capture ELISA (Ag-ELISA), as previously described (27, 28). VLP and NS1 antigens were captured using rabbit anti-JEV or anti-WNV VLP and NS1 polyclonal sera from rabbits immunized with VLPs or soluble NS1 proteins of JEV or WNV. Captured antigens were detected with anti-JEV or anti-WNV murine hyperimmune ascitic fluid (MHIAF) from mice immunized with live JEV or WNV virions. The antigens used for all downstream experiments were standardized at a single concentration, producing an optical density at 450 nm (OD450) of 1.4.
VLP- and NS1-MAC-ELISAs were performed to analyze the presence of prM/E- and NS1-specific IgM antibodies in human serum, as previously described (24, 27). Briefly, 96-well plates were coated with goat anti-human IgM (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:2,000 in coating buffer (0.015 M NaCO3, 0.035 M NaHCO3 [pH 9.6]) and incubated overnight in a humidified chamber at 4°C. JEV- or WNV-infected patient sera and positive- and naive human serum samples diluted 1:2,000 in wash buffer (phosphate-buffered saline [PBS] with 0.05% Tween 20) were added to the wells and incubated for 2 h at 37°C. JEV or WNV VLP and NS1 antigens were diluted appropriately in wash buffer, tested in duplicate against each serum sample, and detected with the homologous MHIAFs. The mock-transfected culture supernatant was used as a negative antigen control. The positive-to-negative (P/N) ratios were determined using the method used by the Diagnostic and Reference Laboratory at the CDC to interpret the test results, as described previously (29). Positive (P) values for each specimen were determined as the average OD450 for the patient serum sample that reacted with positive viral antigen. Negative (N) values were determined for individual 96-well plates as the average OD450 for the normal human serum control that reacted with the positive viral antigen. A P/N value of <3.0 or ≥3.0 for a given specimen was classified as negative or positive, respectively. For each test specimen, the ratios of JEV-to-WNV P/N value (JEV/WNV IgM ratio) and WNV-to-JEV P/N value (WNV/JEV IgM ratio) were calculated (30). A value of zero was assigned for ratios in which the numerator of the P/N value was <3.0 (and thus negative for JEV or WNV IgM). A JEV/WNV or WNV/JEV IgM ratio of >1.0 indicated that a test specimen contained JEV- or WNV-specific IgM antibodies.
All serum specimens were preabsorbed with VLP antigens to deplete anti-prM/E antibodies before performing NS1-MAC-ELISAs, according to the previously described procedure (24). Briefly, the patient or naive serum was diluted 1:2,000 in phosphate-buffered saline (PBS) premixed with VLP antigens, and 50 μl was added immediately to wells of the Ag-ELISA plate precoated with rabbit anti-JEV or anti-WNV VLP polyclonal serum, as described above, and incubated for 1 h at 37°C. Next, all sera after absorption were transferred to the plates precoated with anti-human IgM for NS1-MAC-ELISAs, as described above.
Human sera.
Human serum specimens, with the status of infection confirmed by a 90% focus-reduction microneutralization test (FRμNT90), were obtained from the archived 1999 to 2008 collections of the Arboviral Diseases Branch, Division of Vector-Borne Infectious Diseases at the CDC. Eighty serum specimens were assembled as the target disease panel, including acute-phase patient sera from JEV (n = 16) and WNV (n = 64) infections. Sixty-seven serum specimens were assembled as a control panel, including yellow fever-17D (YF-17D) postvaccination sera (n = 10); acute-phase patient sera from Dengue virus (n = 5), Zika virus (n = 4), Chikungunya virus (n = 6), and Hantaan virus (n = 7) infections; and normal human sera (n = 35) as a naive serum control.
Statistical analysis.
Student's t test was used for comparisons between normally distributed continuous variables. Bland-Altman analysis was used to determine the agreement between VLP- and NS1-MAC-ELISAs by calculating the differences between the log-transformed values of the two methods (log P/N ratio of NS1-MAC-ELISA minus the log P/N ratio of VLP MAC-ELISA) against the averages of the two methods. It generated a plot showing horizontal lines drawn at the mean difference (bias), the 95% confidence interval (CI) of the mean difference, and the 95% limits of agreement as the mean difference ±1.96 its standard deviation (SD). A significant systematic difference was established when the 95% CI of the mean difference contained the line of equality (zero difference). The comparative receiver operating characteristic (ROC) curve analysis was used to compare the paired-assay performance, and the area under the ROC curve (AUC) was used to quantify the accuracy of discrimination. Two-by-two contingency tables were prepared to determine the sensitivities and specificities of the tests based on the positive-cutoff criterion (P/N ≥ 3.0) as the evidence of infection. All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA), and the significance level was set at a P value of <0.05.
RESULTS
Serum preabsorption with VLPs enhances detection of JEV and WNV anti-NS1 IgM antibodies by MAC-ELISA.
We previously demonstrated (24) that preabsorption of sera from DENV-infected individuals with DENV VLP antigens could improve the sensitivity for detecting anti-NS1 antibodies. To rigorously test this novel approach, we further applied this method to presumptive JEV and WNV infections. Due to a limited volume of available specimens, one from each of the JEV- and WNV-infected serum samples was first used to preliminarily compare the results of NS1-MAC-ELISAs with and without preabsorption with JEV and WNV VLPs, respectively.
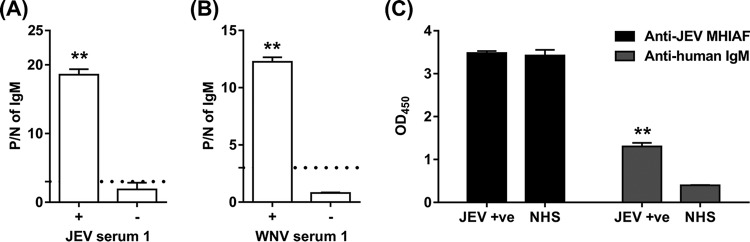
For the JEV-infected serum (Fig. 1A), preabsorption with JEV VLPs significantly increased the P/N value of IgM from 1.86 to 18.57 (P = 0.0057). For the WNV-infected serum (Fig. 1B), preabsorption with WNV VLPs also significantly increased the P/N value of IgM from 0.80 to 12.25 (P = 0.0013). Taken together, these results consistently support our recent finding that serum preabsorption with VLP antigens enhances the detection of anti-NS1 antibodies in flavivirus infections.
FIG 1.
Comparison of NS1-MAC-ELISAs with and without serum preabsorption with VLP antigens. (A) P/N values of anti-NS1 IgM on JEV-infected human serum with (+) or without (−) preabsorption with JEV VLPs before JEV NS1-MAC-ELISA. (B) P/N values of anti-NS1 IgM on WNV-infected human serum with (+) or without (−) preabsorption with WNV VLPs before WNV NS1-MAC-ELISA. (C) Detection of VLP antigens by anti-JEV mouse hyperimmune ascitic fluid (MHIAF) and prM/E antibody-VLP antigen immune complexes by anti-human IgM on the Ag-ELISA plate used in the preabsorption of the JEV-infected serum (JEV +ve) and normal human serum (NHS). The dotted lines indicate the P/N cutoff value of ≥3.0 for the positive detection of serum IgM. All data were obtained from the results from two independent experiments, and the error bars represent the standard deviations. Statistical significance is indicated with two asterisks (P < 0.01).
Serum preabsorption depletes prM/E antibodies.
We hypothesized that the enhanced sensitivity of our NS1-MAC-ELISA after serum preabsorption with VLPs was due to the depletion of the relatively abundant anti-prM/E antibodies that would outcompete the anti-NS1 antibodies from being captured on the plate coated with goat anti-human IgM antibodies. To confirm that the increase in P/N ratio was due to the depletion of anti-prM/E antibodies in the serum after the preabsorption step, the JEV VLP-Ag-ELISA plate used in the preabsorption of the JEV-infected serum and normal human serum (NHS) was further detected separately with anti-JEV MHIAF and anti-human IgM antibody. As detected by anti-JEV MHIAF, high OD readings in the JEV-infected serum and NHS wells (OD, 3.48 and 3.43, respectively) indicated that JEV VLP antigens were indeed captured on the wells (Fig. 1C). As detected by anti-human IgM antibody, significantly higher OD readings in the JEV-infected serum wells compared to those in the NHS wells (OD, 1.30 versus 0.40, P = 0.0081) further indicated that the serum prM/E antibodies against JEV remained on the preabsorption plate after forming an immune complex with the captured JEV VLP antigens; thus, the JEV-infected serum was depleted of anti-prM/E antibodies before testing with NS1-MAC-ELISA.
Diagnostic performances of VLP- and NS1-MAC-ELISAs.
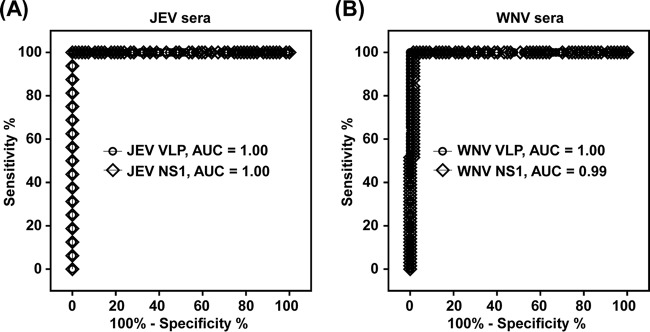
VLP- and NS1-MAC-ELISAs were applied simultaneously to a total of 147 human serum specimens, consisting of 16 JEV-infected specimens, 64 WNV-infected specimens, and 67 control specimens. The diagnostic accuracies of the two methods were then determined using various combined serum specimens as the control panel when applied in a geographic context wherein only JEV or WNV is endemic or both cocirculate. In the setting of one being endemic, either JEV or WNV sera were excluded from the control panels, whereas in the setting of them cocirculating, either JEV or WNV sera were included to take cross-reactivity into account. The results, expressed as P/N values, were analyzed on a continuous rating scale by ROC curves for assay discriminatory ability. For the JEV serum panel, paired-ROC curve analysis revealed no significant differences between the performances of the JEV VLP- and NS1-MAC-ELISAs (AUCs, 1.00; 95% CI, 1.00 to 1.00) when WNV sera were excluded from the controls (Table 1 and Fig. 2A). Comparable performances were also observed between the JEV VLP-MAC-ELISA (AUC, 0.99; 95% CI, 0.99 to 1.00) and NS1-MAC-ELISA (AUC, 0.99; 95% CI, 0.96 to 1.01) when WNV sera were included in the controls. For the WNV serum panel, paired-ROC curve analysis also revealed no statistical difference between the WNV VLP-MAC-ELISA (AUC, 1.00; 95% CI, 1.00 to 1.00) and NS1-MAC-ELISA (AUC, 0.99; 95% CI, 0.98 to 1.01) when the JEV sera were excluded from the controls (Table 1 and Fig. 2B). However, significantly greater performance (P < 0.05) was observed with the WNV VLP-MAC-ELISA (AUC, 0.99; 95% CI, 0.98 to 1.00) than that with the NS1-MAC-ELISA (AUC, 0.89; 95% CI, 0.83 to 0.94) when JEV sera were included in the controls.
TABLE 1.
Test accuracy of MAC-ELISA using VLP and/or NS1 antigensa
| Control panel by disease panel | Antigen | ELISA result | True status |
AUC (95% CI) | % sensitivity (95% CI) | % specificity (95% CI) | |
|---|---|---|---|---|---|---|---|
| Disease | No disease | ||||||
| JEV | |||||||
| Other groupsb | JEV VLP | Positive | 16 | 5 | 1.00 (1.00–1.00) | 100.00 (79.41–100.00) | 92.54 (83.44–97.53) |
| Negative | 0 | 62 | |||||
| JEV NS1 | Positive | 16 | 0 | 1.00 (1.00–1.00) | 100.00 (79.41–100.00) | 100.00 (94.64–100.00) | |
| Negative | 0 | 67 | |||||
| JEV VLP and NS1 | Positive | 16 | 0 | NAc | 100.00 (79.41–100.00) | 100.00 (94.64–100.00) | |
| Negative | 0 | 67 | |||||
| WNV and other groups | JEV VLP | Positive | 16 | 23 | 0.99 (0.99–1.00) | 100.00 (79.41–100.00) | 82.44 (74.83–88.53) |
| Negative | 0 | 108 | |||||
| JEV NS1 | Positive | 16 | 41 | 0.99 (0.96–1.01) | 100.00 (79.41–100.00) | 68.70 (60.02–76.52) | |
| Negative | 0 | 90 | |||||
| JEV VLP and NS1 | Positive | 16 | 13 | NA | 100.00 (79.41–100.00) | 90.08 (83.63–94.61) | |
| Negative | 0 | 118 | |||||
| WNV | |||||||
| Other groups | WNV VLP | Positive | 64 | 0 | 1.00 (1.00–1.00) | 100.00 (94.40–100.00) | 100.00 (94.64–100.00) |
| Negative | 0 | 67 | |||||
| WNV NS1 | Positive | 64 | 2 | 0.99 (0.98–1.01) | 100.00 (94.40–100.00) | 97.01 (89.63–99.63) | |
| Negative | 0 | 65 | |||||
| WNV VLP and NS1 | Positive | 64 | 0 | NA | 100.00 (94.40–100.00) | 100.00 (94.64–100.00) | |
| Negative | 0 | 67 | |||||
| JEV and other groups | WNV VLP | Positive | 64 | 8 | 0.99 (0.98–1.00)d | 100.00 (94.40–100.00) | 90.36 (81.89–95.75) |
| Negative | 0 | 75 | |||||
| WNV NS1 | Positive | 64 | 16 | 0.89 (0.83–0.94) | 100.00 (94.40–100.00) | 80.72 (70.59–88.56) | |
| Negative | 0 | 67 | |||||
| WNV VLP and NS1 | Positive | 64 | 8 | NA | 100.00 (94.40–100.00) | 90.36 (81.89–95.75) | |
| Negative | 0 | 75 | |||||
Accuracy was defined as the ability to distinguish the disease panel (JEV or WNV serum panels) from the control panel using the positive-cutoff criterion (P/N ≥ 3.0) as the evidence of infection.
Other groups include DENV, YFV, Zika, HTN, CHIKV, and negative serum panels.
NA, not available.
P < 0.05.
FIG 2.
Fitted ROC curves of VLP- and NS1-MAC-ELISAs on JEV-infected (A) and WNV-infected (B) human sera. Assay performances between JEV VLP- and NS1-MAC-ELISAs on the target JEV-infected serum panel (A) or between WNV VLP- and NS1-MAC-ELISAs on the target WNV-infected serum panel (B) against the control panel, including DENV, YFV, Zika, HTN, CHIKV, and negative panels, were compared.
Two-by-two contingency tables were prepared to analyze the sensitivities and specificities of the VLP- and NS1-MAC-ELISAs using the positive-cutoff criterion (P/N ≥ 3.0) (Table 1). For the JEV serum panel, the sensitivities of the JEV VLP- and NS1-MAC-ELISAs were 100%, while the specificities were 92.54% and 100%, respectively, when WNV sera were excluded from the controls. However, the specificities of the JEV VLP- and NS1-MAC-ELISAs were lower, at 82.44% and 68.70%, respectively, when WNV sera were included in the controls. For the WNV serum panel, the sensitivities of the WNV VLP- and NS1-MAC-ELISAs were also 100%, while the specificities were 100% and 97.01%, respectively, when the JEV sera were excluded from the controls. In the same manner, the specificities of WNV VLP- and NS1-MAC-ELISAs were lower, at 90.36% and 80.72%, respectively, when JEV sera were included in the controls.
Agreement between VLP- and NS1-MAC-ELISAs.
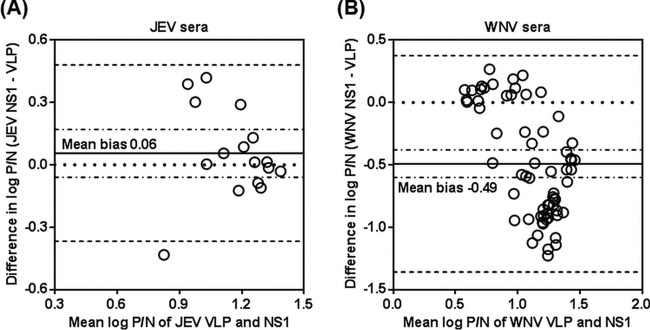
Bland-Altman plots were also constructed to analyze the agreement between VLP- and NS1-MAC-ELISAs in the diagnosis of JEV and WNV infections. In the JEV MAC-ELISAs, the mean bias on the log scale was 0.06 (95% CI, −0.06 to 0.17), with the limits of agreement ranging from −0.37 to 0.48 (Fig. 3A). The line of equality (zero difference) fell within the 95% CI of the mean bias; hence, there was no statistical evidence of bias (P = 0.3126) between the JEV VLP- and NS1-MAC-ELISAs. In the WNV MAC-ELISAs, the mean bias on the log scale was −0.49 (95% CI, −0.60 to −0.38), with the limits of agreement ranging from −1.36 to 0.38) (Fig. 3B). The negative mean bias between the WNV VLP- and NS1-MAC-ELISAs indicated that the WNV NS1-MAC-ELISA generated a lower measurement than that with the WNV VLP-MAC-ELISA, which was statistically significant (P < 0.001), as depicted by the 95% CI of the mean bias that did not contain zero. However, the systematic differences between the two methods were only about 1-fold different or smaller, and the limits of agreement were also small. Taken together, the Bland-Altman analyses showed good agreement between the VLP- and NS1-MAC-ELISAs.
FIG 3.
Bland-Altman analyses between VLP- and NS1-MAC-ELISAs on JEV- and WNV-infected human sera. (A and B) Bland-Altman plots showing the differences in the average log-transformed P/N values of VLP- and NS1-MAC-ELISAs on JEV-infected (A) and WNV-infected (B) human serum specimens. The solid horizontal lines indicate the mean bias of the systematic difference between the two methods. The dashed horizontal lines indicate the 95% limits of agreement as the mean bias ±1.96 its standard deviation (SD). The dotted lines indicate the line of equality or zero difference between the two methods. The dashed and dotted horizontal lines indicate the 95% CI of the mean bias.
Comparison between VLP- and NS1-MAC-ELISAs using different antigens.
The clinical signs and symptoms caused by encephalitic flaviviruses closely resemble each other. To facilitate a differential diagnosis of JEV and WNV infections, without prior knowledge of the type of infection, the results from the VLP- and NS1-MAC-ELISAs using homologous and heterologous antigens were compared.
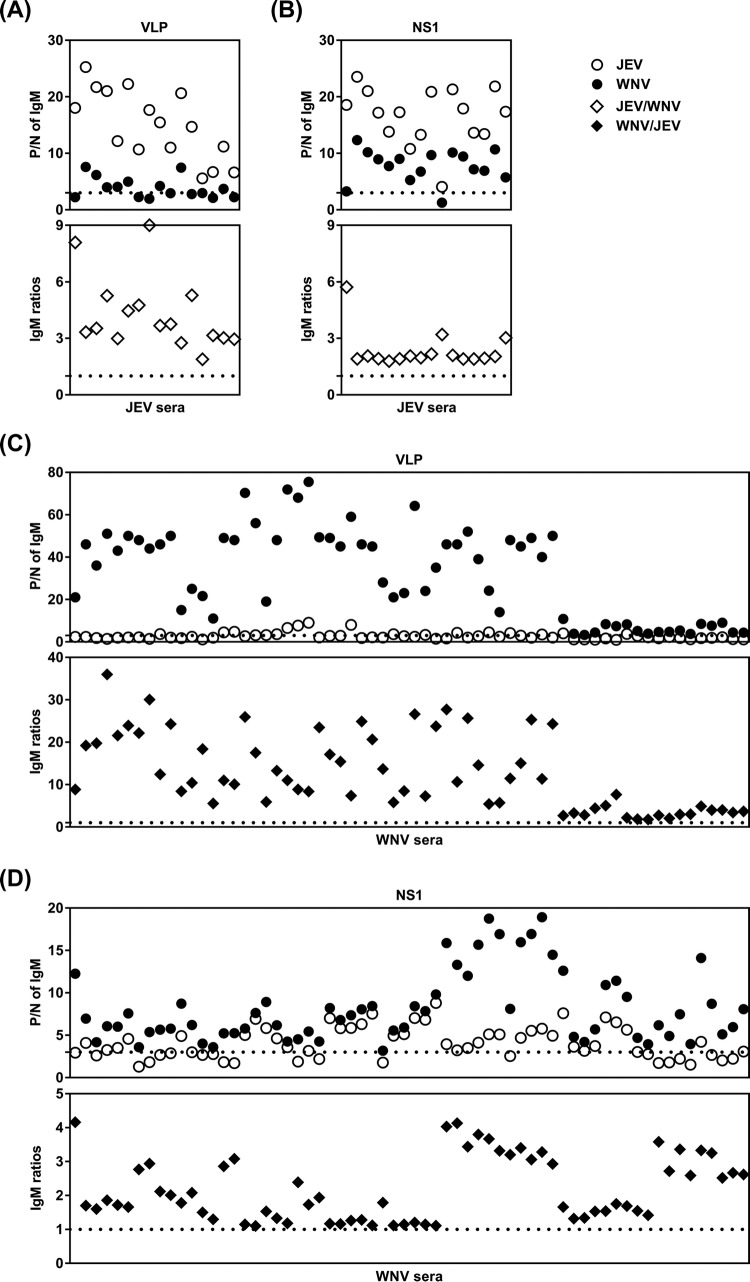
For the JEV serum panel, the VLP-MAC-ELISA results (Fig. 4A, upper panel) showed that the P/N values were higher with the use of homologous JEV VLP antigens (average P/N, 15.04; range, 5.57 to 25.25) than those with the use of heterologous WNV VLP antigens (average P/N, 3.85; range, 1.96 to 7.59). The NS1-MAC-ELISA results (Fig. 4B, upper panel) also showed that the P/N values were similarly higher with the use of homologous JEV NS1 antigens (average P/N, 16.62; range, 4.07 to 23.53) than those with the use of heterologous WNV NS1 antigens (average P/N, 7.78; range, 1.27 to 12.34). Of the 16 JEV-infected serum samples, 8 (50%) and 15 (94%) samples were cross-reactive to the heterologous WNV antigens in the VLP- and NS1-MAC-ELISAs, respectively (Fig. 4A and B, upper panels). Further calculation of the IgM ratios in the VLP-MAC-ELISA, determined by dividing the P/N values from the JEV VLP-MAC-ELISA with those from the WNV VLP-MAC-ELISA and vice versa, showed that the JEV/WNV IgM ratios of all JEV serum specimens were >1.0 (range, 1.89 to 9.01) (Fig. 4A, lower panel). Similar results were found when calculating the JEV/WNV IgM ratios in the NS1-MAC-ELISA (range, 1.79 to 5.72) (Fig. 4B, lower panel).
FIG 4.
P/N values of VLP- and NS1-MAC-ELISAs and the ratios of JEV-to-WNV P/N value (JEV/WNV IgM ratio) and WNV-to-JEV P/N value (WNV/JEV IgM ratio). (A and B) P/N values (upper panel) and JEV/WNV IgM ratios (lower panel) of VLP- (A) and NS1-MAC-ELISAs (B) using JEV and WNV antigens on JEV-infected human sera. (C and D) P/N values (upper panel) and WNV/JEV IgM ratios (lower panel) of VLP-MAC-ELISA (C) and NS1-MAC-ELISA (D) using JEV and WNV antigens on WNV-infected human sera. The open circles indicate the P/N values obtained by using JEV antigens, and the closed circles indicate the P/N values obtained by using WNV antigens. The JEV/WNV and WNV/JEV IgM ratios are indicated with open and closed diamonds, respectively. All data points were obtained from the results from two independent experiments in duplicates. The dotted lines denote the cutoff values of ≥3.0 for P/N and >1.0 for JEV/WNV and WNV/JEV IgM ratios.
For the WNV serum panel, the VLP-MAC-ELISA results (Fig. 4C, upper panel) demonstrated that the P/N values were higher with the use of homologous WNV VLP antigens (average P/N, 32.24; range, 3.33 to 75.47) than those with the use of heterologous JEV VLP antigens (average P/N, 2.75; range, 0.97 to 9.00). The NS1-MAC-ELISA results (Fig. 4D, upper panel) also demonstrated that the P/N values were similarly higher with the use of homologous WNV NS1 antigens (average P/N, 8.16; range, 3.18 to 18.92) than those with the use of heterologous JEV NS1 antigens (average P/N, 4.06; range, 1.30 to 8.80). Of the 64 WNV-infected serum specimens, 18 (28%) and 41 (64%) specimens were cross-reactive to the heterologous JEV antigens in the VLP- and NS1-MAC-ELISAs, respectively (Fig. 4C and D, upper panels). Further calculation of the IgM ratios in the VLP-MAC-ELISA, determined by dividing the P/N values from the WNV VLP-MAC-ELISA with those from the JEV VLP-MAC-ELISA and vice versa, likewise demonstrated that the WNV/JEV IgM ratios of all WNV serum specimens were >1.0 (range, 1.78 to 35.97) (Fig. 4C, lower panel). Similar results were found when calculating the WNV/JEV IgM ratios in the NS1-MAC-ELISA (range, 1.10 to 4.16) (Fig. 4D, lower panel). However, the WNV NS1-MAC-ELISA demonstrated a borderline discriminatory capacity (WNV/JEV IgM ratios between 1.10 and 1.33) in 16/64 (25%) of the WNV-infected serum specimens due to high cross-reactivity in these specimens.
Sequential MAC-ELISAs increase the specificity of serodiagnosis.
Although both VLP- and NS1-MAC-ELISAs were highly sensitive for diagnosing WNV and JEV infections, false positivity was also inevitable among the control panels due to the nonspecific binding of IgM antibodies to the antigens. To increase the assay specificity, we further analyzed the sensitivities and specificities when VLP- and NS1-MAC-ELISAs were applied simultaneously and with both having satisfied the positive-cutoff criterion (P/N ≥ 3.0). For the JEV serum panel, the sensitivity and specificity were both 100% when WNV sera were excluded from the control panel, whereas the sensitivity and specificity were 100% and 90%, respectively, when WNV sera were included in the controls (Table 1). Similarly, the sensitivity and specificity for the WNV serum panel were 100% when JEV sera were excluded from the control panel, and the sensitivity and specificity were 100% and 90%, respectively, when JEV sera were included in the controls.
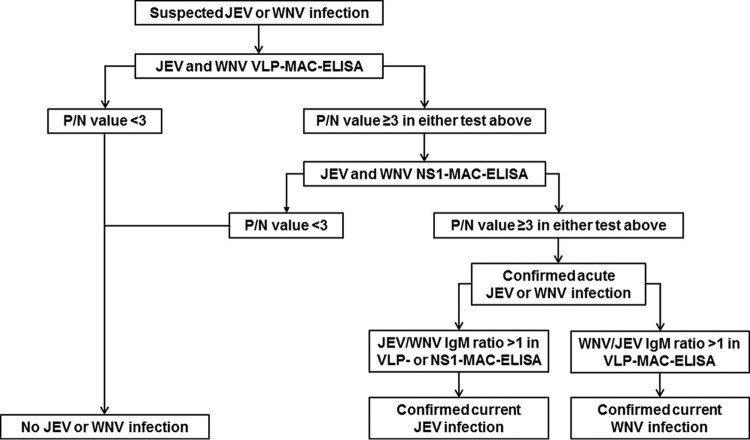
An algorithm using a sequential multiantigen MAC-ELISA was established to make an accurate and economical differential diagnosis and to confirm an infection without the need for a neutralization test (Fig. 5). Initially, a suspected JEV- or WNV-infected serum specimen was tested for the presence of anti-prM/E IgM antibodies with VLP-MAC-ELISA using JEV and WNV VLPs. If the P/N value was ≥3.0 in the JEV and/or WNV VLP-MAC-ELISAs, the sample was considered positive; if not, the sample was considered negative for JEV or WNV. To confirm unequivocally the presence of virus-specific antibodies, a positive sample was tested further for the presence of anti-NS1 IgM antibodies, which are present only during an active flavivirus infection, using the NS1-MAC-ELISA with JEV and WNV NS1 antigens. If the P/N value was ≥3.0 in the JEV and/or WNV NS1-MAC-ELISAs, the sample was confirmed to be from JEV or WNV acute infection; if not, the reactivity in the VLP-MAC-ELISA was false positive due to nonspecific binding of heterophile antibodies to the VLP antigens. Finally, by utilizing the IgM cross-reactivity, the ratios of the JEV-to-WNV P/N value and vice versa in VLP- or NS1-MAC-ELISAs were calculated to discriminate the type of flavivirus infection. The results indicated that JEV could be sufficiently determined as the currently infecting virus with a JEV/WNV IgM ratio of >1.0 in both the VLP- and NS1-MAC-ELISAs for all JEV serum specimens (Fig. 4A and B, lower panels), whereas WNV could be reliably identified with a WNV/JEV IgM ratio of >1.0 in the VLP-MAC-ELISA only (Fig. 4C, lower panel) but not in NS1-MAC-ELISA because of the lower discriminatory power of the assay, as observed in 16 (25%) of the 64 WNV-infected serum specimens (Fig. 4D, lower panel).
FIG 5.
Diagnostic algorithm for confirmation of acute JEV or WNV infection in human serum using multiantigen VLP- and NS1-MAC-ELISAs.
After confirmation and discrimination between JEV and WNV infection, upon employing the sequential multiantigen approach from the algorithm, the results of the homologous VLP- and NS1-MAC-ELISAs were combined to give a final cumulative result that might increase the specificity of serodiagnosis, particularly for JEV infection, to 90% in a setting where both JEV and WNV geographically overlap or to 100% in areas where cross-reactivity to JEV or WNV would not be an issue (Table 1).
DISCUSSION
In most peripheral health centers with resource-limited diagnostic laboratory settings, a diagnostic test ideally should be affordable for most of the population afflicted with infectious diseases, sensitive, specific, user-friendly, rapid, robust, equipment-free, and delivered to those who need it (31). Nearly all of these characteristics can be satisfied by VLP-MAC-ELISA for diagnosing flaviviral encephalitis with high sensitivity and specificity. The assay can also quickly be performed using little equipment and requires only minimal training, especially when performed using commercially available test kits that readily include all reagents and instructions that are simple and easy to follow but nevertheless are not designed for the type of analysis recommended in the present study. Although IgM is usually less cross-reactive than IgG to other flaviviruses, and hence a higher specificity of the IgM ELISA is found compared to that with the IgG ELISA, the routine PRNT procedure is also required for confirmation at the CDC (32). In the present study, we developed an NS1-MAC-ELISA with sensitivity and specificity comparable to those of the VLP-MAC-ELISA. By combining the two assays, 100% sensitivity and specificity can be reached without the need for PRNT. Therefore, a diagnostic algorithm has been developed for the confirmation and differentiation of positive JEV or WNV infection. This algorithm will be useful not only in countries that are endemic for JEV or WNV but also in the Indian subcontinent, Indonesian archipelago, and northern Australia, where the two encephalitic flaviviruses cocirculate.
The immunodominant flavivirus E structural protein, which is highly conserved among members of the same serocomplex, is the major target of the antibody response in humans and animals. Hence, serological diagnosis in flaviviral infections relies primarily on the detection of antibodies to this antigen. However, interpretation of the results can be confounded by the presence of a large proportion of cross-reactive antibodies developed from repeated exposures to flaviviruses, sequential infection with related flaviviruses, or vaccination. The gold standard used for a definitive diagnosis of flavivirus infections is the PRNT (12). However, because PRNT is laborious and requires expertise and a high-biosafety-level facility for manipulation of infectious viruses, it is not a routine test and is performed only to confirm ELISA-positive samples. Moreover, PRNT requires testing of paired acute- and convalescent-phase serum samples against a battery of flaviviruses cocirculating in a certain area to distinguish between previous and recent infections, and it takes several days to obtain a diagnosis. The protocol currently used by the CDC for serological testing of acute JEV and WNV infections involves the screening of serum or cerebrospinal fluid specimens, if the central nervous system is involved in the infectious process, by a prM/E-specific MAC-ELISA, which is followed by PRNT if enough sample remains (32).
The soluble NS1 protein is also known to be actively secreted at high levels during flavivirus infection and as immunogenic as the viral prM/E surface proteins. An indirect monoclonal antibody capture and epitope-blocking ELISAs have been developed to detect and differentiate anti-NS1 antibodies in flavivirus-infected human sera, but these approaches inherently possess lower sensitivity (17, 21), like with the ordinary indirect ELISA. In the present study, we evaluated the applicability of our novel NS1-specific MAC-ELISA as an alternative rapid method for the clinical detection of IgM antibodies to acute JEV and WNV infections in humans by using a panel of well-characterized arbovirus-infected serum specimens. Here, we show that the newly developed JEV and WNV NS1-MAC-ELISAs displayed 100% sensitivity. The novelty in our NS1-MAC-ELISA is the preabsorption of the patient serum with VLP antigens to deplete the anti-prM/E antibodies. This is based on the rationale that the binding of a low level of anti-NS1 antibodies, which are captured on the MAC-ELISA plate coated with goat anti-human IgM antibodies, to the NS1 antigens would be dramatically hampered by the presence of relatively abundant anti-prM/E IgM antibodies; hence, we see its poor detectability, particularly in asymptomatic cases (16, 33, 34). Furthermore, detection of the formed prM/E antibody-VLP antigen immune complexes in the preabsorption plate demonstrated that a single preabsorption step sufficiently depleted the serum anti-prM/E antibodies and consequently allowed for the optimal detection of anti-NS1 antibodies. The performance of the NS1-MAC-ELISA was also evaluated in parallel with that of the VLP-MAC-ELISA. The overall diagnostic accuracies of the anti-prM/E and anti-NS1 antibody detections were comparably robust, as illustrated by the large AUCs for both VLP and NS1 antigens in the JEV and WNV MAC-ELISAs (Fig. 2A and B). By Bland-Altman comparison, the negligible systematic biases of the VLP- and NS1-MAC-ELISAs depicted a high degree of agreement between the two methods (Fig. 3A and B). Thus, the two methods can be used interchangeably for the diagnosis of acute JEV and WNV infections.
In the present study, it should be noted that the antibody response against the secreted NS1 protein is relatively weaker than that against the prM/E protein after WNV infection, as evident by the lower P/N values of the WNV NS1-MAC-ELISA (average P/N, 8.16) (Fig. 4D, upper panel) than those of WNV VLP-MAC-ELISA (average P/N, 32.24) (Fig. 4C, upper panel) on the WNV serum panel, making the utility of NS1-MAC-ELISA for the diagnosis of WNV infection extremely limited. However, a similar situation was not observed with JEV VLP- and NS1-MAC-ELISAs (average P/N, 15.04 and 16.62, respectively) (Fig. 4A and B, upper panels) on the JEV serum panel. The relatively weak reactivity of the WNV-infected sera in the WNV NS1-MAC-ELISA might be attributed to an insufficient anti-NS1 antibody response due to the characteristic delayed secretion of WNV NS1 protein. Previous studies have demonstrated that WNV NS1 can be detected in the supernatant of mammalian cells beginning at 12 to 16 h postinfection and in the sera of animals at 3 days postinfection (35, 36). This is in contrast to the secretion kinetics of JEV NS1, which is efficient and takes only 2 h before it can be detected in the culture fluid of infected mammalian cells (37). Indeed, a recent study has identified two critical amino acids (residues 10 and 11) of a short peptide motif at the N terminus of WNV NS1 that direct its greater retention time in the endoplasmic reticulum (ER) and preferential plasma membrane expression, resulting in the accumulation of more NS1 on the surface of the infected cell with decreased levels of secretion (38). Also, a unique feature of flaviviruses in the JEV serocomplex is the presence of an additional form of NS1 with a carboxy-terminal extension, termed NS1′, as a product of a programmed −1 ribosomal frameshift (PRF) at the start of the NS2A gene (37, 39). In WNV, PRF occurs in 30 to 50% of translation events in vitro (39) and affects the synthesis of NS1 protein, since translatable molecules producing NS1′ do not produce NS1 due to the deficiency in NS1/NS2A cleavage (40). Although it could not be ruled out, there is the possibility that a substantial proportion of the antibodies produced against the secreted nonstructural protein after WNV infection are anti-NS1′ antibodies. If such a phenomenon is true, these anti-NS1′ antibodies also could not possibly bind to NS1 protein, which might have a conformational structure different from that of the NS1′ protein. Therefore, the utility of our developed WNV NS1-MAC-ELISA for detecting an already inherent small proportion of anti-NS1 IgM antibodies in WNV-infected serum specimens would be extremely limited and thus result in a weak-positive reactivity in the assay. Additionally, the results demonstrated that the NS1-MAC-ELISA had lower discriminatory power for WNV-infected sera, as observed in the marginal WNV/JEV IgM ratios between 1.10 and 1.33 in 16/64 (25%) of the WNV serum samples (Fig. 4D, lower panel; see also Table S1 in the supplemental material), suggesting that the cross-reactivity of WNV anti-NS1 antibodies is unexpectedly higher than that of anti-E antibodies. Since the amino acid sequence similarity between JEV and WNV in the NS1 gene (∼65%) is lower than that in the E gene (∼78%), the high cross-reactivity of the anti-NS1 antibodies among these WNV sera could not be explained by the sequence similarity between the genes, but it might be due to the immunodominance that was directed against the highly conserved JEV serocomplex cross-reactive NS1 epitopes after WNV infection. In contrast, the discriminatory power of the NS1-MAC-ELISA was as good as that of the VLP-MAC-ELISA for all JEV serum samples (JEV/WNV IgM ratios of >1.5 in both assays) (Fig. 4A and B, lower panels; see also Table S1 in the supplemental material).
In the present study, the use of the novel NS1-MAC-ELISA in tandem with VLP-MAC-ELISA strongly increased the specificity of our assay, particularly for JEV infection, to 90% when applied in areas where JEV cocirculates with WNV, or to 100% when applied in areas that are endemic for JEV (Table 1); it was incorporated into the first part of our diagnostic algorithm to confirm JEV or WNV infection (Fig. 5). A similar diagnostic algorithm has been described (30, 41); however, the present study demonstrates that sequential use of the NS1-MAC-ELISA, as per command of the initial condition we set in our algorithm, has effectively served as a confirmatory assay. Because NS1 proteins are secreted from the host's infected cells only during an active viral replication, the detection of NS1-specific IgM antibodies in acute-phase serum samples might help confirm a current infection in lieu of PRNT. Also, as an apparent beneficial result, the NS1-MAC-ELISAs might complement serodiagnosis in ruling out false positives in the VLP-MAC-ELISA that might occur in instances of nonspecific binding of heterophile antibodies, like in some of the sera we tested (1 YFV-infected serum sample, 2 Chikungunya virus [CHIKV]-infected serum samples and 2 negative-control serum samples) (see Table S1 in the supplemental material). Moreover, this sequential screening will be more cost-effective than the simultaneous application of VLP- and NS1-MAC-ELISAs. Assay specificity is of utmost significance in the diagnosis and surveillance of infectious diseases, since some encephalitides can be treated with drugs or prevented with vaccines (42). The wrong identification of a case as JEV infection may come up when diagnosis is made using a laboratory test with low specificity, especially when further confirmatory testing is not performed (32, 42, 43). As a result, patients with treatable infections (such as bacterial, fungal, and parasitic encephalitides) would be deprived of immediate and necessary treatment. Overestimation of the true disease burden in a population may also happen as an outcome of a misdiagnosis of JEV, leading to perhaps futile vaccination policies, which can impose unwarranted efforts and costs on already weak public health programs (32, 42). The algorithm developed in the present study fills this gap and might enhance diagnostic accuracy in the event of the emergence of encephalitic flaviviruses.
The results of the present study also show that our VLP- and NS1-MAC-ELISAs have acceptable specificities, at >80%, although varied cross-reactivities were observed in the JEV- and WNV-infected sera that we tested with both JEV and WNV antigens (Fig. 4A to D). In the same manner, limited cross-reactivity to the JEV and WNV antigens was observed in few YF-17D-vaccinated human serum specimens (see Table S1 in the supplemental material). These observations are consistent with previous reports that antibodies to prM/E and NS1 proteins cross-react highly to flaviviruses within the same serocomplex, and poorly to those from different serocomplexes (24, 27, 29, 44). In the United States, the recommended test protocol to distinguish WNV infections from St. Louis encephalitis virus (SLEV) infections involves the initial screening of specimens by MAC-ELISA, and sometimes in tandem with IgG ELISA, followed by PRNT to confirm positive MAC-ELISA results (45); this might have a turnaround time of about 2 weeks. This testing regimen has been simplified by using WNV/SLEV IgM ratios to differentiate the two flaviviruses during outbreak situations (30). In the present study, the simultaneous testing of multiple antigens has permitted easier analysis of MAC-ELISA results, in accordance with a clearly outlined diagnostic algorithm (Fig. 5). We propose that the NS1-MAC-ELISA can be used to increase the specificity of diagnosis, especially with its application to JEV infection, but for discriminating JEV or WNV, the VLP-MAC-ELISA would be a better choice. Based on our results, differential diagnosis has fairly been resolved upon comparison of the IgM ratios of P/N values derived from cross-reactions to both JEV and WNV antigens in the VLP-MAC-ELISAs. Thus, the higher JEV/WNV or WNV/JEV IgM ratio would reliably indicate either JEV or WNV, respectively, as being the virus responsible for the current infection.
In addition, the algorithm established in this study would have good geographical application. For areas that are endemic for JEV, the specificity of the JEV VLP-MAC-ELISA would already be good, at 92%; however, combining it with the JEV NS1-MAC-ELISA might further increase the specificity for diagnosis to 100%. For areas that are endemic for WNV, the WNV VLP-MAC-ELISA alone already achieved 100% sensitivity and specificity; adding the WNV NS1-MAC-ELISA would not confer any advantages. In a setting where there is a geographic overlap between JEV and WNV, the algorithm might also provide the capacity to increase the accuracy of serodiagnosis and surveillance as well, particularly in areas where JEV and WNV are newly emerging. For these areas, the JEV VLP-MAC-ELISA would have a lower specificity, at 82%, but it could still be sufficiently increased to 90% with the combined use of the NS1-MAC-ELISA. Similarly, the specificity of the WNV VLP-MAC-ELISA would be lower, at 90%, and adding the WNV NS1-MAC-ELISA would offer no beneficial use. Although there are only very small differences in surveillance, vector control, and patient treatment concerning JEV and WNV, the discrimination of these two flaviviruses is important for defining its clinical and epidemiological characteristics in areas where the two cocirculate, and for tracing its spread (30).
The present study has several main limitations. First, archived clinical specimens with limited information on the patients' clinical and demographic information were used. Second, the algorithm developed in this study required the uniform standardization of all reagents used and was established using a panel of well-characterized serum specimens containing JEV- or WNV-specific IgM antibodies, as confirmed by PRNT. Third, indeterminate or unconfirmed samples were not included in the analysis, which might lower the positive predictive value (PPV) of the algorithm, as was previously suggested (30). The proportion of indeterminate or unconfirmed samples is affected by factors that change the pretest likelihood of flavivirus infection, like time of year or the manifestation of clinically similar symptoms and previous flavivirus exposure. Fourth, an appropriate cutoff of the JEV/WNV or WNV/JEV IgM ratio would need to be determined in the future. Normally, the PPV of the JEV/WNV or WNV/JEV IgM ratio is calculated by the underlying proportion of infections due to JEV against WNV, or vice versa, in the population and the chosen cutoff. To determine the optimal cutoff in the algorithm, an adequate number of samples must be tested by PRNT to confirm that infections are due to JEV or WNV. Thus, a comprehensive validation experiment is recommended.
Overall, we evidently demonstrated here that the practical use of our novel NS1-specific MAC-ELISA as a complement to the prM/E-specific MAC-ELISA might provide a more specific and reliable result in the serodiagnosis of current JEV and WNV infections in humans. Furthermore, the algorithm we developed might enhance test accuracy in diagnostic and surveillance activities, wherein assay specificity is of utmost significance, as some causative agents of encephalitides can be treated with drugs and prevented with vaccines.
Supplementary Material
ACKNOWLEDGMENTS
Travel grants to support D.-Y.C. and J.U.G. as guest researchers at the DVBD, CDC, were supported by grants from Ministry of Science and Technology, Taiwan (grants 98-2320-B-005-003-MY3 and 101-2321-B-005-018-MY2).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02469-15.
REFERENCES
- 1.Lindenbach BD, Murray CL, Thiel HL, Rice CM. 2013. Flaviviridae: the viruses and their replication, p 712–746. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Tiwari S, Singh RK, Tiwari R, Dhole TN. 2012. Japanese encephalitis: a review of the Indian perspective. Braz J Infect Dis 16:564–573. doi: 10.1016/j.bjid.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Lwande OW, Mosomtai G, Symekher S. 2015. West Nile virus, a reemerging virus. Precis Med 2:e604. doi: 10.14800/pm.604. [DOI] [Google Scholar]
- 4.Mackenzie JS. 2005. Emerging zoonotic encephalitis viruses: lessons from Southeast Asia and Oceania. J Neurovirol 11:434–440. doi: 10.1080/13550280591002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 6.Gubler DJ. 2007. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis 45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10:S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 8.Dash AP, Bhatia R, Sunyoto T, Mourya DT. 2013. Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis 50:77–84. [PubMed] [Google Scholar]
- 9.Solomon T, Vaughn DW. 2002. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol 267:171–194. [DOI] [PubMed] [Google Scholar]
- 10.Busch MP, Tobler LH, Saldanha J, Caglioti S, Shyamala V, Linnen JM, Gallarda J, Phelps B, Smith RI, Drebot M, Kleinman SH. 2005. Analytical and clinical sensitivity of West Nile virus RNA screening and supplemental assays available in 2003. Transfusion 45:492–499. doi: 10.1111/j.0041-1132.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- 11.Halpin KWJ, Smith IL. 2011. Japanese encephalitis virus, p 201–208. In Liu D. (ed), Molecular detection of human viral pathogens. CRC Press, Boca Raton, FL. [Google Scholar]
- 12.Kuno G. 2003. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv Virus Res 61:3–65. doi: 10.1016/S0065-3527(03)61001-8. [DOI] [PubMed] [Google Scholar]
- 13.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. 2007. Manual for the laboratory diagnosis of Japanese encephalitis virus infection. World Health Organization, Geneva, Switzerland: http://www.wpro.who.int/immunization/documents/Manual_lab_diagnosis_JE.pdf. [Google Scholar]
- 15.Lindenbach BD, Rice CM. 2003. Molecular biology of flaviviruses. Adv Virus Res 59:23–61. doi: 10.1016/S0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 16.Konishi E, Suzuki T. 2002. Ratios of subclinical to clinical Japanese encephalitis (JE) virus infections in vaccinated populations: evaluation of an inactivated JE vaccine by comparing the ratios with those in unvaccinated populations. Vaccine 21:98–107. doi: 10.1016/S0264-410X(02)00433-4. [DOI] [PubMed] [Google Scholar]
- 17.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. 2001. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine 19:1753–1763. doi: 10.1016/S0264-410X(00)00391-1. [DOI] [PubMed] [Google Scholar]
- 18.Yeh JY, Chung KM, Song J. 2012. Differentiation of West Nile virus-infected animals from vaccinated animals by competitive ELISA using monoclonal antibodies against non-structural protein 1. Vector Borne Zoonotic Dis 12:380–387. doi: 10.1089/vbz.2011.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitai Y, Shoda M, Kondo T, Konishi E. 2007. Epitope-blocking enzyme-linked immunosorbent assay to differentiate West Nile virus from Japanese encephalitis virus infections in equine sera. Clin Vaccine Immunol 14:1024–1031. doi: 10.1128/CVI.00051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh JY, Lee JH, Park JY, Seo HJ, Moon JS, Cho IS, Kim HP, Yang YJ, Ahn KM, Kyung SG, Choi IS, Lee JB. 2012. A diagnostic algorithm to serologically differentiate West Nile virus from Japanese encephalitis virus infections and its validation in field surveillance of poultry and horses. Vector Borne Zoonotic Dis 12:372–379. doi: 10.1089/vbz.2011.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi E, Konishi M. 2011. Nonstructural protein 1 antibody-based epitope-blocking enzyme-linked immunosorbent assay to differentiate Japanese encephalitis virus from dengue virus infections in humans. Jpn J Infect Dis 64:284–291. [PubMed] [Google Scholar]
- 22.Shu PY, Chen LK, Chang SF, Su CL, Chien LJ, Chin C, Lin TH, Huang JH. 2004. Dengue virus serotyping based on envelope and membrane and nonstructural protein NS1 serotype-specific capture immunoglobulin M enzyme-linked immunosorbent assays. J Clin Microbiol 42:2489–2494. doi: 10.1128/JCM.42.6.2489-2494.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi E, Kitai Y. 2009. Detection by ELISA of antibodies to Japanese encephalitis virus nonstructural 1 protein induced in subclinically infected humans. Vaccine 27:7053–7058. doi: 10.1016/j.vaccine.2009.09.064. [DOI] [PubMed] [Google Scholar]
- 24.Chao DY, Galula JU, Shen WF, Davis BS, Chang GJ. 2015. Nonstructural protein 1-specific immunoglobulin m and g antibody capture enzyme-linked immunosorbent assays in diagnosis of flaviviral infections in humans. J Clin Microbiol 53:557–566. doi: 10.1128/JCM.02735-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang GJ, Davis BS, Stringfield C, Lutz C. 2007. Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine 25:2325–2330. doi: 10.1016/j.vaccine.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 26.Chang GJ, Hunt AR, Holmes DA, Springfield T, Chiueh TS, Roehrig JT, Gubler DJ. 2003. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology 306:170–180. doi: 10.1016/S0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 27.Holmes DA, Purdy DE, Chao DY, Noga AJ, Chang GJ. 2005. Comparative analysis of immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay using virus-like particles or virus-infected mouse brain antigens to detect IgM antibody in sera from patients with evident flaviviral infections. J Clin Microbiol 43:3227–3236. doi: 10.1128/JCM.43.7.3227-3236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin DA, Biggerstaff BJ, Allen B, Johnson AJ, Lanciotti RS, Roehrig JT. 2002. Use of immunoglobulin m cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin Diagn Lab Immunol 9:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin DA, Noga A, Kosoy O, Johnson AJ, Petersen LR, Lanciotti RS. 2004. Evaluation of a diagnostic algorithm using immunoglobulin M enzyme-linked immunosorbent assay to differentiate human West Nile Virus and St. Louis encephalitis virus infections during the 2002 West Nile Virus epidemic in the United States. Clin Diagn Lab Immunol 11:1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabey D, Peeling RW, Ustianowski A, Perkins MD. 2004. Diagnostics for the developing world. Nat Rev Microbiol 2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JS, Featherstone D, Vasanthapuram R, Biggerstaff BJ, Desai A, Ramamurty N, Chowdhury AH, Sandhu HS, Cavallaro KF, Johnson BW. 2010. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg 83:1146–1155. doi: 10.4269/ajtmh.2010.10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi E, Shoda M, Ajiro N, Kondo T. 2004. Development and evaluation of an enzyme-linked immunosorbent assay for quantifying antibodies to Japanese encephalitis virus nonstructural 1 protein to detect subclinical infections in vaccinated horses. J Clin Microbiol 42:5087–5093. doi: 10.1128/JCM.42.11.5087-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konishi E, Kitai Y, Kondo T. 2008. Utilization of complement-dependent cytotoxicity to measure low levels of antibodies: application to nonstructural protein 1 in a model of Japanese encephalitis virus. Clin Vaccine Immunol 15:88–94. doi: 10.1128/CVI.00347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung KM, Diamond MS. 2008. Defining the levels of secreted non-structural protein NS1 after West Nile virus infection in cell culture and mice. J Med Virol 80:547–556. doi: 10.1002/jmv.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macdonald J, Tonry J, Hall RA, Williams B, Palacios G, Ashok MS, Jabado O, Clark D, Tesh RB, Briese T, Lipkin WI. 2005. NS1 protein secretion during the acute phase of West Nile virus infection. J Virol 79:13924–13933. doi: 10.1128/JVI.79.22.13924-13933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason PW. 1989. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology 169:354–364. doi: 10.1016/0042-6822(89)90161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn S, Cho H, Fremont DH, Diamond MS. 2010. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J Virol 84:9516–9532. doi: 10.1128/JVI.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melian EB, Hinzman E, Nagasaki T, Firth AE, Wills NM, Nouwens AS, Blitvich BJ, Leung J, Funk A, Atkins JF, Hall R, Khromykh AA. 2010. NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J Virol 84:1641–1647. doi: 10.1128/JVI.01979-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young LB, Melian EB, Khromykh AA. 2013. NS1′ colocalizes with NS1 and can substitute for NS1 in West Nile virus replication. J Virol 87:9384–9390. doi: 10.1128/JVI.01101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberson JA, Crill WD, Chang GJ. 2007. Differentiation of West Nile and St. Louis encephalitis virus infections by use of noninfectious virus-like particles with reduced cross-reactivity. J Clin Microbiol 45:3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giri A, Arjyal A, Koirala S, Karkey A, Dongol S, Thapa SD, Shilpakar O, Shrestha R, van Tan L, Thi Thuy Chinh BN, Krishna KCR, Pathak KR, Shakya M, Farrar J, Van Doorn HR, Basnyat B. 2013. Aetiologies of central nervous system infections in adults in Kathmandu, Nepal: a prospective hospital-based study. Sci Rep 3:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, White N. 2008. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ 86:178–186. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiou SS, Crill WD, Chen LK, Chang GJ. 2008. Enzyme-linked immunosorbent assays using novel Japanese encephalitis virus antigen improve the accuracy of clinical diagnosis of flavivirus infections. Clin Vaccine Immunol 15:825–835. doi: 10.1128/CVI.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gubler DJ, Campbell GL, Nasci R, Komar N, Petersen L, Roehrig JT. 2000. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol 13:469–475. doi: 10.1089/vim.2000.13.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.