Abstract
Introduction
Maroteaux–Lamy syndrome, or mucopolysaccharidosis (MPS) type VI, is an autosomal recessive lysosomal storage disease caused by a deficient activity of the enzyme arylsulfatase B (ARSB), required to degrade dermatan sulfate. The onset and progression of the disease vary, producing a spectrum of clinical presentation. So far, 133 mutations have been reported. The aim of this study is to determine the mutations in the ARSB gene that are responsible for this disease in Colombian patients.
Results
Fourteen patients with clinical manifestations and biochemical diagnosis of MPS VI were studied, including two siblings. The 8 exons of the gene were directly sequenced from patients' DNA, and 14 mutations were found. 57% of these mutations had not been previously reported (p.H111P, p.C121R, p.G446S, p.*534W, p.S334I, p.H147P, c.900T > G, and c.1531_1553del) and 43% had been previously reported (p.G144R, p.W322*, p.G302R, p.C447F, p.L128del, and c.1143-1G > C). Of the previously reported mutations, 80% have been associated with severe phenotypes and 20% with intermediate-severe phenotypes. Bioinformatic predictions indicate that the new mutations reported in this paper are also highly deleterious.
Conclusions
Most of the Colombian patients in this study had private mutations.
Keywords: Mucopolysaccharidosis type VI, ARSB deficiency, Lysosomal storage disease, Maroteaux–Lamy syndrome, Mutation
1. Introduction
Mucopolysaccharidoses are a group of congenital metabolic disorders caused by the deficiency of a specific lysosomal enzyme that affects normal catabolism of glycosaminoglycans (GAGs). Accumulation of GAGs in different organs and tissues leads to the complex signs and symptoms of these multisystemic diseases (Giugliani et al., 2010, Neufeld and Muenzer, 2001). Mucopolysaccharidosis type VI (MPS VI; MIM no. 253200) or Maroteaux–Lamy syndrome is a rare genetic disease with recessive autosomal inheritance caused by deficiency of the N-acetylgalactosamine-4-sulfatase enzyme, also known as arylsulfatase B (ARSB). This enzyme is required to degrade dermatan sulfate and chondroitin sulfate [review in 3]. The disease is characterized by progressive, systemic clinical manifestations that cause significant functional impairment. The rapidly progressive form is typically characterized by a slowing of the growth rate, skeletal and joint deformities, coarse facies, and obstruction and recurrent infections of the upper airway. Later on, patients require wheelchair support or are bed-ridden due to bone deformities, cardiopulmonary disease, blindness, or compression of the spinal canal. Patients with the rapidly progressive disease die in adolescence or by age 20, while individuals with slowly progressive forms have a life expectancy of approximately 40–50 years. Although cognitive impairment is not usually described, physical and functional limitations affect psychomotor development and learning (Giugliani et al., 2007). A global incidence of MPS VI between 1 in 248,000 and 1 in 300,000 live births is estimated (El Dib and Pastores, 2009), but population data indicate that incidence may be higher in Brazil. In a high-risk screening of a Brazilian population with MPS diagnoses, 19% were identified as MPS VI (Giugliani et al., 2007). In the city of Monte Santo, State of Bahia, northeastern Brazil, actual prevalence is estimated to be much higher than the prevalence reported in the literature; a founder effect of the p.H178L mutation has been described in a cluster of patients in this area (Costa-Motta et al., 2011). In 2012, 27 MPS VI cases were identified in Colombia, of which 10 occurred in native groups (Rosselli et al., 2012). Additionally, a series of 20 ceramic artifacts from the Tumaco-La Tolita culture, more than 200 years old, were found. It is possible that these artifacts represent individuals with Maroteaux–Lamy syndrome. The artifacts depict phenotypic characteristics, such as skeletal dysplasia, macrocephaly, coarse features, wide mouths, prominent chests, kyphosis, and scoliosis. These ceramics are likely evidence of this disease occurring in pre-Columbian populations in Colombia (Pachajoa and Rodriguez, 2014). Patients with MPS VI have widely variable multisystemic symptoms, with typically chronic and progressive courses, that mainly affect the cardiorespiratory and skeletal systems, corneas, skin, liver, spleen, meninges, and brain (Azevedo et al., 2004). Although the systemic involvement is very similar to the clinical profile of MPS I, intelligence quotient is not affected by MPS VI because there is no accumulation of heparan sulfate, which is predominantly responsible for neurological damage (Neufeld and Muenzer, 2001).
The ARSB gene, located on chromosome 5 (5q13-q14), is made up of 8 exons and synthesizes a 2228-bp mRNA that encodes a precursor protein of 533 amino acids (Valayannopoulos et al., 2010, Litjens et al., 1989). As of October 2015, 165 mutations were reported by The Human Gene Mutation Database (HGMD Professional 2015.3) (www.hgmd.cf.ac.uk.) including missense mutations (102), nonsense mutations (9), splice site mutations (18), small deletions (3), small insertions (1), insertion–deletion (indel) mutations, and large deletions (2). In South America, various studies have been conducted on patients with MPS VI, including the first molecular study of South American patients (12 Brazilian and 1 Chilean) by Petry et al. in 2005 that identified 7 new mutations (Petry et al., 2005). Other studies involving South American patients include Karageorgos et al. study in 2007 (Karageorgos et al., 2007) and Garrido et al. study in 2007 (4 Argentinian patients) (Garrido et al., 2007). As mentioned earlier, some studies have been conducted on South American population, but to date, no molecular study has been conducted on Colombian patients.
A correlation between excreted urinary GAGs and phenotype was found by Swiedler et al. in 2005 (Giugliani et al., 2010, Swiedler et al., 2005), but no direct correlation has been established between genotype and phenotype thus far (Giugliani et al., 2010, Litjens et al., 1996). Identification of the genotype may be important for predicting phenotype and, in some MPS cases, making treatment decisions; it is also useful for providing family genetic counseling on reproductive risks, prenatal diagnosis, and prevention of genetic diseases (Giugliani et al., 2010). The aim of this study is to identify the molecular alterations responsible for Maroteaux–Lamy syndrome in Colombian patients.
2. Material and methods
2.1. Patients
Fourteen patients with MPS VI, or Maroteaux–Lamy syndrome, biochemically confirmed by enzyme activity analysis, entered the study after signing an informed consent. All patients came from different areas of Colombia; 50% (7 patients) from Bogotá and 50% (7 patients) from other regions of the country (Cartagena, Ipiales, Funza, and Medellín). In regards to gender distribution, 50% were women and 50% were men. Of the 14 patients, 2 were siblings (patients 4 and 5) and the rest of the patients were not related. This study complied with the provisions established by resolution No. 008430 of 1993 of Colombia Ministry of Health, and it was conducted under the Ethical Principles for Medical Research Involving Humans established by the Declaration of Helsinki. Finally, it was approved by the Ethics Committee of Universidad El Bosque and Pontificia Universidad Javeriana.
2.2. Genomic DNA extraction, polymerase chain reaction (PCR), and sequencing
Genomic DNA was obtained from peripheral blood in EDTA tubes using the salting-out method of extraction (Miller et al., 1988). Each of the 8 exons, including their adjacent intronic regions (approximately 30 bp 5′ and 3′ of each exon), was amplified by PCR. Primers were based on those reported by Garrido et al. (Garrido et al., 2007) and were verified by the Primer 3 program (http://primer3.sourceforge.net). The primer sequences and annealing temperatures are shown in Table 1. The PCR consisted of 40 ng of template DNA, 1.25 U of Taq DNA polymerase (Bioline Ltd., London, UK), 1 × buffer, 1 mM MgCl2, 0.08 mM dNTPs, 0.2 mM primers, and 4% DMSO in a final volume of 25 μl. The reactions underwent initial denaturation for 5 min at 95 °C and 35 cycles of denaturation at 95 °C for 30 seconds, annealing at 59–62 °C (depending of the primer) for 30 seconds, elongation at 72 °C for 30 seconds, and a final extension step at 72 °C for 5 min. The PCR products were purified using the EXOSAP-IT® enzymatic column method, and sequencing was performed using an ABI PRISM 3730XL Analyzer® (96 capillary type).
Table 1.
Sequence of the primers, size of the product, and the annealing temperature in PCR protocols.
| Exon | Primers | Expected product (bp) | Annealing temperature |
|---|---|---|---|
| Exon 1 | TTCCTCATTCTATCAGCGGTACAAG | 522 | 59.8 °C |
| GAGAAGCCGCCGGGACCCATAACT | |||
| Exon 2 | GAAGGCCATTTTATCTGCTTGT | 337 | 59 °C |
| TGATTGCACTTGGGTGTGTT | |||
| Exon 3 | TAGCCTCGTCACGGGTAATC | 382 | 59.3 °C |
| CAACAATGGCCTTTTCCTACA | |||
| Exon 4 | GCATAAATCTGAACTGTCTTATCCT | 378 | 63 °C |
| GCTAACCGCTCCAATTTGTC | |||
| Exon 5 | GGGAATTTAGGGTGGGAAAA | 444 | 59 °C |
| TCAGGCTGCTCTTGGAGTTTT | |||
| Exon 6 | CTGGCAGGTTTGTTATTTCC | 236 | 61.5 °C |
| AATCAAACCATCTTGGTGGG | |||
| Exon 7 | CACATTTGCACTCCAGTGTTG | 333 | 61.9 °C |
| CAGGAGGGCAGATAGACTGG | |||
| Exon 8 | ATGTTTCCACACCCACAACC | 430 | 62 °C |
| AAAAGGCCTGAGGTCCAACT |
2.3. Bioinformatic tools
The program Sequencher 5.2.4 (Gene Codes Corporation) was used for sequence analysis. Upon discovery of any discrepancy between the reference sequence (NG_007089.1) and a patient's sequence, a search was conducted at the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/gene/411) to determine whether the variant had been previously reported. If it proved to be a single nucleotide polymorphism (SNP), the allelic frequency of this change was searched in the 1000 Genomes database (http://www.1000genomes.org/). Previous reporting of the mutation was corroborated in the database of specific MPS6 mutations (http://mps6-database.org/) and the human gene mutations database (http://www.hgmd.cf.ac.uk/ac/index.php). If the variant had not been reported, bioinformatic predictions of the defects in the protein variant were performed using the following bioinformatic tools: PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) (Adzhubei et al., 2010), MutationTaster (http://www.mutationtaster.org/) (Schwarz et al., 2014), MuStab (http://bioinfo.ggc.org/mustab/) (Teng et al., 2010), SNPs&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go/) (Calabrese et al., 2009), and Provean (http://provean.jcvi.org/index.php) (Choi et al., 2012). Novel mutated amino acids were located on a 3D ARSB protein graphic using modeling of the enzyme arylsulfatase B (1FSU PDB) described by Bond et al. (1997) for Pymol v1.7.4 free accesses software (http://www.pymol.org/) (The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC). Adaptive Poisson–Boltzmann Solver (APBS) and PDB2PQR packages were used to calculate the electrostatic potentials of all the protein atoms (Baker et al., 2001, Dolinsky et al., 2004). The APBS default parameters were set. The thermodynamic stability changes of mutations were computed using the force-field FoldX (http://foldx.crg.es/) (Schymkowitz et al., 2005). The MutationTaster was used to compare the Arylsulfatase B amino acids of different species with mutated proteins, and the conservation scores were calculated by ConSurf (http://consurf.tau.ac.il/) (Ashkenazy et al., 2010).
3. Results
In the sequencing analysis of the coding region and all the exon–intron boundaries of the ARSB gene in Colombian patients, 8 SNPs were detected, and 62.5% were located in intronic regions (5 out of 8) (Fig. 1). A total of 14 mutations were found (Table 2), including 9 missense, 2 nonsense, 2 deletions, and 1 intronic, of which 5 (36%) were found on exon 2, 4 (29%) on exon 5, 3 (21%) on exon 8, 1 (7%) on exon 7, and 1 (7%) on intron 5 (Fig. 1). Six of the 14 mutations had been previously described, including c.430G > A (p.G144R), c.1143-1G > C, c.966G > A (p.W322*), c.904G > A (p.G302R), c.384_386delCCT (p.L128del), and c.1340G > T (p.C447F); 80% of the previously reported mutations are known to result in a severe phenotype and 20% in an intermediate-severe phenotype (p.W322*). On the other hand, the remaining 8 mutations (57%) had not been previously reported, including c.332A > C (p.H111P), c.361T > C (p.C121R), c.900T > G (p.D300E), c.1336G > A (p.G446S), c.1601A > G (p.*534W), c.1531_1553del (p.Y513*), c.1001G > T (p.S334I), and c.440A > C (p.H147P). All these mutations were analyzed by bioinformatic tools; the results are shown in Table 3.
Fig. 1.
Locations of mutations in the ARSB gene identified in the Colombian MPS VI patients. The exons are represented by boxes. The novel mutations are in red, previously reported mutations are in black, and the SNPs are in blue.
Table 2.
Mutations in ARSB gene in Colombian patients with clinical and biochemical diagnosis of MPS VI; each allele has the corresponding location (exonic or intronic).*
| Patient | Mutation – Allele 1 | Location | Mutation – Allele 2 | Location | Origin |
|---|---|---|---|---|---|
| 1 | c.430G > A (p.G144R) |
Exon 2 | c.361 T > C (p.C121R) |
Exon 2 | Bogotá |
| 2 | c.332 A > C (p.H111P) | Exon 2 | c.1143-1G > C (IVS5-1g > c) |
Intron 5 | Bogotá |
| 3 | c.332A > C (p.H111P) |
Exon 2 | c.332A > C (p.H111P) |
Exon 2 | Bogotá |
| 4⁎ | c.332 A > C (p.H111P) | Exon 2 | c.1143-1G > C (IVS5-1g > c) |
Intron 5 | Funza |
| 5⁎ | c.332 A > C (p.H111P) | Exon 2 | c.1143-1G > C (IVS5-1g > c) |
Intron 5 | Funza |
| 6 | c.384_386delCCT (p.L129del) |
Exon 2 | c.966G > A (p.W322*) | Exon 5 | Bogotá |
| 7 | c.904G > A (p.G302R) |
Exon 5 | c.904G > A (p.G302R) |
Exon 5 | Bogotá |
| 8 | c.900T > G (p.D300E) | Exon 5 | c.900T > G (p.D300E) | Exon 5 | Bogotá |
| 9 | c.1336G > A (p.G446S) | Exon 7 | c.1336G > A (p.G446S) | Exon 7 | Medellín |
| 10 | c.440A > C (p.H147P) | Exon 2 | c.1601A > G (p.*534W) | Exon 8 | Cartagena |
| 11 | c.440A > C (p.H147P) | Exon 2 | c.1531_1553del (p.Y513*) |
Exon 8 | Cartagena |
| 12 | c.1001G > T (p.S334I) |
Exon 5 | c.1340G > T (p.C447F) | Exon 8 | Ipiales |
| 13 | c.1001G > T (p.S334I) |
Exon 5 | c.1001G > T (p.S334I) |
Exon 5 | Ipiales |
| 14 | c.904G > A (p.G302R) | Exon 5 | c.904G > A (p.G302R) | Exon 5 | Bogotá |
This two patients are siblings.
Table 3.
Predictions of the effect of novel mutations in Colombian MPSVI patients, all bioinformatics tools showed that the variants are deleterious and causing disease mutations. c.1531_1553del could not be assessed by these bioinformatics tools because the prediction programs are not designed for this type of mutations; this variant is a frameshift mutation that causes a premature stop codon. The Foldx for the wild type is − 47.39 kcal/mol.
| Mutation | Predictions using bioinformatics tools |
|||||
|---|---|---|---|---|---|---|
| PolyPhen-2 | Mutation taster | Mustab | SNP&GO | Provean | FoldX | |
| c.332A > C (p.H111P) |
Deleterious Score: 1.00 |
Disease causing Probability: 0.99 |
Decrease in protein stability PC: 94.6% |
Disease causing RI: 6 Probability: 0.78 |
Deleterious Score: − 9.82 |
− 55.83 kcal/mol |
| c.361T > C (p.C121R) |
Deleterious Score: 0.99 |
Disease causing Probability: 0.99 |
Decrease in protein stability PC: 83.6% |
Disease causing RI: 5 Probability: 0.75 |
Deleterious Score: − 8.41 |
− 39.63 kcal/mol |
| c.900T > G (p.D300E) |
Deleterious Score: 1.00 |
Disease causing Probability: 0.99 |
Increased in protein stability PC: 26.8% |
Disease causing RI: 10 Probability: 0.99 |
Deleterious Score: − 3.87 |
− 33.35 kcal/mol |
| c.1336G > A (p.G446S) |
Deleterious Score: 0.96 |
Disease causing Probability: 0.99 |
Decrease in protein stability PC: 78.4% |
Disease causing RI: 3 Probability: 0.64 |
Deleterious Score: − 4.47 |
− 49.44 kcal/mol |
| c.1601A > G (p.*534W) |
NA | Disease causing Probability: 0.99 |
NA | NA | NA |
NA |
| c.1531_1553del | NA | NA | NA | NA | NA | NA |
| c.1001G > T (p.S334I) |
Deleterious Score: 1.00 |
Disease causing Probability: 0.99 |
Decrease in protein stability PC: 78.4% |
Disease causing RI: 8 Probability: 0.92 |
Deleterious Score: − 5.46 |
− 48.8 kcal/mol |
| c.440A > C p.H147P |
Deleterious Score: 1.00 |
Disease causing Probability: 0.99 |
Decrease in protein stability PC: 81.6% |
Disease causing RI: 9 Probability: 0.94 |
Deleterious Score: − 9.95 |
− 44.18 kcal/mol |
NA: The programs could not perform the prediction of the effect due to the type of mutation.
RI: Reliability Index; PC: Prediction Confidence.
The most frequent mutations in this study were p.H111P and p.G302R, accounting each one for 15.4% of the mutated alleles (4 out of 26). They were followed by p.S334I with 11.5% (3 out of 26 alleles), and p.D300E, p.H147P, p.G446S, and c.1143-1G > C each one with 7.7% (2 alleles); other mutations were found in only one allele.
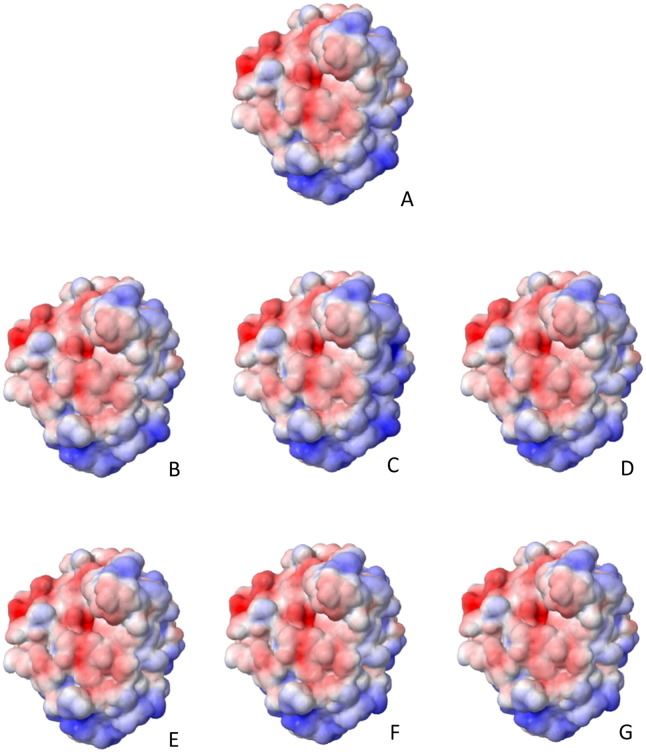
Novel missense mutations p.H111P, p.C121R, p.H147P, p.D300E, p.S334I, and p.G446S had an abnormal electrostatic surface potential as it is shown in Fig. 2. Since electrostatic interaction plays a significant role in diffusion-influenced reactions (Zhou, 1993), it was possibly affected in the mutant protein. These mutations also had thermodynamic stability changes in comparison with the wild type protein (Table 3).
Fig. 2.
Electrostatic surface potential of the mutant proteins, products of the novel missense mutations. The color scale ranges from − 5 KT/e (red) to 5 KT/e (blue). A) Wild type protein; B) p.H111P; C) p.C121R; D) p.H147P; E) p.D300E; F) p.S334I; G) p.G446S. There are changes in the electrostatic surface potential in all mutant proteins.
Most of the affected novel mutations conserved amino acid residues. Fig. 3 shows that histidine at position 111, cysteine at position 121, aspartic acid at position 300, glycine at position 446, serine at position 334, histidine at position 147, and leucine at position 129 are highly conserved in arylsulfatase B of different species. The conservation score for these amino acids was 9 (1 less conserved and 9 fully conserved) except for histidine at position 147 whose score was 7.
Fig. 3.
Conservation analysis. An amino acid comparison of Arylsulfatase B of different species whit mutant proteins, using MutationTaster. A) p.H111P; B) p.C121R; C) p.D300E; D) p.G446S; E) p.S334I; F) p.H147P; G) p.L129del. All these amino acids are highly conserved.
4. Discussion
Of the previously described mutations, the c.430G > A (p.G144R) mutation was reported (Isbrandt et al., 1994) in a patient with a severe phenotype in the United States of America. c.1143-1G > C (IVS5-1G > C), an intronic mutation (Garrido et al., 2007), alters the splicing acceptor site, causing the loss of exon 6 and a truncated protein of 391 amino acids. It was the most common mutation in a study of Argentinian and Spanish patients, representing 21.9% (7/32) of the mutant alleles and the patients with this mutation had a severe phenotype. The nonsense mutation c.966G > A (p.W322*) was also reported in the Argentinian and Spanish patients study (Garrido et al., 2007). This mutation causes a truncation of the ARSB enzyme, eliminating the 212 carboxy-terminal residues; it was reported in a heterozygous patient with a severe-intermediate phenotype. The missense mutation c.904G > A (p.G302R) was described in an Italian patient with a severe phenotype in a homozygous form (Villani et al., 1998), and the amino acid at position 302 may be particularly important for this polypeptide as it is fully conserved in all of the sulfatases of eukaryotic lineage. The c.1340G > T (p.C447F) mutation results in the substitution of cysteine for phenylalanine (Karageorgos et al., 2007); since amino acid 447 is a potential glycosylation site, this change affects its affinity for the mannose-6-phosphate receptor and enzyme uptake.
Of the 8 new mutations reported in this study (Table 3), 6 were missense mutations. For the c.332A > C (p.H111P) mutation, bioinformatic programs predicted that it was a deleterious and disease-causing mutation. Amino acid 111 is conserved in the arylsulfatase B of different vertebrate species (Fig. 3). The positive charge is missing with the loss of histidine, and this change could result in alteration of contacts. In addition, this mutation could alter the electrostatic potential of the protein, affecting its functioning and interactions. The bioinformatic programs predicted that c.361T > C (p.C121R) was a deleterious and disease-causing mutation; amino acid 121 is conserved in all sulfatases, and there is a disulfide bond at this position. In the literature, a similar variant was reported by Wei-Dong et al. in 1992. They described a mutation in a disulfide bond (p.C117R) (Jin et al., 1992) and the loss of protein stability and enzymatic activity. Bioinformatic programs also showed that c.900T > G (p.D300E) was a deleterious and disease-causing mutation because the aspartic acid at position 300 is one of the 10 residues involved in the active site. The p.D300E mutation was reported by Karageorgos but one caused by a different mutation c.900T > A, which results in a severe phenotype (Karageorgos et al., 2007). c.1336G > A (p.G446S) was found to be a deleterious and disease-causing mutation, according to the bioinformatic programs; the amino acid 446 is conserved in all of the sulfatases. Regardless, both amino acids are neutral, glycine is apolar, and serine is polar, which disrupts the three-dimensional structure of the protein; a similar change in the protein was also reported (Karageorgos et al., 2007) for the p.G446R mutation that causes a severe phenotype. The bioinformatic programs predicted that the c.440A > C (p.H147P) was a deleterious and disease-causing mutation. Histidine 147 is conserved in all of the sulfatases, and this amino acid is in the active site of the ARSB enzyme. Exchanging histidine for proline makes it lose its positive charge, which affects the active site. Finally, c.1001G > T (p.S334I) was found to be also a deleterious and disease-causing mutation according to the bioinformatic programs; serine in the 334 position is conserved in all of the sulfatases. Since serine is polar and isoleucine is apolar, the substitution of a polar amino acid for a hydrophobic amino acid could affect the residue contacts.
4.1. Stop-loss mutation
According to MutationTaster, the c.1601A > G (p.*534W) mutation causes disease and a change in the amino acid sequence, elongating the protein and therefore resulting in a protein with 584 amino acids (the normal protein has 533). The same effect on the length of protein was described by Arl et al. (Arlt et al., 1994) who reported the p.*533Q mutation that results in a protein with 584 amino acids. Although most precursors of higher size are proteolytically degraded before reaching the trans-Golgi network (Arlt et al., 1994, Lippincott-Schwartz et al., 1988), some proteins are able avoid this mechanism. Changes in the kinetic and conformational structure could increase the enzymatic activity; this last effect could partially compensate the first effect and be associated to an intermediate phenotype, but an altered three-dimensional structure of the mutant polypeptide causing a higher susceptibility to proteinase. The phenotype described for this mutation is intermediate (Arlt et al., 1994).
4.2. Deletions
According to MutationTaster, c.384_386delCCT causes disease, and a leucine is lost at position 129 (p.L129del), without altering the reading frame (non-frameshift). It is the same c.382_384delCTC mutation that has been described by Fernández-Marmiesse et al (Fernández-Marmiesse et al., 2014) which produces the same change in the electropherogram and in the protein. This loss is located near the active site, and such deletion mutations cause structural changes (Jurecka et al., 2012). The c.1531_1553del(CCCGTGTACTTCCCTGCACAGGA) is a 23 bp deletion. This deletion changes the reading frame (frameshift), causing a premature stop codon that results in a 513-amino acid protein (truncated protein). A similar protein abnormality was reported by Voskoboeva et al. in 2000; they described the p.Y513* (c.1539C > G) nonsense mutation in Russian patients, which results in a truncated protein of 513 amino acids (Voskoboeva and Krasnopol'skaia, 2000).
The most common SNPs were rs1065757 and rs25415. Two patients carried the minor allele of 5 SNPs and another 2 carried the minor allele of 4 SNPs. This finding and the high frequency of some novel mutations suggest that a haplotype analysis should be performed. Although this study is not sufficient to determine the founder effect of new mutations found in the Colombian population, it constitutes the first step in understanding the population genetics of Colombian patients with MPS VI. A genotype–phenotype correlation analysis was not performed.
5. Conclusions
Molecular analysis allowed the identification of mutations in Colombian patients. Some of the mutations in our patients were identified as private as described in previous studies. This is the first molecular study of patients with MPS VI in the Colombian population, and 9 of the mutations had not been previously reported. As described in the literature, most mutations have been found in exons 1, 2, 5, and 8 (Karageorgos et al., 2007); in this study, most mutations were found in exons 2 and 5 (65%). Unlike prior reports, no mutations were found in exon 1. Of the 8 new mutations, 3 of them were found in more than one Colombian patient; p.H111P was found in 4 patients from Bogota, p.H147P in 2 patients from Cartagena, and p.S334I in 2 patients from Ipiales (Fig. 4A). The other 5 mutations were only present in one patient each one (Fig. 4B). Of the mutations that had not been previously reported, bioinformatics predicted that they caused significant changes to the enzyme, and most of these mutations were located near the active site of ARSB (Fig. 5). Additional studies to analyze the possible founder effect of these mutations are required. Furthermore, we will continue to study patients diagnosed with MPS VI through in silico, in vitro, and ancestry studies as well as genotype–phenotype correlation studies.
Fig. 4.
Map of Colombia with the locations of the novel mutations. A) Map of Colombia indicating three novel mutations with possible founder effect, p.H147P was observed in two patients from Cartagena, p.H111P in four patients from Bogotá, and the p.S334I in two patients from Ipiales. B) Map of Colombia indicating 6 novel mutations observed in different patients from Cartagena, Medellín, Funza, Bogotá, and Ipiales.
Fig. 5.
Locations of novel mutations in the enzyme model. Modeling of the enzyme arylsulfatase B (1FSU PDB) (Bond et al., 1997) for Pymol, in which it observed the locations of mutations and proximity to the active site of the enzyme (In the white circle), these changes may affect the enzyme activity.
Declaration of interest
The authors declare that they have no competing interests. The translation of this article was sponsored by Biomarin Colombia LTDA.
Funding
This project was funded by the Research Division of Universidad El Bosque (project ID PCI 2012-278) in collaboration with the Pontificia Universidad Javeriana (Project: “Determination of the molecular profile of Colombian patients diagnosed with mucopolysaccharidosis type VI Maroteaux Lamy” ID 00005838).
Acknowledgments
We appreciate the participation of Colombian patients with MPS VI and their families because each day they show us strength in the face of adversity and are the reason why we performed these studies to better understand and manage their diseases and provide appropriate counseling. We would also like to recognize the assistance of the Nutrition, Genetics and Metabolism Research Institute of the Universidad El Bosque and the Division of Molecular Biology of the Institute of Genetics of the Pontificia Universidad Javeriana.
References
- Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt G., Brooks D.A., Isbrandt D., Hopwood J.J., Bielicki J., Bradford T.M., Bindloss-Petherbridge C.A., von Figura K., Peters C. Juvenile form of mucopolysaccharidosis VI (Maroteaux–Lamy syndrome). A C-terminal extension causes instability but increases catalytic efficiency of arylsulfatase B. J Biol Chem. 1994;269:9638–9643. [PubMed] [Google Scholar]
- Ashkenazy H., Erez E., Martz E., Pupko T., Ben-Tal N. Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;2010(38):W529–W533. doi: 10.1093/nar/gkq399. (ConSurf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo A.C., Schwartz I.V., Kalakun L., Brustolin S., Burin M.G., Beheregaray A.P., Leistner S., Giugliani C., Rosa M., Barrios P. Clinical and biochemical study of 28 patients with mucopolysaccharidosis type VI. Clin. Genet. 2004;66:208–213. doi: 10.1111/j.1399-0004.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Baker N.A., Sept D., Joseph S., Holst M.J., McCammon J.A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C.S., Clements P.R., Ashby S.J., Collyer C.A., Harrop S.J., Hopwood J.J., Guss J.M. Structure of a human lysosomal sulfatase. Structure. 1997;5:277–289. doi: 10.1016/s0969-2126(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Calabrese R., Capriotti E., Fariselli P., Martelli P.L., Casadio R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum. Mutat. 2009;30:1237–1244. doi: 10.1002/humu.21047. [DOI] [PubMed] [Google Scholar]
- Choi Y., Sims G.E., Murphy S., Miller J.R., Chan A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Motta F.M., Acosta A.X., Abé-Sandes K., Bender F., Schwartz I.V., Giugliani R., Leistner-Segal S. Genetic studies in a cluster of mucopolysaccharidosis type VI patients in Northeast Brazil. Mol. Genet. Metab. 2011;104:603–607. doi: 10.1016/j.ymgme.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Dolinsky T.J., Nielsen J.E., McCammon J.A., Baker N.A. PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–W667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Dib R.P., Pastores G.M. A systematic review of new advances in the management of mucopolysaccharidosis VI (Maroteaux–Lamy syndrome): focus on galsulfase. Biologics. 2009;3:459–468. doi: 10.2147/btt.2009.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Marmiesse A., Morey M., Pineda M., Eiris J., Couce M.L., Castro-Gago M., Fraga J.M., Lacerda L., Gouveia S., Pérez-Poyato M.S. Assessment of a targeted resequencing assay as a support tool in the diagnosis of lysosomal storage disorders. Orphanet J Rare Dis. 2014;9:59. doi: 10.1186/1750-1172-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido E., Chabás A., Coll M.J., Blanco M., Domínguez C., Grinberg D., Vilageliu L., Cormand B. Identification of the molecular defects in Spanish and Argentinian mucopolysaccharidosis VI (Maroteaux–Lamy syndrome) patients, including 9 novel mutations. Mol. Genet. Metab. 2007;92:122–130. doi: 10.1016/j.ymgme.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Giugliani R., Harmatz P., Wraith J.E. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- Giugliani R., Federhen A., Rojas M.V., Vieira T., Artigalás O., Pinto L.L., Azevedo A.C., Acosta A., Bonfim C., Lourenço C.M. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment. Genet. Mol. Biol. 2010;33:589–604. doi: 10.1590/S1415-47572010005000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrandt D., Arlt G., Brooks D.A., Hopwood J.J., von Figura K., Peters C. Mucopolysaccharidosis VI (Maroteaux–Lamy syndrome): six unique arylsulfatase B gene alleles causing variable disease phenotypes. Am. J. Hum. Genet. 1994;54:454–463. [PMC free article] [PubMed] [Google Scholar]
- Jin W.D., Jackson C.E., Desnick R.J., Schuchman E.H. Mucopolysaccharidosis type VI: identification of three mutations in the arylsulfatase B gene of patients with the severe and mild phenotypes provides molecular evidence for genetic heterogeneity. Am. J. Hum. Genet. 1992;50:795–800. [PMC free article] [PubMed] [Google Scholar]
- Jurecka A., Piotrowska E., Cimbalistiene L., Gusina N., Sobczyńska A., Czartoryska B., Czerska K., Õunap K., Węgrzyn G., Tylki-Szymańska A. Molecular analysis of mucopolysaccharidosis type VI in Poland, Belarus, Lithuania and Estonia. Mol. Genet. Metab. 2012;105:237–243. doi: 10.1016/j.ymgme.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Karageorgos L., Brooks D.A., Pollard A., Melville E.L., Hein L.K., Clements P.R., Ketteridge D., Swiedler S.J., Beck M., Giugliani R. Mutational analysis of 105 mucopolysaccharidosis type VI patients. Hum. Mutat. 2007;28:897–903. doi: 10.1002/humu.20534. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J.S., Yuan L.C., Klausner R.D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988;54:209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Litjens T., Baker E.G., Beckmann K.R., Morris C.P., Hopwood J.J., Callen D.F. Chromosomal localization of ARSB, the gene for human N-acetylgalactosamine-4-sulphatase. Hum. Genet. 1989;82:67–68. doi: 10.1007/BF00288275. [DOI] [PubMed] [Google Scholar]
- Litjens T., Brooks D.A., Peters C., Gibson G.J., Hopwood J.J. Identification, expression, and biochemical characterization of N-acetylgalactosamine-4-sulfatase mutations and relationship with clinical phenotype in MPS-VI patients. Am. J. Hum. Genet. 1996;58:1127–1134. [PMC free article] [PubMed] [Google Scholar]
- Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E., Muenzer J. In: The mucopolysaccharidoses. 8 ed. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Kinzler K.W., Vogelstein B., editors. McGraw-Hill; New York: 2001. [Google Scholar]
- Pachajoa H., Rodriguez C.A. Mucopolysaccharidosis type VI (Maroteaux–Lamy syndrome) in the pre-Columbian culture of Colombia. Colomb Med (Cali) 2014;45:85–88. [PMC free article] [PubMed] [Google Scholar]
- Petry M.F., Nonemacher K., Sebben J.C., Schwartz I.V., Azevedo A.C., Burin M.G., de Rezende A.R., Kim C.A., Giugliani R., Leistner-Segal S. Mucopolysaccharidosis type VI: identification of novel mutations on the arylsulphatase B gene in South American patients. J. Inherit. Metab. Dis. 2005;28:1027–1034. doi: 10.1007/s10545-005-0020-2. [DOI] [PubMed] [Google Scholar]
- Rosselli D., Rueda J.D., Solano M. Ethical and economic considerations of rare diseases in ethnic minorities: the case of mucopolysaccharidosis VI in Colombia. J. Med. Ethics. 2012;38:699–700. doi: 10.1136/medethics-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- Schymkowitz J., Borg J., Stricher F., Nys R., Rousseau F., Serrano L. The FoldX web server: an online force field. Nucleic Acids Res. 2005;33:W382–W388. doi: 10.1093/nar/gki387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiedler S.J., Beck M., Bajbouj M., Giugliani R., Schwartz I., Harmatz P., Wraith J.E., Roberts J., Ketteridge D., Hopwood J.J. Threshold effect of urinary glycosaminoglycans and the walk test as indicators of disease progression in a survey of subjects with Mucopolysaccharidosis VI (Maroteaux–Lamy syndrome) Am J Med Genet A. 2005;134A:144–150. doi: 10.1002/ajmg.a.30579. [DOI] [PubMed] [Google Scholar]
- Teng S., Srivastava A.K., Wang L. Sequence feature-based prediction of protein stability changes upon amino acid substitutions. BMC Genomics. 2010;11(Suppl. 2):S5. doi: 10.1186/1471-2164-11-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valayannopoulos V., Nicely H., Harmatz P., Turbeville S. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5:5. doi: 10.1186/1750-1172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G.R., Balzano N., Di Natale P. Two novel mutations of the arylsulfatase B gene in two Italian patients with severe form of mucopolysaccharidosis. Mutations in brief no. 127. Online. Hum. Mutat. 1998;11:410. doi: 10.1002/(SICI)1098-1004(1998)11:5<410::AID-HUMU9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Voskoboeva E.I., Krasnopol'skaia K.D., Peters K., von Figura K. [Identification of mutations in the arylsulfatase B gene in Russian mucopolysaccharidosis type VI patients] Genetika. 2000;36:837–843. [PubMed] [Google Scholar]
- Zhou H.X. Brownian dynamics study of the influences of electrostatic interaction and diffusion on protein-protein association kinetics. Biophys. J. 1993;64:1711–1726. doi: 10.1016/S0006-3495(93)81543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]