Abstract
The selectivity with which proprioceptive sensory neurons innervate their central and peripheral targets implies that they exhibit distinctions in muscle-type identity. The molecular correlates of proprioceptor identity and its origins remain largely unknown, however. In screens to define muscle-type proprioceptor character we find all-or-none differences in gene expression for proprioceptors that control antagonistic muscles at a single hindlimb joint. Analysis of three of these genes, cadherin13 (cdh13), semaphorin5a (sema5a) and cartilage-acidic protein-1 (crtac1), reveals expression in proprioceptor subsets that supply muscle-groups located at restricted dorso-ventral and proximo-distal domains of the limb. Genetically altering the dorso-ventral character of the limb mesenchyme elicits a change in the profile of proprioceptor cdh13, sema5a and crtac1 expression. These findings indicate that proprioceptors acquire aspects of their muscle-type identity in response to mesenchymal signals expressed in restricted proximo-distal and dorso-ventral domains of the developing limb.
Introduction
The diverse repertoire of limb movements available to terrestrial mammals is directed by motor circuits in the spinal cord. Spinal motor neurons innervate individual muscle targets and in turn, receive instructive input from sensory feedback pathways and descending commands that act directly or via local circuit interneurons to specify patterns of motor neuron and muscle activation. Of these inputs, proprioceptive sensory neurons alone are assigned the job of conveying information about the state of muscle activation to central neurons, most immediately through the formation of monosynaptic connections with selected pools of motor neurons (Baldissera et al., 1981).
Proprioceptors are highly diverse. Each of the sixty or so muscles in the mammalian hindlimb is innervated by a single dedicated pool of motor neurons, and in turn each motor pool receives specialized inputs from selected group Ia proprioceptive sensory neurons. These inputs derive from sensory neurons that supply the muscle target of a given motor pool, and to a lesser extent from proprioceptors that supply muscles with biomechanically-related functions at a limb joint. But motor neurons rarely if ever, receive input from sensory neurons supplying muscles with antagonist functions (Eccles et al., 1957; Mears and Frank, 1997). Indeed, many aspects of this sensory-motor connectivity matrix are assembled in the absence of patterned neural activity (Mendelson and Frank 1991; Mendelsohn et al., 2015), implying that sensory neurons possess diverse molecular characters.
Within sensory-motor circuits, molecular programs that specify the identity and connectivity of motor neurons have been documented (Stifani, 2014). Motor neurons acquire subtype identities before the innervation of limb target muscles, a state reflected in the expression of distinct transcription factors and downstream effectors that permit motor axons to respond to guidance cues expressed by the limb mesenchyme en route to specific muscle targets (Stifani, 2014). The limb mesenchyme also contains positional signals that determine the cleavage pattern of individual muscles (Kardon et al., 2003), in this way matching the guidance and termination of motor axons to the position of their target muscles (Tosney and Landmesser, 1984). These analyses argue for the existence of sequentially implemented programs of motor neuron specification and connectivity. An early, limb-independent program confers major distinctions in motor neuron subtype and trajectory, whereas a later specification program requires exposure to limb signals for induced gene expression (Stifani, 2014).
By contrast with the extensive information on motor neuron programming, only fragmentary information is available on strategies of proprioceptor specification (Arber, 2012; Usoskin et al., 2015). The transcription factors Brn3a, Neurogenin2 and Runx3 direct the differentiation of DRG sensory neurons toward a generic proprioceptor fate, and the neurotrophin NT-3 ensures proprioceptor survival, in part by inducing expression of the ETS transcription factor Etv1 (De Nooij et al., 2013; Lallemend and Ernfors, 2012). But how proprioceptor identities are assigned in a manner that matches their muscle targets remains unclear. A single study, performed in embryonic chick, has addressed the strategy for muscle-type proprioceptor specification at a molecular level, and describes a gene, lmo4, that is broadly expressed by proprioceptors in the absence of limb-derived signals (Chen et al, 2002). Yet, other studies in chick have implicated limb muscle-derived signals as determinants of the fine pattern of proprioceptive sensory connections with motor pools (Wenner and Frank, 1995). This latter observation, taken together with the precedent of both limb-dependent and -independent programs of motor neuron specification (Stifani, 2014), implies that certain features of proprioceptor specification are induced by limb-derived signals.
To clarify the developmental strategies of muscle-type proprioceptor specification we performed molecular screens to identify genetic distinctions in proprioceptors supplying two muscles with antagonist functions at the ankle joint - the flexor tibialis anterior (TA) and extensor gastrocnemius (GS) muscles. These two muscles are found at a common proximal-distal position within the limb, with the TA muscle positioned dorsally and the GS muscle ventrally. These molecular screens identify several genes expressed in an all-or-none manner by proprioceptors innervating TA or GS muscles. Analysis of the patterns of expression of three of these genes - the adhesion and recognition molecules cdh13, sema5a and crtac1– provide insight into the principles of muscle-type proprioceptor specification. We find that cdh13 and sema5a are expressed preferentially by proprioceptors supplying dorsal-distal hindlimb muscles, whereas crtac1 is expressed by proprioceptors supplying ventral-distal hindlimb muscles.
To pinpoint the source of signals that induce proprioceptor identity we explored how the profile of expression of these genes is influenced by genetic manipulations that differentially affect motor neuron, muscle and limb mesenchyme. The pattern of cdh13 expression is unaffected by the loss of motor neuron or muscle. But when the dorsal mesenchyme acquires a ventral positional character, proprioceptors that project into dorsally-positioned but ventrally-specified mesenchyme lack expression of cdh13. Conversely, when the ventral mesenchyme acquires a dorsal character, cdh13 and sema5a are expressed in proprioceptors that project through ventrally-positioned but dorsally-specified limb mesenchyme, while the expression of crtac1 in ventrally-projecting proprioceptors is abolished. We conclude that molecular features defining the muscle-type identity of proprioceptors are imposed by spatially-confined signals from the limb mesenchyme.
Results
Genetic distinctions in muscle-defined proprioceptors
We set out to define genes that distinguish sensory neurons supplying muscles that exert antagonist activities at a single hindlimb joint, focusing initially on the profile of proprioceptors that convey feedback from the ankle flexor tibialis anterior (TA) and extensor gastrocnemius (GS) muscles.
Proprioceptive neurons can be marked by expression of Parvalbumin (Pv), which also labels a small population of low-threshold cutaneous mechanoreceptors (de Nooij et al., 2013). Parvalbumin::Cre (Pv::Cre) mice were crossed with a Thy1::lox-STOP-lox::YFP reporter line (Hippenmeyer et al 2005; Buffeli et al 2003; Figure 1A–B), to generate Pv::YFP mice. In lumbar DRG we observed that ~96% of p1 Pvon sensory neurons expressed YFP, and conversely that ~90% of YFP+ DRG neurons expressed Pv (Figure S1A–B). The high coincidence in Pv and YFP protein expression indicates that Pv::YFP provides a reliable reporter of endogenous Pv expression.
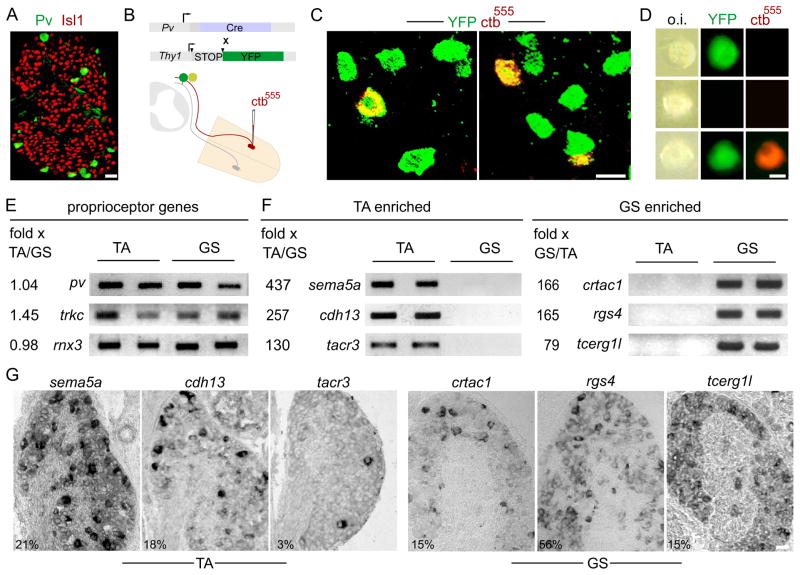
Figure 1. Molecular distinctions in TA and GS proprioceptors.
(A–D) Purification of proprioceptors.
(A) Pv immunostaining in p0 lumbar DRG (L4) distinguishes proprioceptors from other sensory neurons. Scale bar, 25μm.
(B) TA or GS proprioceptors retrogradely-labeled via muscle injection of ctb555 in Pv::Cre; Thy1::lox-STOP-lox::YFP mice (arrowheads represent loxP sites).
(C) ctb555-labeled YFPon pSNs innervating TA (left) or GS (right) muscle in p1 L5 DRG. Scale bar, 20μm.
(D) Dissociated sensory neurons. Top: generic proprioceptor. Middle: non-proprioceptive DRG sensory neuron. Bottom: proprioceptor identified by muscle innervation; o.i: oblique illumination. Scale bar, 12μm.
(E) Generic proprioceptor-expressed genes do not vary significantly between TA and GS samples.
(F) TA (left) or GS (right) proprioceptor genes upregulated >50 fold in an Affymetrix screen (one-way ANOVA; p<0.005) and validated by RNA-Seq analysis (fold change>6, p<0.00005), semi-quantitative PCR and in situ immunohistochemistry (see also Fig. S1). Fold change values shown are from the Affymetrix screen.
(G) TA- and GS-upregulated genes are expressed in subsets of DRG neurons. Numbers at bottom represent the percentage of total L4 DRG neurons at p0 expressing each gene. Scale bar, 30 μm. See also Figure S1 and Table S1.
To identify proprioceptors on the basis of their muscle targets we assessed the selectivity of muscle and sensory labeling after cholera toxin B subunit-Alexa 555 (ctb555) injections into the TA or GS muscles of p0 Pv::YFP mice. With TA muscle targeting ctb555 was confined to the injected muscle, whereas injections targeting the GS muscle also resulted in some ctb555 labeling of adjacent soleus (SOL) and plantaris (PL) muscles, likely a consequence of their close apposition. Lumbar DRG were dissociated 24h after muscle injection, individual YFP+, ctb555+ neurons were isolated, and 25 to 30 neurons were pooled to generate TA and GS (or more accurately GS/SOL/PL) muscle target-defined samples (Figure 1C–D). After extraction of total RNA, cDNA libraries were prepared for analysis of differential gene expression by Affymetrix microarray and RNA sequencing (RNA-Seq).
We first assessed the validity of proprioceptor gene expression in TA and GS samples by comparing the ratio of three generic proprioceptor markers - parvalbumin (pv), runx3, and trkc. This analysis failed to detect consistent differences in the abundance of these three transcripts in TA and GS cDNA samples by micro-array or semi-quantitative PCR (p>0.15; Figure 1E, S1C). Thus, DRG cDNA libraries preserve the representation of proprioceptor genes.
We next searched for genes expressed differentially by TA and GS proprioceptors. Analysis of array data revealed 18 genes with >50-fold TA enrichment, and 23 genes with >50-fold GS enrichment (p<0.05; Table S1). RNA-Seq analysis confirmed that 20 of these TA and GS enriched genes were expressed at 6-fold or greater levels in one or other population (p<0.05). Semi-quantitative PCR analysis confirmed the differential expression of 4 TA and 6 GS candidate genes (Figure 1F, S1C and data not shown). More definitively, high-level neuronal subset-restricted expression of 3 TA and 4 GS genes was detected by in situ hybridization histochemistry, with the incidence of expression ranging from ~3% to ~56% of all L4/L5 DRG neurons (Figure 1G). The predicted products of this set of seven genes crtac1, rgs4, pcdh17 and tcerg1l for GS group proprioceptors, and sema5a, tacr3, and cadherin13 (cdh13) for TA proprioceptors encode cell and matrix adhesion and signaling proteins. Thus the identity of TA and GS proprioceptors is marked by all-or-none differences in gene expression.
Muscle-type proprioceptors defined by cdh13, sema5a or crtac1 expression
We chose to analyze two TA-enriched genes cdh13 and sema5a and one GS-enriched gene crtac1, determining first their incidence in DRG neurons. We found that cdh13 is expressed by 48% of L1-L6 pvon proprioceptors, sema5a by 22% and crtac1 by 38% (Figure 2A–B, E–F, I–J). All three genes were also expressed by subsets of trkAon or trkBon cutaneous sensory neurons (Figure S1D). Thus, all three genes are expressed by subsets of proprioceptors (Figure 2; Lallemend and Ernfors, 2012).
Figure 2. cdh13, sema5a and crtac1 proprioceptor expression pattern.
(A–B, E–F, I–J) cdh13on (A–B), sema5aon (E–F) and crtac1on (I–J) proprioceptors (pSNs) at p0 in lumbar level DRG (L, lumbar level; n=6; >300 neurons/level). Data are represented as mean +/− SD. *: significant difference detected in pairwise comparisons with all other levels; ** significant difference detected in pairwise comparisons with L1, L2, L3 and L4 (Student’s t-test, p<0.001).
(C–D, G–H, K–L) cdh13, sema5a and crtac1 status in proprioceptors. ctb555-labeled TA, GS, RF/V, and AD/GR/ST/SM proprioceptors linked to pv and cdh13 expression (C–D), sema5a (G–H) or ctac1 (K–L) at p1. (D) Marker expression in TA, GS, RF/V and AD/GR/ST/SM proprioceptors (cdh13, TA: n=5; GS: n=6; RF/V: n=3; AD/GR/ST/SM: n=6; sema5a and crtac1: TA: n=3; GS: n=3; RF/V: n=3; AD/GR/ST/SM: n=3). Data represented as mean +/− SD. *: significant difference in TA vs GS and RF/V vs AD samples (Student’s t-test, p<0.005). Scale bar, 20μm. See also Figure S2.
We examined whether expression of cdh13 and sema5a exhibit selectivity for proprioceptors innervating TA muscle and conversely, whether expression of crtac1 is selective for GS proprioceptors. To evaluate this issue we injected ctb555 into TA or GS muscles at p0 and assessed the status of cdh13, sema5a or crtac1 expression in retrogradely-labeled proprioceptors at p1. We found that 96% and 95% respectively of pvon, ctb555-labeled TA neurons expressed cdh13 and sema5a, whereas GS neurons were devoid of cdh13 or sema5a transcript expression (Figure 2C–D, G–H). The rostrocaudal distribution of pvon DRG neurons that co-expressed cdh13 exhibited a progressive caudal decline, from 66% of pv-labeled neurons in L1 DRG to 21% in L6 DRG. Similarly, sema5a was expressed by 33% of pv-labeled neurons in L1 DRG but only by 8% in L6 DRG (Figure 2B,F). Conversely, 65% of GS neurons expressed crtac1 and TA neurons lacked crtac1 expression (Figure 2K–L). The rostrocaudal segmental distribution of pvon DRG neurons that co-expressed crtac1 also showed a progressive caudal decline, from 38% of pv-labeled neurons in L1 DRG to 20% in L4 DRG, although fractional expression increased to ~50% in L5 and L6 DRG (Figure 2J).
The cell bodies of TA and GS proprioceptors are located in L3 to L6 DRG. These four ganglia contain ~630, 280 and 560 cdh13on, sema5aon and crtac1on proprioceptors respectively (see Moqrich et al., 2004 for an estimation of total pvon neurons in lumbar DRG). To estimate TA and GS/SOL/PL proprioceptor number we noted that each spindle typically receives innervation from one group Ia and two group II afferents with each Golgi tendon organ (GTO) receiving only a single Ib fiber (Hunt, 1974). On this basis, the detection of 18 spindles in the TA and 39 spindles in the GS/SOL/PL muscles indicates that a total of ~60 and ~130 proprioceptors respectively supply the TA or GS/SOL/PL muscles. These numbers represent many fewer than the total number of cdh13on, sema5aon or crtac1on proprioceptors observed in L3-L6 DRG (Figure 2), implying that other limb muscles are supplied by lumbar cdh13on, sema5aon and crtac1on proprioceptors.
To identify the additional muscle targets of cdh13on, sema5aon and crtac1on proprioceptors we labeled sensory neurons by injection of ctb555 into defined hip, thigh, shank and foot muscles that occupy distinct dorso-ventral and proximo-distal positions within the limb (see Figure 3I for scheme depicting muscle location). Proprioceptors supplying the proximo-dorsal gluteus (GL) hip muscle lacked cdh13, sema5a or crtac1 expression, whereas 64%, 2% and 1% respectively, of proprioceptors supplying the dorsal rectus femoris (RF) and vastus (V) thigh muscles expressed cdh13, sema5a and crtac1 (Figure 2 C–D,G–H,K–L, S2A). Proprioceptors supplying ventrally positioned adductor (AD), gracilis (GR), semitendinosus (ST) and semimembranosus (SM) thigh muscles lacked cdh13 or crtac1 expression, although ~25% of them did express sema5a (Figure 2C–D,G–H,K–L). Injections targeting dorsal shank TA, extensor digitorius longus (EDL) and peroneus longus (PER) muscles, revealed that ~95% and 97% of pvon, ctb555 neurons expressed cdh13 or sema5a, whereas TA/EDL/PER proprioceptors lacked crtac1 expression.
Figure 3. Selective expression of cdh13 in proprioceptors supplying the dorso-distal hindlimb.
(A) Cdh13::CreERT2 and Tau::lox-STOP-lox:mGFP mice were crossed (Cdh13::GFP) and tamoxifen injected into pregnant females at day e16.5 of gestation to induce Cre activity.
(B) GFP expression in p2 DRG of Cdh13::GFP mice. Long arrows: Pvon,GFP+ neurons. Short arrows: Pvoff,GFP+ neurons. Scale bar, 50μm.
(C) GFP expression in TA proprioceptors in Cdh13::GFP mice. TA or GS proprioceptors in p0 Cdh13::GFP mice were retrogradely labeled by ctb555 muscle injection. At p1, DRG were removed and expression of GFP and Pv assessed by immunostaining. Arrows: Pvon, GFP+, ctb555+ TA proprioceptors. Scale bar, 10μm.
(D–E) TA but not GS MNs receive cdh13on input from TA synergist afferents. Immunostaining for GFP and vGluT1 shows GFP+ proprioceptive boutons on TA (D) but not GS (E) MNs. Scale bar, 20μm.
(F) TA and GS retrogradely labeled MNs receiving GFP+, vGlut1+ input (left) and GFP+ proprioceptive inputs to TA and GS MNs (n= 3 mice; TA: n=36 MNs, 836 boutons; GS: n=61 MNs, 1287 boutons). Data represented as mean +/− SD.
(G) GFP labels TA muscle spindle terminals in Cdh13::GFP mice. p7 TA muscles immunostained for vGluT1+ and GFP+. Scale bar, 40μm.
(H) Cdh13on proprioceptors supply dorsal-distal hindlimb muscles. Hindlimb muscles along the rostro-caudal and proximo-distal axes of the limb in Cdh13::GFP mice (tamoxifen induction at ~e14.5–16.5) were assessed for GFP expression at spindle terminals by immunostaining at p7 (n≥3 mice; additional muscles innervated by cdh13off proprioceptors include: pectineus, flexor digitorium longus, tibialis posterior, semimembranosus, semitendinosus, quadratus femoris, biceps femoris, and body wall). Scale bar, 50μm.
(I) Hindlimb domains innervated by cdh13on proprioceptors. Muscles shaded yellow are supplied by cdh13on proprioceptors. Those in grey are innervated by cdh13off proprioceptors. See also Figure S2, S3 and S4.
The small size of foot muscles prevented us from targeting dorsal or ventral limb domains with absolute precision. Nevertheless, dorsally-directed ctb555 injections revealed that 91%, 40% and 14% of retrogradely-labeled proprioceptors expressed cdh13, sema5a and crtac1, whereas 24%, 10% and 89% of proprioceptors expressed cdh13, sema5a and crtac1 respectively after ventrally-directed foot injections (Figure S2A). These findings support the notion that dorsal-distal muscles are supplied preferentially by proprioceptors that express cdh13. Conversely, crtac1 is expressed preferentially by proprioceptors that innervate ventral-distal limb muscles, and sema5a is expressed in proprioceptors that supply dorsal or ventral domains at particular proximo-distal positions. Thus, there is a clear link between proprioceptor expression of cdh13, sema5a and crtac1 and muscle position along the dorso-ventral and proximo-distal axes of the limb.
Genetic labeling of cdh13-expressing neurons
To provide a more detailed evaluation of the identity and position of muscles innervated by cdh13on proprioceptors, we used a genetic strategy to mark the cell bodies and peripheral and central axons of cdh13on neurons. We generated a “knock-in” mouse line in which a tamoxifen-inducible Cre recombinase/Estrogen Receptor fusion construct (CreERT2) is expressed under regulatory control of the TA-enriched gene cdh13 (Cdh13::CreERT2). We crossed Cdh13::CreERT2 mice with a Tau::lox-STOP-lox::mGFP line to generate Cdh13::GFP tracer mice (Hippenmeyer et al, 2005, Figure 3A). Tamoxifen delivery to pregnant females at embryonic day 16.5 resulted in GFP expression in Pvon lumbar DRG neurons (Figure 3B). The fraction of GFPon proprioceptors in DRG L3-L5 was ~8%, whereas ~45% of proprioceptors express cdh13 at these levels (Figure 2A–B), indicating a tamoxifen induction efficiency of ~18% (Figure 3B, S2B). The low induction efficiency likely reflects a high level of Hsp-90 expression in DRG neurons, which retains the CreERT2 fusion protein in the cytoplasm (Zhao et al., 2006).
We determined the fidelity of GFP expression in Cdh13::GFP mice by analyzing TA and GS proprioceptors. At p1, 15% of ctb555-labeled TA proprioceptors, but no GS proprioceptors, expressed GFP (Figure 3C, S2C), as with endogenous cdh13 expression. The central connectivity of cdh13on proprioceptors (TA/EDL/PER) was assessed by monitoring GFP expression in vGluT1+ proprioceptor terminals in the ventral spinal cord of Cdh13::GFP mice. 92% of TA motor neurons received inputs from GFP-labeled vGluT1+ proprioceptor terminals and inversely, 14% of all vGluT1+ synaptic contacts found on TA motor neuron somata and the proximal 100μm of the dendritic tree co-expressed GFP (Figure 3D–F). This low fraction presumably reflects mosaicism in GFP expression after tamoxifen induction. In contrast, none of the vGluT1+ terminals on the somata and proximal dendrites of GS motor neurons expressed GFP (Figure 3E–F). Since GS motor neurons also receive heteronymous input from soleus (SOL) and plantaris (PL) proprioceptors (Eccles et al., 1957), we conclude that these two classes also lack cdh13 expression.
Thus, the pattern of sensory-motor contacts in Cdh13::GFP mice conforms to the agonist-antagonist rules of synaptic patterning.
Transient erosion of sensory-motor target specificity in cdh13 mutant mice
The recognition functions proposed for many type II cadherins (Duan et al., 2014) led us to examine whether elimination of Cdh13 function has any impact on the selectivity of sensory-motor connections. We analyzed homozygous Cdh13::CreERT2 mice in which expression of cdh13 is eliminated, termed Cdh13mut::GFP mice. In Cdh13mut::GFP animals the number of muscle spindles in dorsal and ventral shank muscles was similar to the number in Cdh13het::GFP and wild type mice. In addition, TA but not GS proprioceptors express GFP, arguing against a role for cdh13 in peripheral sensory targeting and muscle spindle formation (Figure S5E and data not shown).
Comparison of the fraction of GFP+ TA/EDL/PER sensory terminals in contact with antagonist GS group motor neurons in Cdh13mut::GFP mice at p7 revealed a ~5-fold increase in GFP+/vGlut1+ proprioceptor sensory neuron boutons on retrogradely-labeled GS/SOL/PL MNs compared with Cdh13het::GFP littermates (Figure S3B; p<0.001). Correcting for differences in Cre dosage between homozygous and heterozygous mice still indicated a ~3-fold increase in ectopic GFP+/vGlut1+ boutons at p7 (p<0.01). By p18, however, mis-targeting to GS MNs in Cdh13mut::GFP mice was much less pronounced (Figure S3B), indicative of a restricted developmental period during which sensory axons mis-project to antagonist motor pools. The density of PVon neurons in p18 lumbar DRG was not significantly different in Cdh13het::GFP and Cdh13mut::GFP mice (Figure S3D), arguing against cell death as the basis of the late correction in mis-projections. Taken together, these findings suggest that proprioceptor cdh13 expression contributes, modestly, to the fidelity with which TA sensory afferents restrict themselves to functionally appropriate synergist motor pool targets.
Mapping proprioceptor cdh13 expression in cdh13::GFP mice
Cdh13::GFP mice permitted analysis of the peripheral terminations of Cdh13on proprioceptors. After retrograde muscle tracer labeling we detected expression of cdh13 in most TA proprioceptors suggesting that all three subtypes of proprioceptors – group Ia, group II and group Ib afferents - express cdh13. In support of this view, analysis of TA muscle revealed annulospiral GFP+, vGluT1+ type Ia and type II sensory endings in muscle spindles, as well as more broadly arborized type Ib endings associated with GTOs (Figure 3G–H, S2D). In contrast, GFP expression was not observed in sensory endings in GS muscle (Figure 3H), consistent with the TA group specificity of Cdh13::GFP mice.
To investigate further the relationship between proprioceptor cdh13 status and the limb position of muscle targets, we assayed the status of sensory GFP expression in individual hip, thigh, shank, and foot muscle spindles in p7 Cdh13::GFP mice exposed to tamoxifen in utero at ~e14.5–16.5. At the dorsal shank level, GFP+ endings were observed in the TA (Figure 3G–H), extensor digitorum longus (EDL) and peroneus longus (PER) muscles (Figure 3H–I). In contrast, none of the spindles of the ventrally-derived GS, SOL, PL, tibialis posterior (TP) or flexor digitorius longus (FDL) shank muscles were innervated by GFP+ proprioceptors (Figure 3H–I).
At more proximal levels, none of the spindles of dorsally- or ventrally-derived hip muscles - the gluteus GL, iliacus (IL), psoas (PS), obturator externus/internus (Oe/i), and caudofemoralis (CF) - contained GFP+ sensory terminals (Figure 3H–I). For dorsally-derived thigh muscles, the RF but not the V group (Vl, Vi, Vm) or pectineus (Pec) muscles contained GFP+ proprioceptor terminals, providing an explanation of the mosaic cdh13 status of RF/V proprioceptors detected in retrograde tracing experiments. Ventrally-derived muscles AD/GR/ST/SM and biceps femoris (BF) did not contain any GFP+ sensory terminals. Finally, we observed that spindles in the most dorsally located intrinsic foot muscles, but not the ventral foot muscles, contained GFP+ terminals (Figure 3H–I). This muscle-by-muscle analysis consolidates the view that cdh13on proprioceptors supply dorsally-derived limb muscles with a distal positional bias.
Onset of proprioceptor cdh13 expression occurs after limb innervation
Certain features of proprioceptor pool identity are acquired in a neuron-autonomous manner, with others dependent on limb signaling. If cell-autonomous signals operate, cdh13 may be expressed prior to contact with the limb mesenchyme. In reality, however, we found that cdh13 expression was initiated only after limb innervation, and its characteristic pattern is not shaped by programmed neuronal cell death (Figure S4A–C).
To explore the involvement of limb inductive signaling, we focused on proprioceptor subtype specification at a molecular level, employing genetic strategies to examine the cellular source of these signals. We considered three sources of proprioceptor gene patterning signals: i/ the motor axons that fasciculate with sensory axons during their limb trajectory, ii/ the target muscles innervated by sensory axons, and iii/ limb mesenchymal tissues traversed by sensory axons.
Motor neurons are not involved in specifying proprioceptor cdh13 expression
During limb innervation, sensory and motor axons fasciculate in peripheral nerves en route to their target muscles (Honig et al., 1998; Huettl et al., 2011). Distinct motor axon-derived signals could impose the sensory pattern of proprioceptor cdh13 expression. Alternatively, a generic permissive signal from motor axons could function with more selective inductive sources to induce pool profiles of cdh13 expression. To test these possibilities we deployed a genetic strategy that erodes the subtype identity of motor neurons and a second strategy that kills motor neurons early in their post-mitotic differentiation (Figure 4A–B).
Figure 4. Motor neurons are dispensable for proprioceptor Cdh13 expression.
A. Erosion of MN identity was achieved by crossing Olig2::Cre with floxed FoxP1 mice (FoxP1MNΔ). B. Post-mitotic MNs were killed by crossing Olig2::Cre with Rosa26::lox-STOP-lox:DTA mice (MNDTA).
(C) cdh13 and pv expression in p0 FoxP1MNΔ L4 DRG. The density of proprioceptors and the percentage of proprioceptors expressing cdh13 do not differ between FoxP1MNΔ and wt mice (see also Supplementary Figure 4). Scale bar, 30μm.
(D) LMC neurons are killed in MNDTA mice, as shown by immunostaining for FoxP1 and Isl1 in e11.5 wt and MNDTA lumbar spinal cords. Note the lack of FoxP1on neurons in the ventro-lateral spinal cord in MNDTA mice (int, interneurons; quantification in Figure S4).
(E) TA or GS proprioceptors in wt and FoxP1MNΔ mice identified by ctb555 and examined for cdh13 expression by double FISH. Arrowheads indicate pvon, ctb555+, cdh13on or pvon, ctb555+, cdh13off TA and GS proprioceptors, respectively. Scale bar, 10μm. (E) The cdh13 status of TA and GS proprioceptors does not differ between wt and FoxP1MNΔ mice (TA: n=3 mice; GS: n=3 mice). Data represented as mean +/− SD. (E–F) Differential cdh13 status is maintained in TA and GS proprioceptors of FoxP1MNΔ mice.
(G–H) The density of proprioceptors and percentage of cdh13on proprioceptors are similar in wt and MNDTA mice. (G) Images show double FISH for pv and cdh13 in e18.5 L4 DRG. Scale bar, 30μm. (H) Quantification of proprioceptors expressing cdh13 in wt and MNDTA L2 and L5 DRG at e18.5 (n=3 mice). Data represented as mean +/− SD. See also Figure S5.
To determine whether distinctions in the identity of limb-innervating motor axons impose proprioceptor subtype identities we inactivated FoxP1, an accessory Hox factor needed for the emergence of pool identities in limb-innervating motor neurons (Dasen et al., 2008). FoxP1 activity was abolished selectively in motor neurons (FoxP1MNΔ mice) by crossing an Olig2::Cre driver line with mice carrying a floxed foxP1 allele (Feng et al., 2010; Figure 4A). In FoxP1MNΔ mice examined at p1, the density of pvon neurons and the proportion of pvon neurons expressing cdh13 was unchanged in L2 and L5 DRG, when compared to wild type littermates (Figure 4C, S5A). Moreover, 100% of TA proprioceptors expressed cdh13 in FoxP1MNΔ mice (Figure 4E–F), and conversely <4% of ctb555-labeled GS proprioceptors expressed cdh13. Thus, motor neuron identity is not involved in the selectivity of proprioceptor cdh13 expression.
We also considered whether a generic signal provided by motor neurons acts with other inductive signals to direct the selectivity of proprioceptor cdh13 expression. To assess this issue we used a genetic strategy to ablate motor neurons, crossing an Olig2::Cre driver line with Rosa::DTA mice (Figure 4B; Wu et al., 2006) to generate MNDTA mice. Over 95% of LMC motor neurons in MNDTA mice were ablated by e11.5, as assessed by expression of FoxP1 and Isl1 (Figure 4D, S5B), and limb muscles in MNDTA mice exhibited atrophy at e18.5 (not shown). Nevertheless, proprioceptor endings were still found in association with muscle spindles (Figure S5C) and the density of pvon neurons in e18.5 lumbar DRG was similar in wild type and MNDTA mice (Figure 4G, Figure S5A).
MNDTA mice die soon after birth, so we were not able to identify proprioceptors by retrograde labeling from individual muscles. Nevertheless rostro-caudally, the variable fraction of proprioceptors expressing cdh13 did not change in MNDTA mice: cdh13 was expressed in ~70% of wild type and ~69% of MNDTA proprioceptors in L2 DRG, and in ~32% of wild type and ~34% of MNDTA proprioceptors in L5 DRG (Figure 4G–H). Together, these data provide evidence that cdh13 induction in proprioceptors is independent of permissive or instructive signals from motor axons.
Persistence of proprioceptor cdh13 pattern in limbs devoid of most muscles
We next examined whether limb muscles might be the source of a cdh13 inductive signal for proprioceptors, a possibility suggested by studies in chick embryos (Wenner and Frank, 1995). To assess this issue, we analyzed proprioceptor cdh13 expression in Lbx1−/− mice in which myogenic precursor migration into the developing limb is impaired such that the hindlimb is virtually devoid of skeletal muscle (Figure 5B; Gross et al., 2000).
Figure 5. Cdh13 expression is conserved in Lbx1−/− lumbar DRG.
(A) MN and proprioceptive sensory neuron innervating a hindlimb muscle. The boxed area highlights putative signaling from muscle to proprioceptive sensory axons.
(B) Developing muscles are absent from Lbx1−/− hindlimbs. Absence of muscle myosin in limbs of Lbx1−/− mice (Prox -Dis, Proximo-distal).
(C–D) The density of proprioceptors is similar in wt and Lbx1−/− DRG. (C) In situ hybridizations for pv in e15.5 L4 DRG of wt and Lbx1−/− mice. Scale bar, 20μm. (D) Proprioceptor density in L2 and L5 DRG of wt and Lbx1−/− mice.
(E–F) cdh13on proprioceptors conserved in Lbx1−/− DRG. (E) Double FISH for pv and cdh13 in e15.5 L4 wt and Lbx1−/− DRG. Scale bar, 10μm. (F) Percentage of proprioceptors expressing cdh13 at L2 and L5 DRG in e15.5 wt and Lbx1−/− mice (n=3 mice/genotype). See also Figure S5.
The major peripheral nerve trajectories in Lbx1−/− mice were similar to those in wild type limbs (data not shown; Phelan and Hollyday, 1990). Dextran injection into the hindlimb of e13.5 Lbx1−/− mice revealed dextran labelled proprioceptors in lumbar DRG, demonstrating sensory axon invasion of the hindlimb (Figure S5D). In Lbx1−/− mice examined at e15.5, the density of pvon neurons in L2 and L5 DRG was unchanged when compared to wild type DRG (203 pvon neurons/1mm2 in wild type vs ~198 pvon neurons/1mm2 in Lbx1−/− at L2, p= 0.68; 334 pvon neurons/1mm2 in wild type vs 320 pvon neurons/1mm2 in Lbx1−/− at L5, p=0.12; Figure 5C–D). We also compared the proportion of proprioceptors expressing cdh13 in e15.5 wild type and Lbx1−/− mice, at different lumbar rostro-caudal levels. In both L2 and L5 DRG, proprioceptor cdh13 expression was similar in Lbx1−/− and wild type mice (~69% in wild type vs ~63% in Lbx1−/− at L2, p=0.18; ~34% in wild type vs ~35% in Lbx1−/− at L5, p=0.89; Figure 5E–F). These data argue against the idea that muscles provide signals required for induction of proprioceptor cdh13 expression.
Proprioceptor gene expression is sensitive to the positional character of limb mesenchyme
We next studied the potential role of limb mesenchyme in establishing proprioceptor gene expression profiles. We analyzed cdh13, as well as sema5a and crtac1 expression, in mice in which the normal dorso-ventral character of the limb mesenchyme has been switched genetically, to either a double-ventral or a double-dorsal fate. We focused this analysis on proprioceptors innervating the shank, a domain of the limb in which all muscles of the dorsally-derived anterior crural group (TA/EDL/PER) are innervated by cdh13on, sema5aon, and crtac1off proprioceptors. Conversely, muscles of the ventrally-derived posterior crural group (GS/SOL/PLAN) are innervated by crtac1on and cdh13off, sema5aoff proprioceptors (Figures 2D,H,L and 3H–I).
To induce dorso-ventral mesenchymal conversion we manipulated expression of the LIM-homeodomain transcription factor Lmx1b, which is restricted to, and specifies dorsal limb identity (Riddle et al., 1995; Vogel et al., 1995; Chen et al., 1998). To generate mice with double-dorsal limb mesenchyme (d/v → d/d′; Li et al., 2010) lmx1b was expressed in ventral limb mesenchyme, achieved by crossing Rosa26lsl.Lmx1b to Prx1::Cre mice, such that Cre expression is directed throughout the limb mesenchyme from ~e9.5 (termed Prx1Lmx1b; Li et al., 2010). Mice with double ventral limb mesenchyme (d/v → v′/v) were examined in an Lmx1b−/− mutant background (Chen et al., 1998).
Such lmx1b manipulations resulted in the transformation of muscle, bone, and connective tissue to double-dorsal (in Prx1Lmx1b mice) or double-ventral (in Lmx1b−/− mice) character (Figure S5F–H; see Chen et al., 1998; Riddle et al., 1995; Vogel et al., 1995; Li et al., 2010). The incidence of pvon neurons in L2 and L5 DRG and the total number of dorsal and ventral shank muscle spindles did not differ significantly in wild type, Prx1Lmx1b and Lmx1b−/− mice (Figure S5A, S5E). Thus, generic aspects of proprioceptor development are unperturbed by encounter with symmetrically duplicated limb mesenchymal character.
We determined the profile of proprioceptor cdh13 expression in mice lacking dorsal limb mesenchymal character. Lmx1b−/− mice die within 24h of birth (Chen et al., 1998), precluding identification of proprioceptors by ctb555 retrograde tracing. We therefore crossed Lmx1b−/− mutants to Cdh13::GFP mice and assayed muscle spindles for the presence of GFP+ afferents at p0, following tamoxifen activation of Cre recombinase at e14.5 (Figure 6A–D). We dissected v and v′ shank muscles from Lmx1b−/−, Cdh13::GFP mice as well as v and d shank muscles from Cdh13::GFP mice and examined the sensory GFP status of spindles in these muscles (Figure 6A–D). In the dorsal limb of wild type mice we found that ~28% of muscle spindles received GFP+ afferent innervation (consistent with the efficiency of tamoxifen-mediated Cre induction). In contrast in Lmx1b−/− mutants, only ~2% of v′ muscle spindles were associated with GFP+ sensory axon terminals (Figure 6A–D; p<0.001). In both wild type and Lmx1b−/− mutant mice spindles supplying ventral shank muscles lacked GFP+ proprioceptor axons (Figure 6H–K).
Figure 6. Limb mesenchyme imposes cdh13, sema5a and crtac1 proprioceptor subtype character.
(A–D) Proprioceptors supplying duplicated ventral shank in Lmx1b−/− mice lack cdh13 expression. Shank muscles of wt Cdh13::GFP (A–B) and Lmx1b−/−, Cdh13::GFP (C–D) mice analyzed for GFP+ spindle afferents. (B, D) Percentage of GFP+ muscle spindles in dorsally or ventrally innervating proprioceptors in Cdh13::GFP (B) and Lmx1b−/−, Cdh13::GFP (D) mice (*, p<0.001, Student’s t-test, when comparing dorsally located muscles in Lmx1b−/− to wild type dorsal muscles; n=3 mice/genotype). (A,C) GFP status of vGluT1+ endings in muscle spindles supplying dorsally located muscles in Cdh13::GFP (A) and Lmx1b−/−, Cdh13::GFP (C) mice. Data represented as mean +/− SD. Scale bars, 50μm.
(E–P) Proprioceptors supplying duplicated dorsal shank in Prx1Lmx1b mice express cdh13 and sema5a but not crtac1. Shank propriocepotrs of wt and Prx1Lmx1b mice were identified by ctb555 and their cdh13 (E–H), sema5a (I–L) or crtac1 (M–P) expression was assessed by double FISH with pv. (E–G, I–K, M–N) Retrogradely-labeled proprioceptors supplying ventrally located muscles in wt and Prx1Lmx1b mice. Scale bars, 10μm. Dorsally or ventrally innervating proprioceptors expressing cdh13 (F–H), sema5a (J–L) or crtac1 (N–P) in wt and Prx1Lmx1b mice (*, p<0.001, Student’s t-test, when comparing ventrally located shank muscles in Prx1Lmx1b to wt ventral muscles; n=3 mice/genotype/gene). See also Figure S5.
We also examined the cdh13 expression status of proprioceptors supplying ventrally-positioned but dorsally specified (d′) shank muscles in Prx1Lmx1b mice. The cdh13 status of retrogradely-labeled proprioceptors was assessed at p1 after injection of ctb555 into d′ shank muscles at p0 (Figure 6E–H). We found that ~66% of pvon neurons innervating d′ muscles in Prx1Lmx1b mice exhibited cdh13 expression, in contrast to wild type mice where none of the proprioceptors innervating ventral shank muscles expressed cdh13 (Figure 6E–H; p<0.001). All proprioceptors innervating dorsal shank muscles in Prx1Lmx1b mice expressed cdh13, indicating no deviation from the normal cdh13 profile (Figure 6G–H). Thus, a ventral to dorsal switch in the positional character of the limb mesenchyme induces cdh13 expression in proprioceptors supplying ventrally-positioned but fate-switched muscles. These findings support the idea that the dorsal shank mesenchyme is the source of a local inductive signal that imposes the selective status of cdh13 expression in limb-innervating proprioceptors.
To determine whether limb mesenchyme also determines the pattern of expression of additional proprioceptor muscle-type genes we investigated the sema5a and crtac1 expression status of proprioceptors supplying ventrally-located but dorsally specified (d′) ventral shank muscles after retrograde labeling in Prx1Lmx1b mice. We found that ~72% of pvon neurons innervating d′ muscles in Prx1Lmx1b mice exhibited sema5a expression, in contrast to wild type mice where none of the proprioceptors innervating ventral shank muscles expressed sema5a (Figure 6I–L; p<0.001). Conversely, the expression of crtac1 in pvon neurons that supply ventral shank muscles was reduced from ~ 67% in wt mice to ~2% in Prx1Lmx1b mice (Figure 6M–P). No deviation from the normal sema5a and crtac1 expression profile was observed in proprioceptors innervating dorsal shank in Prx1Lmx1b mice (Figure 6K–L, 6O–P).
Together, these results indicate that limb mesenchymal signals differentially distributed along the dorso-ventral and proximo-distal axes of the limb impose the expression of many proprioceptor genes.
Discussion
Neither the molecular character of proprioceptive sensory neurons, nor the source of signals that direct their muscle-type programs of differentiation, have been defined. We document all-or-none molecular distinctions in muscle-target defined proprioceptors and show that three of the genes with such discriminative potential, cdh13, sema5a and crtac1, are confined to proprioceptors supplying muscles located in distinct domains along the dorso-ventral and proximo-distal axes of the hindlimb. Cdh13 is expressed by group Ia, group II and group Ib proprioceptors – assigning them a common muscle identity. Manipulating the dorso-ventral identity of the limb mesenchyme elicits a marked change in the profile of proprioceptor gene expression, documenting an instructive role for limb mesenchyme-derived inductive signals in patterning proprioceptor muscle-type identity.
Regionally-restricted limb mesenchymal signals induce proprioceptor gene expression
The emergence of muscle-selective proprioceptor identity appears intimately linked to the positional specification of limb mesenchyme. Patterning the limb mesenchyme involves signaling systems that operate along the proximo-distal, dorso-ventral and anterio-posterior limb axes (Bénazet and Zeller, 2009). The outgrowth and patterning of thigh, shank and foot domains is assigned along the proximo-distal axis, whereas the orthogonal dorso-ventral axis primarily delineates antagonist flexor and extensor muscle compartments. The profiles of proprioceptor cdh13, sema5a and crtac1 expression indicate that the molecular features of proprioceptor identity can be considered as a neuronal response to signals that operate independently along these orthogonal axes of the limb mesenchyme.
Gain or loss of proprioceptor cdh13, sema5a and crtac1 expression in mice with genetically-induced double-dorsal or double-ventral limbs could have its basis in inductive and/or repressive signals. The simplest of scheme holds that inductive signals expressed selectively by dorsal and distal limb mesenchyme induce cdh13 and sema5a expression in proprioceptors that innervate this mesenchymal domain (Figure 7). Conversely, an inductive signal expressed selectively by ventral-distal limb mesenchyme may induce crtac1 proprioceptor expression. Nevertheless, more complex scenarios are possible. The intersection of repressive and inductive signals could form grid-like systems of positional information, such that cdh13 expression is shaped by an inductive signal expressed along the entire proximo-distal axis of the dorsal limb mesenchyme, together with an independent proximal repressive signal.
Figure 7. Signals from limb mesenchyme control proprioceptor identity.
A. Limb mesenchyme signals pattern proprioceptor gene expression (c.t., muscle connective tissue, a putative source of proprioceptor specification signals; MN, motor neuron; pSN, proprioceptive sensory neuron).
B. Patterning mechanisms for proprioceptor gene expression include cdh13 or crtac1 inductive signals in dorso-distal or ventro-distal hindlimb respectively. Inhibitory and inductive signals may pattern proprioceptor gene expression along these limb axes.
Neurotrophin-3 (NT3) acting through proprioceptor TrkC has been suggested to control proprioceptor subtype and central connectivity (De Nooij et al., 2013; Wang et al., 2007). However, the sensory profile of cdh13 is unaltered in mice in which NT-3 is ectopically expressed in all limb muscles (data not shown), arguing against the sufficiency of muscle NT-3 as a cdh13 inducer. The secreted cerebellin (Cbln) family contains candidates for induction of cdh13 and sema5a in proprioceptors, given their restricted expression by dorsal limb mesenchyme, and down-regulation from limb mesenchyme in Lmx1b−/− mice (Feenstra et al., 2012; Haddick et al., 2014). It is also possible that mesenchymal cues responsible for guiding motor axons have a dual role in specifying proprioceptor muscle-type identity, in that ephrins, glial derived neurotrophic factor (GDNF) and netrin-1 are expressed in a restricted manner by dorsal or ventral limb mesenchyme and regulate the dorso-ventral choice of motor axons (Stifani, 2014, Poliak et al., 2015). The precise mesenchymal cell type that represents the source of proprioceptor inductive signals is also uncertain. Based on the timing of onset of cdh13 expression, undifferentiated limb mesenchymal cells or one of their derivative tissues could be sources. For example, TCF4-expressing limb connective tissue which has been proposed to set the pattern of muscle cleavage (Kardon et al., 2003) could also specify proprioceptor positional identity.
Linking peripheral specification and central connectivity
Grafting methods in chick embryos have been used to generate limb tissue with double dorsal character and examine proprioceptor identity indirectly through assessment of the selectivity of sensory-motor connections (Wenner and Frank, 1995). Proprioceptors supplying ventrally-positioned, but dorsally-specified limb tissue formed ectopic connections with motor neurons in the lateral division of the LMC, the normal target of proprioceptors innervating dorsal muscles. These results support the idea that peripheral limb signaling specifies aspects of proprioceptor identity involved in motor neuron connectivity as well as genetic identity.
Could the genes that mark muscle-type proprioceptors be involved in motor neuron connectivity? Some support for this view is provided by an analysis of cdh13 mutant mice, which exhibit a modest incidence of aberrant sensory-motor connections. The nature of the observed changes in connectivity in cdh13 mutants could reflect the combinatorial or redundant roles of other molecules in establishing final patterns of sensory-motor connectivity (Schwabe et al., 2013). Moreover, since cdh13 is not expressed by GS or TA motor neurons (data not shown), heterophilic interactions may link Cdh13 to other type II cadherins (Duan et al., 2014). Limb inductive signals that specify dorso-ventral proprioceptor identity are likely to instruct the recognition of motor neurons in medial and lateral divisions of the LMC, whereas proximo-distal limb signaling may confer recognition of the tier domains that appear to contribute to the dorso-ventral patterning of sensory-motor connections (Sürmeli et al., 2011). Together, our results establish a link between the specification of proprioceptor identity and patterns of central connectivity.
Proprioceptor diversity assigned in limb motor coordinates
The distinct molecular character of TA and GS proprioceptors, exemplified by cdh13, sema5a and crtac1 expression, reveals molecular differences that correlate with muscle positional character. Genes that mark defined subsets of proprioceptors have been identified, without examining the link between gene expression and the domain of limb innervation. Thus, plexinD1 and lmo4 are expressed by proprioceptors supplying both dorsal and ventral muscles, but the status of molecular expression with regard to proximo-distal limb target domain has not been analyzed in any detail (Fukuhara et al., 2011; Chen et al., 2002). Moreover, the maintenance of lmo4 expression after limb ablation (Chen et al., 2002) suggests that certain aspects of proprioceptor identity could be specified through cell-autonomous programs.
A muscle-by-muscle analysis of proprioceptor cdh13 expression indicate that the muscle-type identity of proprioceptors is assigned in a positional manner that conforms to the commonalities of function exhibited by synergistic muscle groups (Eccles et al., 1957; Nichols, 1994). They also imply that proprioceptor and motor pool identities adhere to the same positional logic, perhaps not surprisingly since the peripheral and central terminals of both sets of neurons occupy the same local microdomains in the limb and spinal cord. The expression of cdh13 by proprioceptors innervating each of the ankle flexor muscles further suggests that functionally related proprioceptors share a common molecular profile. Nevertheless in certain instances, proprioceptor identity does appear to segregate with individual muscles. Thus cdh13 is expressed by proprioceptors innervating the rectus femoris, but not the vastii muscles, potentially a reflection of the fundamentally different biomechanical features and activity profiles of these two muscles (Eccles et al., 1957; Nichols, 1994).
Other proprioceptor subtype genes identified in our screens - notably sema5a and crtac1 - exhibit patterns of expression distinct from that of cdh13, yet conform to the core principle of positional distinctions for individual limb domains. The fact that cdh13 and sema5a are expressed by proprioceptors supplying partially overlapping muscle groups suggests that the logic of proprioceptor pool identity may emerge through programs of combinatorial specification induced by limb mesenchyme. Thus, the limb mesenchyme may coordinate multiple aspects of motor circuit assembly - by regulating muscle cleavage and motor axon guidance as well as the specification of proprioceptor muscle-type identity.
Experimental procedures
Mice
Mouse strains: Cdh13::CreERT2 mice described in Supplemental Information. Pv::Cre (Hippenmeyer et al., 2005); Thy1::lox-STOP-lox::YFP (line 15; Buffeli et al., 2003); Tau::lox-STOP-lox::mGFP (Hippenmeyer et al., 2005); Olig2::Cre (Dessaud et al., 2007); floxed FoxP1 (Feng et al., 2010); Rosa26::lox-STOP-lox::DTA (Wu et al., 2006); Lbx1−/− (Gross et al., 2000); Prx1::Cre (Logan et al., 2000); Rosa26::lox-STOP-lox::Lmx1b (Li et al., 2010); Lmx1b−/− (Chen et al., 1998). Experiments were performed according to Columbia University (IACUC) guidelines.
Purification and expression profiling of TA and GS proprioceptors
TA or GS muscles of p0 Pv::YFP pups were injected with ctb555. The next day, DRG containing ctb555-labeled neurons were removed, dissociated (Malin et al., 2007), and individual YFP+, ctb555+ proprioceptors identified and purified by aspiration (Hempel et al. 2007). RNA was extracted from three samples of TA and GS proprioceptors, each containing 25–30 neurons, using a PicoPure RNA isolation kit (Arcturus). See Supplemental Experimental Procedures.
Retrograde labeling of proprioceptors
p0–p3 pups were anesthetized and muscles injected with cholera toxin B subunit-Alexa555 (ctb555; 1% dilution in PBS; Life Technologies). The following day, spinal cord and DRG were dissected: fresh frozen for fluorescent in situ hybridization, or 4% paraformaldehyde fixed for immunohistochemistry.
In Situ Hybridization and Immunohistochemistry
In situ hybridization and immunohistochemistry were performed on 16–25 μm sections (Schaeren-Wiemers and Gerfin-Moser, 1993; Dasen et al., 2005). Primary antibodies: rabbit anti-PV (1/5000, Swant); guinea pig anti-Islet1/2, (1/16000; Dasen et al., 2005); rabbit anti-GFP (1/1500, Life Technologies); guinea-pig anti vGlut1 (1/8000; Betley et al., 2009) and rabbit anti-FoxP1 (1/16000; Dasen et al., 2008). FITC, Cy3 and Cy5 secondary antibodies were used at 1/1000, 1/1000 and 1/500 dilutions, respectively. Double fluorescent in situ hybridization was performed with digoxigenin- and fluorescein-labeled cRNA probes, detected with a FITC/Cy-5 tyramide signal amplification (TSA) system (Perkin Elmer). Images were acquired and quantified on a Zeiss LSM510 confocal microscope (Sürmeli et al, 2011).
Supplementary Material
Acknowledgments
We are grateful to Qiaolian Liu for assistance, Gulsen Sürmeli for help with muscle injections and DRG dissections, Sean O’Keeffe for RNA-Seq data analysis, and Randy Johnson and Rajeshwar Awatramani for Rosa26::lox-STOP-lox::Lmx1b mice. We thank Nikolaos Balaskas, Joriene de Nooij, Andrew Murray and David Ng for critical comments on the manuscript; Kathy McArthur for secretarial assistance, and Ira Schieren for graphics. Erica Famojure, Barbara Han, Susan Brenner-Morton, Monica Mendelsohn and Nataliya Zabello provided lab support. S.P. was supported by a Helen Hay Whitney Foundation fellowship. T.M.J. is an HHMI investigator and is supported by grants from NINDS, The Leila and Harold Mathers Foundation, and ProjectALS.
Footnotes
Author Contributions
S.P. and A.N. performed experiments. S.P., A.N. and T.M.J. designed experiments and wrote the manuscript, with input from all authors. M.Y. and J.S. generated the Cdh13::CreERT2 mouse line.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, The Nervous System. 1981. pp. 509–595. [Google Scholar]
- Bénazet JD, Zeller R. Vertebrate Limb Development: Moving from Classical Morphogen Gradients to an Integrated 4-Dimensional Patterning System. Cold Spring Harb Perspect Biol. 2009;1(4):a001339. doi: 10.1101/cshperspect.a001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yip JW, Stewart AF, Frank E. Differential expression of a transcription regulatory factor, the LIM domain only 4 protein Lmo4, in muscle sensory neurons. Development. 2002;129:4879–4889. doi: 10.1242/dev.129.21.4879. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Doobar S, Jessell TM. Etv1 inactivation reveals proprioceptor subclasses that reflect the level of NT3 expression in muscle targets. Neuron. 2013;77:1055–1068. doi: 10.1016/j.neuron.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, Sanes JR. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell. 2014;158(4):793–807. doi: 10.1016/j.cell.2014.06.047. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physio. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra JM, Kanaya K, Pira CU, Hoffman SE, Eppey RJ, Oberg KC. Detection of genes regulated by Lmx1b during limb dorsalization. Dev Growth and Diff. 2012;54:451–462. doi: 10.1111/j.1440-169X.2012.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, Yoshida Y. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep. 2013;5:748–758. doi: 10.1016/j.celrep.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddick PC, Tom I, Luis E, Quinones G, Wranik BJ, Ramani SR, Stephan JP, Tessier-Lavigne M, Gonzalez LC. Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PloS One. 2014;9:e84823. doi: 10.1371/journal.pone.0084823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Honig MG, Frase PA, Camilli SJ. The spatial relationships among cutaneous, muscle sensory and motoneuron axons during development of the chick hindlimb. Development. 1998;125:995–1004. doi: 10.1242/dev.125.6.995. [DOI] [PubMed] [Google Scholar]
- Huettl RE, Soellner H, Bianchi E, Novitch BG, Huber AB. Npn-1 contributes to axon-axon interactions that differentially control sensory and motor innervation of the limb. PLoS biology. 2011;9:e1001020. doi: 10.1371/journal.pbio.1001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC. Handbook of Sensory Physiology. II/2 Springer-Verlag; Berlin-Heidelberg-New York: 1974. Muscle Receptors. [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5:937–944. doi: 10.1016/s1534-5807(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends in Neurosci. 2012;35:373–381. doi: 10.1016/j.tins.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lance Jones C. The morphogenesis of the thigh of the mouse with special reference to the tetrapod muscle homologies. JMorph. 1979;162:275–310. doi: 10.1002/jmor.1051620207. [DOI] [PubMed] [Google Scholar]
- Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci. 1997;17:3128–3135. doi: 10.1523/JNEUROSCI.17-09-03128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B, Frank E. Specific monosynaptic sensory-motor connections form in the absence of patterned neural activity and motoneuronal cell death. J Neurosci. 1991;11:1390–1403. doi: 10.1523/JNEUROSCI.11-05-01390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ, Jessell TM. Activity Regulates the Incidence of Heteronymous Sensory-Motor Connections. Neuron. 2015;87(1):111–23. doi: 10.1016/j.neuron.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Earley TJ, Watson J, Andahazy M, Backus C, Martin-Zanca D, Wright DE, Reichardt LF, Patapoutian A. Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nat Neurosci. 2004;7:812–818. doi: 10.1038/nn1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat. 1994;151:1–13. doi: 10.1159/000147637. [DOI] [PubMed] [Google Scholar]
- Phelan KA, Hollyday M. Axon guidance in muscleless chick wings: the role of muscle cells in motoneuronal pathway selection and muscle nerve formation. J Neurosci. 1990;10:2699–2716. doi: 10.1523/JNEUROSCI.10-08-02699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Morales D, Croteau LP, Krawchuk D, Palmesino E, Morton S, Jean-François C, Charron F, Dalva MB, Ackerman SL, Kao TJ, Kania A. Synergistic integration of Netrin and ephrin axon guidance signals by spinal motor neurons. Elife. 2015;4 doi: 10.7554/eLife.10841. pii: e10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci. 2014;8:293. doi: 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Neuert H, Clandinin TR. A network of cadherin-mediated interactions polarizes growth cones to determine targeting specificity. Cell. 2013;154(2):351–64. doi: 10.1016/j.cell.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Pattern and specificity of axonal outgrowth following varying degrees of chick limb bud ablation. J Neurosci. 1984;4(10):2518–27. doi: 10.1523/JNEUROSCI.04-10-02518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–53. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li LY, Taylor MD, Wright DE, Frank E. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci. 2007;27(14):3686–94. doi: 10.1523/JNEUROSCI.0197-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P, Frank E. Peripheral target specification of synaptic connectivity of muscle spindle sensory neurons with spinal motoneurons. J Neurosci. 1995;15:8191–8198. doi: 10.1523/JNEUROSCI.15-12-08191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Nassar MA, Gavazzi I, Wood JN. Tamoxifen-inducible NaV1.8–CreERT2 recombinase activity in nociceptive neurons of dorsal root ganglia. Genesis. 2006;44:364–371. doi: 10.1002/dvg.20224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.