Abstract
The hospital discharge of premature infants in neonatal intensive care units is often delayed due to their inability to feed by mouth safely and competently. With immature physiologic functions, infants born prematurely cannot be expected to readily feed by mouth at the equivalent age of a third trimester of gestation as the majority of their term counterparts do. Consequently, it is crucial that health care professionals gain an adequate knowledge of the development of preterm infants’ oral feeding skills so as to optimize their safety and competency as they transition to oral feeding. With a greater sensitivity toward their immature skills, we can offer these infants a safer and smoother transition to independent oral feeding than is currently observed. This review article is an overview of the evidence-based research undertaken over the past 2 decades on the development of very-low-birth-weight infants’ oral feeding skills. The description of the different functional levels where these infants can encounter hurdles may assist caregivers in identifying a potential cause or causes for their individual patients’ oral feeding difficulties.
Keywords: prematurity, suck-swallow-respiration coordination, oral feeding issues, neonatal intensive care unit, NICU, dysphagia
INTRODUCTION
The March of Dimes has reported that more than half a million births occur prematurely (<37 wk gestational age), representing 11.7% of the live births in the United States (www.marchofdimes.com). Because the survival of preterm infants continues to increase as a result of medical advances, the difficulties often encountered by these infants to readily feed by mouth are gaining attention.
The care of preterm infants initially rests in the hands of the medical team in neonatal intensive care units (NICUs).4 After overcoming life-threatening morbidities and developing chronic conditions associated with prematurity, such as bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, and/or necrotizing enterocolitis, these infants’ hospital discharge is often delayed because of their inability to feed by mouth safely and efficiently. Because attainment of independent oral feeding is one of the criteria recommended by the American Academy of Pediatrics for hospital discharge (1), the longer these infants’ transition from tube to independent oral feeding, the longer their hospitalization (2, 3). Consequently, prolonged oral feeding difficulties increase medical costs and potential long-term oral feeding aversion and further increase maternal stress as mother-infant reunion is delayed (4–7).
Much of the understanding we have to date with regard to infant oral feeding skills has been gained from bottle feeding. This is not based, by any means, on the presumption that bottle feeding is better than breastfeeding but rather on the technicality that an infant’s performance during bottle feeding is a more accurate reflection of his or her inherent skills because milk availability is held constant. Indeed, during breastfeeding, an infant’s oral feeding skills will be affected by maternal milk availability, be it milk supply and/or ejection (letdown). From a different perspective, one may consider “inherent” oral feeding skills as the “mechanical” skills an infant has developed, specifically the ability of the different musculatures (sets of muscles) implicated in sucking, swallowing, breathing, and esophageal transport that need to work in an appropriate temporal synchrony to prevent food penetration into the lungs and to minimize unnecessary energy expenditure. On the other hand, infant oral feeding performance is the resultant of these skills plus the external factors imposed on the infant that may enhance or hamper the use of his or her inherent skills (e.g., milk availability, NICU environment, caregiver feeding approach).
DEVELOPMENT OF ORAL FEEDING SKILLS
Over the past 2 decades, research studies on the development of infant oral feeding skills have focused primarily on the major concerns of oral feeding issues in NICUs, namely infants’ ability to suck, swallow, and breathe and their coordinated activities. During the past decade, research on the development of esophageal function has identified esophageal bolus transport as an equally important component of infant oral feeding skills. However, the impact that this component has on the safety and competency of infant oral feeding has not yet been fully recognized in clinical practices.
Development of sucking
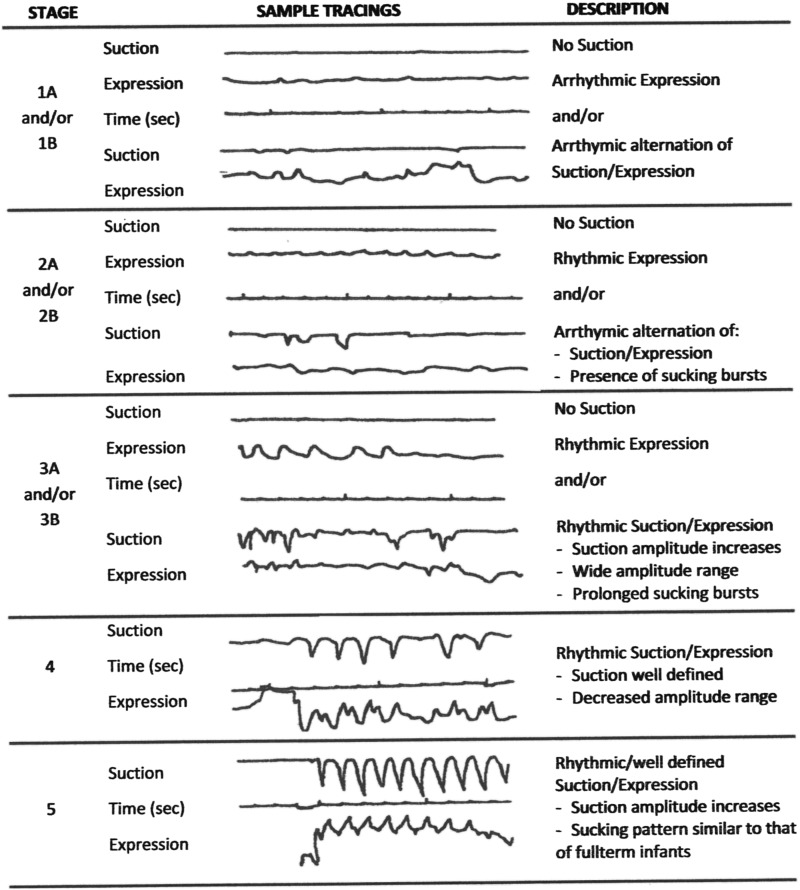
Sucking may be nutritive or nonnutritive in nature with liquid/milk ingestion involved or not involved, respectively. Wolff (8) described nutritive sucking as occurring at 1 cycle/s and nonnutritive sucking at 2 cycles/s. This difference may likely be due to the fact that during nonnutritive sucking minimal swallows occur, except for infants’ own saliva. For both types of sucking, mature sucking comprises 2 components: suction and expression (9, 10). Suction corresponds to the intraoral negative pressure that draws liquid into the mouth, an action similar to drinking with a straw. The lowering of the lower mandible increases the volume of the oral cavity while the closure of the nasal passages by the soft palate and the tight seal of the lips around the breast or bottle nipple prevent air inflow (11). Expression corresponds to the compression and/or stripping of the tongue against the hard palate to eject liquid into the mouth (12, 13). In following the development of nutritive sucking in very-low-birth-weight infants, we developed a descriptive scale based on the presence and absence of the suction and expression components, their respective rhythmicity, and the eventual attainment of a mature rhythmic alternation of suction/expression (14). Figure 1 presents examples of the evolution of infant nutritive sucking patterns as they mature. Stage 1, the most immature, is characterized by the absence of suction and the arrhythmic presence of expression only; stage 5, the most mature, is characterized by the rhythmic alternation of suction/expression. From these studies, it has become apparent that maturation of the expression component occurs before that of suction. It is of interest to note that the presence of suction alone is rarely observed in contrast to that of expression alone (C Lau, unpublished observation, 1995), suggesting that maturation of the musculature implicated in the generation of expression occurs before that of suction (15).
FIGURE 1.
Five-stage descriptive scale of the development of infant nutritive sucking characterized by the presence or absence of the suction and expression components of sucking and the sequential appearance of their respective rhythmicity and frequency.
With bottle feeding, safe and successful oral feeding does not require mature sucking with the rhythmic alternation of suction/expression. Infants who use an immature suck (i.e., expression alone) can complete a feeding, albeit not as efficiently (12, 14). On the other hand, with breastfeeding, it is unclear that successful breastfeeding can be achieved with the use of expression alone. This is anecdotally supported by the observation that preterm infants have difficulty latching onto the breast early on. Elliott (16) showed that the use of a nipple shield can facilitate the latching-on process to the breast. In our own institution, a nipple shield is provided early to mothers in the NICU as a “lactation tool” to preserve mother-infant breastfeeding relation, namely maternal interest in breastfeeding, maintaining lactation, and expressing milk. A number of studies have shown the benefits of the use of nipple shields, although their use has not been readily advocated (17–21). These studies, however, did not investigate the potential reason or reasons for such advantages. Taking the above together, the author speculates that the failure of very-low-birth-weight infants to breastfeed successfully likely results from their inability to remain latched onto the breast during a breastfeeding session. In the absence of the suction component, infants likely cannot retain the maternal nipple in their mouth for prolonged time periods because it does not have the rigidity of a bottle nipple or that of a nipple shield.
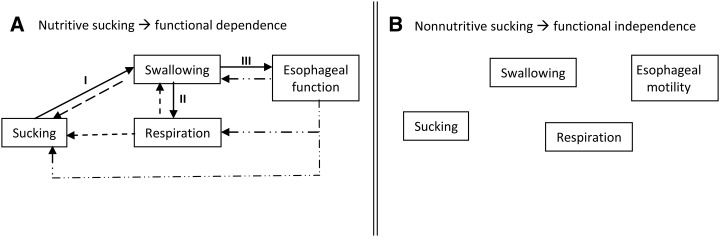
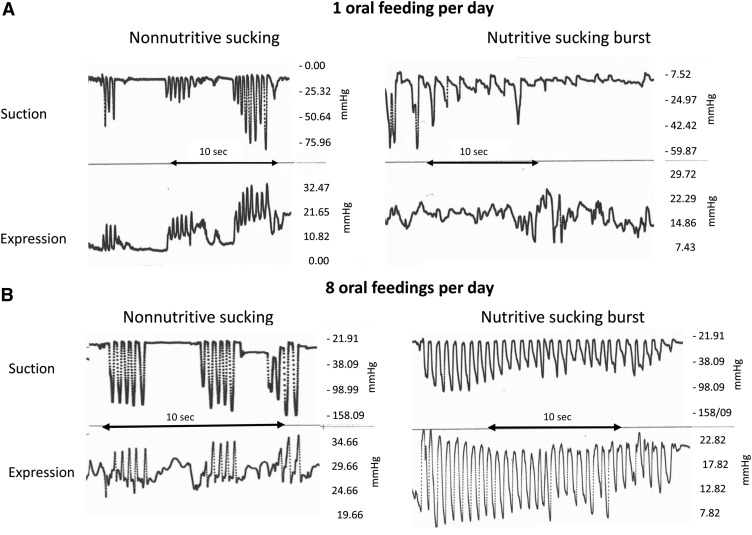
Nutritive sucking can be looked on as a closed-loop system. Indeed, safe and successful transport of the bolus depends on 2 essential events: 1) a timely “downstream” synchronization of sucking, swallowing, respiratory, and esophageal functions to prevent liquid penetration and aspiration, respiratory disruption, and sluggish esophageal transport of the bolus, respectively, to the stomach and 2) an appropriate feedback at the level of each of these functions to send back “upstream” the proper signal of whether sucking need to be stopped, delayed, or maintained (Figure 2A). It has been proposed that the rhythmicity of these functions is controlled by central pattern generators (CPGs) in the brain, with the CPGs for sucking, swallowing, and respiration anatomically located in the medulla (15). If correct, one needs to presume that nutritive sucking will be safe and efficient when the maturity level of these CPGs is sufficiently adequate to allow for the smooth passage of a bolus from mouth to stomach. In contrast, as mentioned above, nonnutritive sucking implicates minimal swallows because no liquid is ingested, except for infants’ own saliva. As such, it is an activity confined within the oral cavity that is independent of the swallow, respiratory, and esophageal functions (Figure 2B). This may be why infant nonnutritive sucking occurs at a faster frequency (2 cycles/s) than does nutritive sucking (at 1 cycle/s) and matures earlier (Figure 3A) (8, 10). Consequently, nonnutritive sucking is a good marker for sucking per se but is not predictive of infants’ nutritive sucking and their readiness to oral feed.
FIGURE 2.
Schematic of the functional dependence (A) and independence (B) of sucking, swallow, respiratory, and esophageal functions during nutritive and nonnutritive sucking, respectively.
FIGURE 3.
Tracings of nonnutritive and nutritive sucking monitored 3 min apart during same-feeding sessions of an infant born at 33 and 1/7 wk gestational age introduced to oral feeding at 34 and 2/7 wk postmentrual age (A) and attaining independent oral feeding at 36 and 1/7 wk postmentrual age (B). The frequency difference in the nonnutritive (2 cycles/s) and nutritive (1 cycle/s) sucking patterns likely results from the absence and presence of subsequent swallowing events, respectively.
Maturation of the swallowing process
The swallowing process comprises the oral preparatory, pharyngeal, and esophageal phases involved in the formation of the bolus and its transport to the stomach through the pharynx and esophagus, respectively. This is a process that infants who are born prematurely have not yet attained (11, 22). Because nutritive sucking occurs at 1 suck/s, the transport of individual boluses must occur rapidly before the next one takes place. Any delay of the bolus passage during the oral, pharyngeal, and/or esophageal phase will disrupt the normal sequence of events and increase the risks of adverse events (e.g., choking, respiratory disruptions, fluid penetration/aspiration into the lungs) (4, 11, 23, 24).
Oral preparatory phase
With maturation, the swallowing process becomes more adaptable and efficient. For instance, infants can handle larger and varying bolus sizes (25, 26). The propulsion force or intrabolus pressure exerted by the tongue in propelling the bolus onto the posterior wall of the oropharynx to initiate the swallow reflex increases as does the swallow rate (26–28). It is of interest to note that when a liquid bolus is held in the mouth, the formation of a “functional” glossopalatal sphincter has been described. This consists of the pinching together of the posterior tongue and soft palate to prevent premature fluid spilling into the pharynx. Pooling of the liquid above this sphincter not only preserves the liquid from dispersing but may also increase the intrabolus pressure above the sphincter, facilitating the propulsion of the bolus into the oropharynx to initiate the swallow reflex when the glossopalatal sphincter relaxes (29). Because safe and swift swallows require an appropriate maturation and synchrony of these functions, it is understandable that the immaturity of preterm infants could lead to unsafe suck-swallow interactions.
Pharyngeal phase
Once the swallow reflex is initiated, appropriate pharyngeal anterograde peristalsis determines the swiftness at which the bolus travels through the pharynx to the upper esophageal sphincter (UES) before the next bolus arrives as well as the rapid clearance of residual fluid around the valleculae and pyriform sinuses to minimize laryngeal penetration. The valleculae are bilateral depressions at the base of the tongue, and the pyriform sinuses are recesses on either side of the laryngeal orifice. Both structures are situated at the level of the epiglottis. Liquid trapped in these recesses increases risks of penetration/aspiration into the larynx when the epiglottis is opened. Consequently, timely closure of the epiglottis determines the safety at which fluid will not enter into the larynx.
Esophageal phase
A better understanding of the maturational process of esophageal function of preterm infants was recently gained with the availability of novel methodologies/tools tailored to their small size: for example, endoscopic manometry, multichannel intraluminal impedance, and pH-multichannel intraluminal impedance. From these advances, the esophagus is now recognized as comprising 3 distinct elements shown to mature at different times and phases as described below:
1) UES: Studies have shown that most preterm infants with weak pharyngeal pressures show poor coordination between pharyngeal bolus propulsion and UES relaxation, thereby delaying timely transport of the bolus from the pharynx into the esophagus. Increased pharyngeal pressure associated with well-developed UES and esophageal motility appears at ∼33–34 wk postmenstrual age (PMA) (30, 31).
2) Esophageal body: In preterm infants, the immaturity of the esophageal body parallels that of the small intestine, both likely a result of the immaturity of the central and peripheral neuromotor properties of these organs (32, 33). Two groups of waves have been characterized: peristaltic and nonperistaltic. The peristaltic waves can be antero- or retrograde in nature, whereas the nonperistaltic waves include synchronous and incomplete wave patterns (34). Proper transport of a bolus toward the stomach requires the presence of anterograde peristaltic waves from the UES down to the lower esophageal sphincter (LES). Retrograde peristaltic waves, in contrast, transport a bolus back upstream and have been blamed for occurrences of regurgitation. However, with maturation, the occurrence of nonperistaltic waves decreases, whereas that of anterograde peristaltic waves increases (35, 36).
3) LES: One of the physiologic functions of the LES is to control the anterograde bolus entry into the stomach and the retrograde nutrient flow back into the esophageal body. Two types of LES relaxation (LESR) have been described: the swallow-related LESR associated with the anterograde bolus transport into the stomach and the transient LESR, independent of swallowing and associated with belching and/or gastroesophageal reflux. Transient LESR is not necessarily associated with feeding, and it is unclear whether its occurrence increases with immaturity (37).
Maturation of the respiratory process
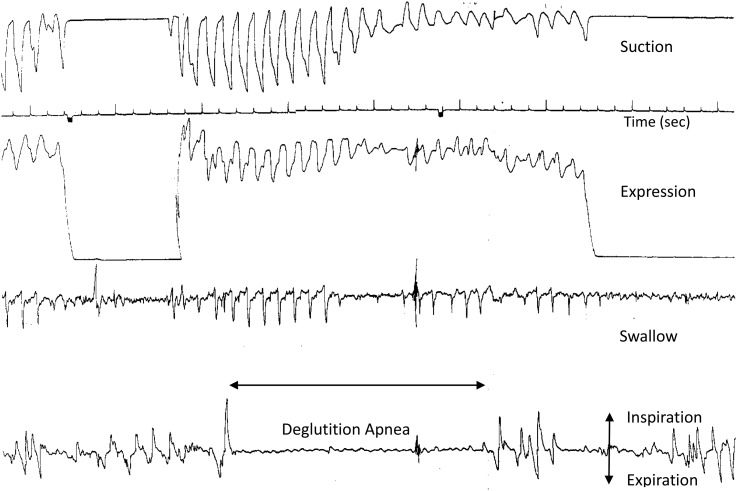
Safe oral feeding entails proper oxygenation. Because the majority of preterm infants mature, their oxygen requirement and episodes of oxygen desaturation and/or apnea likely decrease. With respiratory rates ranging between 40 and 60 breaths/minute, preterm infants average ∼1–1.5 breaths/s. However, given that a swallow event may last between 0.35 and 0.7 s (38), some infants may not have sufficient time between swallows to appropriately breathe, thereby threatening the balance of oxygenation and ventilation. In addition, during oral feeding, minute ventilation decreases, whereas expiration is prolonged and inspiration shortened (39–41). All of these events occurring together threaten balanced oxygen–carbon dioxide exchanges. As such, some preterm infants may have difficulty tolerating oral feeding for a prolonged time period. It is conceivable that episodes of deglutition apnea commonly observed in preterm infants may facilitate oral feeding but at the precarious expense of respiration (Figure 4).
FIGURE 4.
Simultaneous monitoring of the nutritive sucking (suction and expression), swallow event (as identified by hyoid upward movement) and respiration in a 29-wk-gestational-age infant at 47 d of life who experienced an episode of deglutition apnea. Feeding was halted when the infant “turned blue.”
An additional safety concern pertains to when swallowing is safe in relation to the respiratory phases or swallow-respiration interfacing. Although swallows can occur at any phase of respiration, for the majority of full-term infants and adults, they primarily occur at respiratory phases that minimize the risks of pulmonary aspiration when no air inflow occurs (e.g., exhalation, end of inspiration or exhalation, and during respiratory pauses) (42, 43). Unfortunately, preterm infants primarily swallow during deglutition apnea and inhalation, increasing their risk of oxygen desaturation and laryngeal penetration/aspiration, respectively (26, 44).
In summary, infants’ readiness to oral feed is a primary concern of caregivers before weaning their patients from tube feeding. However, the criteria that define such readiness remain elusive. With the current practice, oral feeding is carefully introduced at a certain PMA by using a “trial-and-error” approach at ∼32–34 wk PMA. If an infant shows any adverse effects, interventions lacking evidence-based support are offered, but any ensuing improvement or improvements cannot rule out infants’ normal maturation process [e.g., side-lying feeding position (45), cheek and chin support, cue-based feeding].
The increase in survival of preterm infants has led to the growing awareness and urgency that evidence-based knowledge of these infants’ physical and physiologic immaturities is germane to our ability to understanding why so many of them have difficulty feeding by mouth. The numerous neuromotor and neurophysiologic functions that can be impaired with immaturity along the path taken by a bolus descending into the stomach are overwhelming, and it is no surprise that so many preterm infants encounter difficulties feeding by mouth. Although the information presented in this review has not fully reached the clinical arena, it would appear that the term “readiness to oral feed” best relates to an infant’s ability to safely and efficiently coordinate sucking, swallowing, respiration, and esophageal functions. Scales to assess “readiness to oral feed” have been developed, but their recognition has not been readily accepted (46–53). On a final note, it is encouraging to know that the research on infant oral feeding has led to the development of oral feeding assessment scales and evidence-based interventions and tools that can enhance preterm infants’ skills at various functional levels (44, 54–58).
Acknowledgments
The sole author had responsibility for all parts of the manuscript. The author had no conflicts of interest to disclose.
Footnotes
Abbreviations used: CPG, central pattern generator; LES, lower esophageal sphincter; LESR, lower esophageal sphincter relaxation; NICU, neonatal intensive care unit; PMA, postmenstrual age; UES, upper esophageal sphincter.
REFERENCES
- 1.American Academy of Pediatrics. Policy statement: hospital discharge of the high-risk neonate. Pediatrics 2008;122:1119–26. [DOI] [PubMed] [Google Scholar]
- 2.Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 1999;103:434–9. [DOI] [PubMed] [Google Scholar]
- 3.Eichenwald EC, Blackwell M, Lloyd JS, Tran T, Wilker RE, Richardson DK. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics 2001;108:928–33. [DOI] [PubMed] [Google Scholar]
- 4.Lau C, Hurst N. Oral feeding in infants. Curr Probl Pediatr 1999;29:105–24. [DOI] [PubMed] [Google Scholar]
- 5.Lau C, Hurst NM, Smith EO, Schanler RJ. Ethnic/racial diversity, maternal stress, lactation and very low birthweight infants. J Perinatol 2007;27:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles MS, Funk SG, Carlson J. Parental Stressor Scale: neonatal intensive care unit. Nurs Res 1993;42:148–52. [PubMed] [Google Scholar]
- 7.Melnyk BM, Crean HF, Feinstein NF, Fairbanks E. Maternal anxiety and depression after a premature infant’s discharge from the neonatal intensive care unit: explanatory effects of the creating opportunities for parent empowerment program. Nurs Res 2008;57:383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff PH. The serial organization of sucking in the young infant. Pediatrics 1968;42:943–56. [PubMed] [Google Scholar]
- 9.Sameroff AJ. The components of sucking in the human newborn. J Exp Child Psychol 1968;6:607–23. [DOI] [PubMed] [Google Scholar]
- 10.Lau C, Kusnierczyk I. Quantitative evaluation of infant’s nonnutritive and nutritive sucking. Dysphagia 2001;16:58–67. [DOI] [PubMed] [Google Scholar]
- 11.Wolf LS, Glass RP. Feeding and swallowing disorders in infancy: assessment and management. Tucson (AZ): Therapy Skills Builders; 1992. [Google Scholar]
- 12.Lau C, Sheena HR, Shulman RJ, Schanler RJ. Oral feeding in low birth weight infants. J Pediatr 1997;130:561–9. [DOI] [PubMed] [Google Scholar]
- 13.Waterland RA, Berkowitz RI, Stunkard AJ, Stallings VA. Calibrated-orifice nipples for measurement of infant nutritive sucking. J Pediatr 1998;132:523–6. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr 2000;89:846–52. [PubMed] [Google Scholar]
- 15.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr 2008;97:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott C. Using a silicone nipple shield to assist a baby unable to latch. J Hum Lact 1996;12:309–13. [DOI] [PubMed] [Google Scholar]
- 17.Meier PP, Brown LP, Hurst NM, Spatz DL, Engstrom JL, Borucki LC, Krouse AM. Nipple shields for preterm infants: effect on milk transfer and duration of breastfeeding. J Hum Lact 2000;16:106–14. [DOI] [PubMed] [Google Scholar]
- 18.Chertok IR, Schneider J, Blackburn S. A pilot study of maternal and term infant outcomes associated with ultrathin nipple shield use. J Obstet Gynecol Neonatal Nurs 2006;35:265–72. [DOI] [PubMed] [Google Scholar]
- 19.Clum D, Primomo J. Use of a silicone nipple shield with premature infants. J Hum Lact 1996;12:287–90. [DOI] [PubMed] [Google Scholar]
- 20.Brigham M. Mothers’ reports of the outcome of nipple shield use. J Hum Lact 1996;12:291–7. [DOI] [PubMed] [Google Scholar]
- 21.McKechnie AC, Eglash A. Nipple shields: a review of the literature. Breastfeed Med 2010;5:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leopold NA, Daniels SK. Supranuclear control of swallowing. Dysphagia 2010;25:250–7. [DOI] [PubMed] [Google Scholar]
- 23.Lau C. Oral feeding in the preterm infant. Neoreviews 2006;7:e19–27. [Google Scholar]
- 24.Lau C. Development of oral feeding skills in the preterm infant In: Preedy VR, editor. The handbook of growth and growth monitoring in health and disease. Part 3. New York: Springer; 2012. p. 499–512. [Google Scholar]
- 25.Buchholz DW, Bosma JF, Donner MW. Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol 1985;10:235–9. [DOI] [PubMed] [Google Scholar]
- 26.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr 2003;92:721–7. [PubMed] [Google Scholar]
- 27.Selley WG, Ellis RE, Flack FC, Brooks WA. Coordination of sucking, swallowing and breathing in the newborn: its relationship to infant feeding and normal development. Br J Disord Commun 1990;25:311–27. [DOI] [PubMed] [Google Scholar]
- 28.Omari T, Snel A, Barnett C, Davidson G, Haslam R, Dent J. Measurement of upper esophageal sphincter tone and relaxation during swallowing in premature infants. Am J Physiol 1999;277:G862–6. [DOI] [PubMed] [Google Scholar]
- 29.Dantas RO, Dodds WJ, Massey BT, Shaker R, Cook IJ. Manometric characteristics of glossopalatal sphincter. Dig Dis Sci 1990;35:161–6. [DOI] [PubMed] [Google Scholar]
- 30.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil 2005;17:663–70. [DOI] [PubMed] [Google Scholar]
- 31.Rommel N, van Wijk M, Boets B, Hebbard G, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil 2011;23:e401–8. [DOI] [PubMed] [Google Scholar]
- 32.Berseth CL. Gastrointestinal motility in the neonate. Clin Perinatol 1996;23:179–90. [PubMed] [Google Scholar]
- 33.Gupta A, Gulati P, Kim W, Fernandez S, Shaker R, Jadcherla SR. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol 2009;104:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet—a model in the study of oesophageal development in preterm neonates. Acta Paediatr 2010;99:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr 2003;143:31–8. [DOI] [PubMed] [Google Scholar]
- 36.Omari TI, Miki K, Fraser R, Davidson G, Haslam R, Goldsworthy W, Bakewell M, Kawahara H, Dent J. Esophageal body and lower esophageal sphincter function in healthy premature infants. Gastroenterology 1995;109:1757–64. [DOI] [PubMed] [Google Scholar]
- 37.Omari T. Lower esophageal sphincter function in the neonate. Neoreviews 2006;7:e13–8. [Google Scholar]
- 38.Koenig JS, Davies AM, Thach BT. Coordination of breathing, sucking, and swallowing during bottle feedings in human infants. J Appl Physiol 1990;69:1623–9. [DOI] [PubMed] [Google Scholar]
- 39.Rosen CL, Glaze DG, Frost JD Jr. Hypoxemia associated with feeding in the preterm infant and full-term neonate. Am J Dis Child 1984;138:623–8. [DOI] [PubMed] [Google Scholar]
- 40.Mathew OP, Clark ML, Pronske MH. Apnea, bradycardia, and cyanosis during oral feeding in term neonates [letter]. J Pediatr 1985;106:857. [DOI] [PubMed] [Google Scholar]
- 41.Miller MJ, DiFiore JM. A comparison of swallowing during apnea and periodic breathing in premature infants. Pediatr Res 1995;37:796–9. [DOI] [PubMed] [Google Scholar]
- 42.Nishino T, Yonezawa T, Honda Y. Effects of swallowing on the pattern of continuous respiration in human adults. Am Rev Respir Dis 1985;132:1219–22. [DOI] [PubMed] [Google Scholar]
- 43.Nishino T. The swallowing reflex and its significance as an airway defensive reflex. Front Physiol 2012;3:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fucile S, McFarland DH, Gisel EG, Lau C. Oral and nonoral sensorimotor interventions facilitate suck-swallow-respiration functions and their coordination in preterm infants. Early Hum Dev 2012;88:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau C. Is there an advantage for preterm infants to feed orally in an upright or sidelying position? J Neonatal Nurs 2013;19:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer MM, Crawley K, Blanco IA. Neonatal Oral-Motor Assessment Scale: a reliability study. J Perinatol 1993;13:28–35. [PubMed] [Google Scholar]
- 47.da Costa SP, van der Schans CP. The reliability of the Neonatal Oral-Motor Assessment Scale. Acta Paediatr 2008;97:21–6. [DOI] [PubMed] [Google Scholar]
- 48.Howe TH, Sheu CF, Hsieh YW, Hsieh CL. Psychometric characteristics of the Neonatal Oral-Motor Assessment Scale in healthy preterm infants. Dev Med Child Neurol 2007;49:915–9. [DOI] [PubMed] [Google Scholar]
- 49.Thoyre SM, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw 2005;24:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaker CS, Woida AM. An evidence-based approach to nipple feeding in a level III NICU: nurse autonomy, developmental care, and teamwork. Neonatal Netw 2007;26:77–83. [DOI] [PubMed] [Google Scholar]
- 51.Als H, Gilkerson L, Duffy FH, McAnulty GB, Buehler DM, Vanderberg K, Sweet N, Sell E, Parad RB, Ringer SA, et al. . A three-center, randomized, controlled trial of individualized developmental care for very low birth weight preterm infants: medical, neurodevelopmental, parenting, and caregiving effects. J Dev Behav Pediatr 2003;24:399–408. [DOI] [PubMed] [Google Scholar]
- 52.Premji SS, McNeil DA, Scotland J. Regional neonatal oral feeding protocol: changing the ethos of feeding preterm infants. J Perinat Neonatal Nurs 2004;18:371–84. [DOI] [PubMed] [Google Scholar]
- 53.Pickler RH. A model of feeding readiness for preterm infants. Neonatal Intensive Care 2004;17:31–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Fucile S, Gisel EG, McFarland DH, Lau C. Oral and non-oral sensorimotor interventions enhance oral feeding performance in preterm infants. Dev Med Child Neurol 2011;53:829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau C, Smith EO. A novel approach to assess oral feeding skills of preterm infants. Neonatology 2011;100:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau C, Smith EO. Interventions to improve the oral feeding performance of preterm infants. Acta Paediatr 2012;101:e269–74. [DOI] [PubMed] [Google Scholar]
- 57.Lau C, Fucile S, Schanler RJ. A self-paced oral feeding system that improves preterm infants’ oral feeding skills. J Neonatal Nurs 2015;21:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lau C, Fucile S, Gisel EG. Impact of nonnutritive oral motor stimulation and infant massage therapy on oral feeding skills of preterm infants. J Neonatal Perinatal Med 2012;5:311–7. [Google Scholar]