Abstract
Neonatal dysphagia, or abnormalities of swallowing, represent a major global problem, and consequences of dysfunctional feeding patterns carry over into infancy and toddler age groups. Growth, development, and independent feeding skills are all delayed among high-risk infants. Such a group comprises premature birth, low-birth-weight, congenital anomalies, perinatal asphyxia, postsurgical, and sepsis categories. The conflict between pathophysiologic and pragmatic feeding strategies remains a major conundrum and is largely due to a lack of validated diagnostic approaches amid heterogeneity of the patient phenotype. Thus, well-tested feeding management strategies that can be generalizable are lacking. Furthermore, the aerodigestive symptoms and signs, potential risk factors, and contributory etiologies remain nonspecific. This article presents mechanistic evidence related to the pathophysiologic basis of neonatal dysphagia as well as potential opportunities to improve feeding abilities and long-term development.
Keywords: dysphagia, feeding disorders, neonate, dysphagia, feeding disorders, neonate, aerodigestive reflexes, gastroesophageal reflux
INTRODUCTION
Feeding problems frequently occur in infants and are highly complex in nature. Furthermore, the aerodigestive symptoms related to swallowing can be heterogeneous and nonspecific to either airway or digestive pathologies. Recent advances in technology and available methods are able to clarify inherent disease and pathophysiologic mechanisms of feeding difficulties; as a result, better therapies and monitoring strategies are possible. The approach to the management of neonatal dysphagia is therefore dependent on primary and secondary symptoms, feeding and growth patterns, identifying the systems or target organs of dysfunction, and clinico-pathologic correlation. Such approaches therefore form the basis for individualized therapies. In this article, I discuss the following: 1) prevalence, aerodigestive symptoms, and significance of feeding difficulties; 2) physiologic considerations; 3) pathophysiologic mechanisms as a basis for symptoms; and 4) the approach to the management of neonatal dysphagia.
PREVALENCE, RISK FACTORS, AERODIGESTIVE SYMPTOMS, AND SIGNIFICANCE OF NEONATAL FEEDING DIFFICULTIES
The exact prevalence of neonatal dysphagia or swallowing problems in neonates is not known. The prevalence of feeding problems in premature infants born at <37 wk of gestation is ∼10.5%, and this frequency increases to ∼24.5% among those born with a very low birth weight (<1500 g) (1). On the other hand, among infants born at <28 wk of gestation, oral feeding is significantly delayed and the length of hospitalization is prolonged in comparison to infants >28 wk of gestational age; however, in this study, a large majority of healthy premature infants did indeed achieve oral feeding skills by 36–38 wk of postmenstrual age (2). Neurodevelopmental problems are increasing among high-risk neonatal intensive care unit (NICU)4 graduates; and overall, 20–80% of such infants have feeding concerns during infancy (3, 4). Approximately 26% of prematurely born infants showed dysphagia or its sequelae, and the underlying condition of bronchopulmonary dysplasia was noted in ∼31% of those with persistent feeding difficulties <1 y age (5).
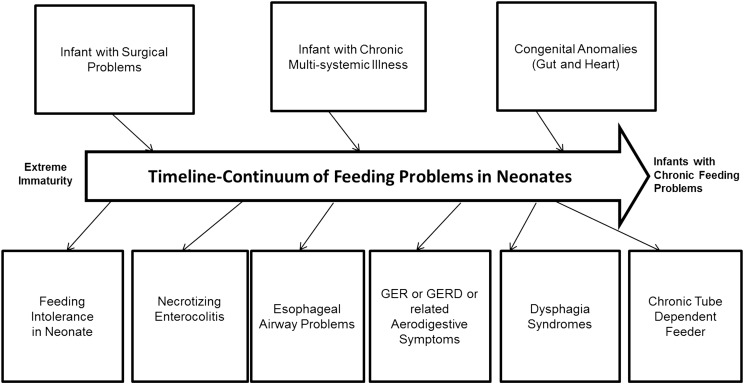
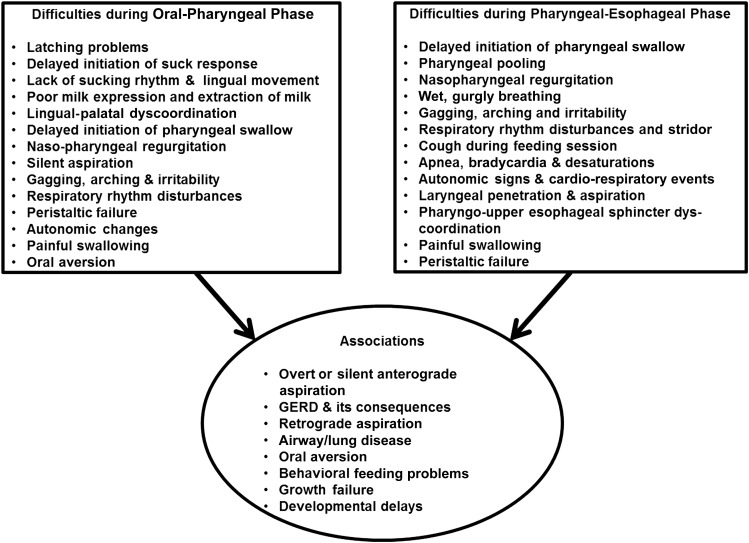
Risk factors for feeding difficulties are several, are related to the underlying primary diagnosis and its sequelae, and are present throughout the continuum of the hospital course (Figure 1). Therefore, the ability to achieve particular feeding milestones such as full enteral feedings, first oral feedings, full oral feedings, and ad libitum oral feedings cannot be met due to the risk factors, variations in ontogeny, or development of new pathology. Hence, the definition or categorization of feeding difficulties or dysphagia in this vulnerable group of infants can be challenging. In this review, I attempted to categorize specific symptoms, signs, and associations of neonatal dysphagia (Figure 2). In most centers, diagnostic and management approaches are often symptom based, and symptoms can be very nonspecific. Attributable dysphagia symptoms are related to prefeeding, feeding, and postprandial periods; thus, the specific diagnosis can be challenging. Although the requirement of safe oral feeding with adequate weight gain is an essential criterion for hospital discharge, these young patients show feeding problems that include feeding-related bradycardia and desaturation, coughing, gagging, arching and irritability, refusal to feed, and inadequate nipple feeding ability associated with failure to thrive (6, 7).
FIGURE 1 .
Risk factors for neonatal feeding difficulties. There are numerous risk factors for neonatal feeding difficulties that are present throughout the continuum of an infant’s hospital course. GER, gastroesophageal reflux; GERD, gastroesophageal reflux disease.
FIGURE 2 .
Symptoms, signs, and associations with neonatal dysphagia. GERD, gastroesophageal reflux disease.
As discussed in this section, abnormalities in oropharyngeal phase or pharyngo-esophageal phases of feeding can delay successful oral feeding. Furthermore, problems with endurance and sensitivity to fluid volume intake due to underlying cardiopulmonary diseases can compromise nutrition, growth, and development. Providers’ multidisciplinary approaches and parental frustration with efforts to feed their infant continue to be a problem in the medically challenged high-risk infant. So as not to compromise on oral feeding safety, gastrostomy tube feedings are often necessary to provide adequate nutrition and maintain growth. The definition of the need and timing for gastrostomy, as well as postgastrostomy rehabilitation to achieve independent oral feeding, remains an unresolved issue. This will undoubtedly escalate health care costs. For example, the approximate health care costs for children with feeding tubes at discharge are $180,000/infant aged >5 y and $46,875 for the first year (8). In infants referred for long-term gastrostomy feeding strategies, with the development and institution of a personalized diagnostic and feeding management program, we were able to avert gastrostomy in 51% of the subjects and achieve independent oral feeding in 84% by their first birthday. The savings in health care costs by avoiding the placement of 51 gastrostomy tubes in this cohort was significant, with an approximate amount of $9.1 million saved over 5 y and $2.1 million over the first year (7). Although feedings (nutrients) are fundamental to overall growth and development, the institution of feeding strategies to attain this goal must go hand-in-hand because feeding targets are constantly changing in the context of variations in the ontogeny of gut and oromotor development, as well as changing patterns of disease in the NICU infant.
PHYSIOLOGIC CONSIDERATIONS
The swallowing function evolves during fetal life as the primitive esophagus transports swallowed amniotic fluid into the stomach. This is called the “primary peristaltic reflex,” generated by coordination with the pharyngeal phase of swallowing, appropriate sequential relaxation of the upper esophageal sphincter (UES), followed by sequential esophageal peristalsis and coordinated relaxation of the lower esophageal sphincter (LES). At the end of this sequence, the integrity of the UES and LES is maintained with adequate resting tone (9–12). The normal neonatal swallowing mechanism is characterized by 3 phases: the oral, pharyngeal, and esophageal phases (13). The oral phase is characterized by the preparation of the bolus with salivary enzymatic action, extraction of the bolus, lingual-palatal coordination, and airway protection via the epiglottis. The pharyngeal phase is characterized by the propulsion of the bolus and airway protection, whereas the main feature of a normal esophageal phase is peristalsis and airway protection. Thus, the regulating neuromotor and neurosensory factors that target safe bolus transit from oromotor apparatus to pharynx to esophagus to stomach, and yet prevent the occurrence of aspiration and gastroesophageal reflux (GER) during the feeding cycle, are important. In that regard, the most important function of the esophagus is to transport swallowed material to the downstream digestive and absorptive organs of the gastrointestinal tract.
Esophageal peristalsis can be classified into primary and secondary peristalsis. Primary peristalsis is triggered in the pharyngeal phase of swallowing and propagates distally into the stomach and is associated with a respiratory pause called “deglutition apnea.” Secondary peristalsis is a swallow-independent sequence and is triggered by esophageal provocation. This reflex can occur due to esophageal distention, chemosensitive stimulation, or osmosensitive stimulation of the esophagus (14–16). Combined, these esophageal peristaltic functions participate in the propulsion of a bolus presented during feeding and swallowing, and during GER events.
An equally important supportive function of the esophagus is its aerodigestive protective function. This function is responsible for vigilance, coordination, and antireflux defenses. The refluxed material during GER events can be injurious to the esophagus and the aerodigestive tract. The properties of the refluxate or of its presence in the esophagus contribute to the injury potential; this property can be related to acidity or alkalinity, enzymatic content, infective nature, or the physical composition of the refluxate. Often, the acidity is balanced by the esophageal mucosal defenses. Mucosal HCO3−, which neutralizes the acidic reflux, is also a key element in preventing epithelial damage (17). GER events increase the production of saliva and primary peristalsis and therefore the further clearance of the refluxate.
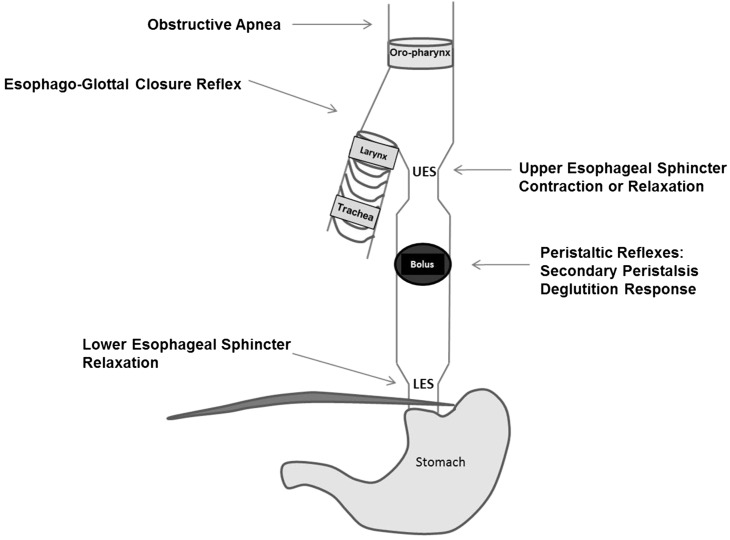
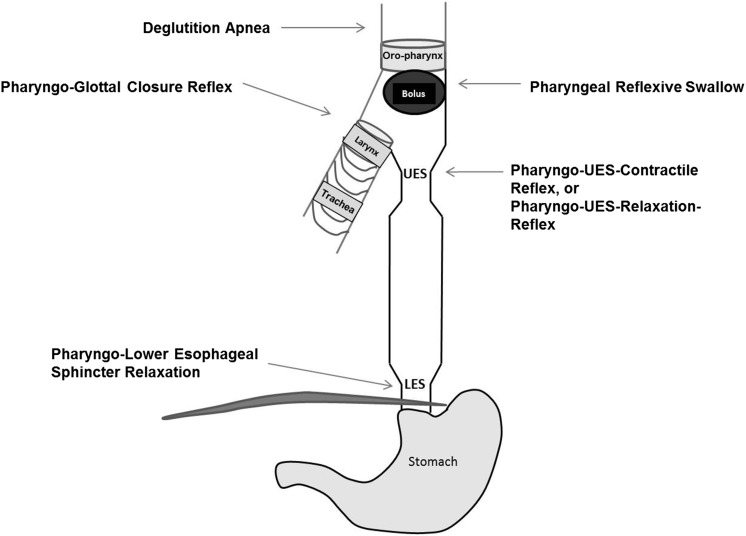
Aerodigestive protection is ensured by several cumulative reflexes as summarized in Figures 3 and 4. The esophagus and airways share similar innervation by the vagus nerve, and the interaction of afferent and efferent neuronal pathways modulate sensory-motor function to ensure safe swallowing and airway protection (18). The following esophageal reflexes are determined to be of interest in ensuring aerodigestive protection in infants: esophago-deglutition reflex, secondary peristaltic reflexes, UES contractile reflex, LES relaxation reflex, and pharyngeal reflexive swallows, in addition to airway-protective pharyngo-glottal closure reflex and esophago-glottal closure reflex (11, 14–16). Collectively, these reflexes prevent the ascending spread of the bolus, favor descending propulsion to ensure esophageal clearance, and enhance aerodigestive vigilance. As part of aerodigestive protective function, the prevention of pulmonary aspiration is an important function of the esophagus. Pulmonary aspiration is a potential cause of mortality and/or morbidity in high-risk infants. Laryngeal adduction in response to pharyngeal or esophageal stimulation results in closure of the glottis and protects against airway aspiration; these reflexes are called the pharyngo-glottal closure reflex and esophago-glottal closure reflex (16, 19). These vigilant reflexes are usually associated with pharyngeal reflexive swallows and deglutition response or secondary peristalsis. Glottal closure accentuates the aspiration-preventing barrier, whereas esophageal peristalsis clears the bolus or perceived stimulus.
FIGURE 3 .
Schematic representation of aerodigestive reflexes evoked on esophageal provocation. An esophageal bolus activates proximal aerodigestive reflexes (e.g., UES contraction or UES relaxation, obstructive apnea, or glottal closure). Similarly, downstream responses or UES relaxation and peristaltic reflex responses are also operational. LES, lower esophageal sphincter; UES, upper esophageal sphincter.
FIGURE 4 .
Schematic representation of aerodigestive reflexes evoked on pharyngeal stimulation. A pharyngeal bolus activates pharyngeal reflexive swallow, PUCR or PURR, deglutition apnea, and pharyngo-glottal closure reflexes. Downstream responses include LES relaxation. LES, lower esophageal sphincter; PUCR, pharyngo-UES contractile reflex; PURR, pharyngo-UES relaxation reflex; UES, upper esophageal sphincter.
Although pharyngeal and esophageal phases of swallowing are important for safe bolus transport, aerodigestive health, and successful feeding, the oral phase is equally important to achieve independent feeding skills. The development of normal oromotor function during perinatal development is essential for normal swallow to occur. Among the oromotor activities that develop during perinatal development are the following: nonnutritive sucking to promote physiologic stability (20), sensory-motor oral stimulation to promote early oral feeding (21), oromotor interventions to enhance feeding and swallowing (22), early oral stimulation and rhythmic alternation of suction to accelerate transition from tube to oral feeding (23) or therapies to entrain neural pathways for integrative biorhythms (24), and coordination of swallowing and adaption of respiration with glottal protective reflexes during maturation (25).
Thus, the development of functional swallowing and aerodigestive protection is a process in a continuum that is modified over time. The factors that modify these functions involve multiple afferents and efferents and feedback systems including, but not limited to, the brain, airway and respiratory system, and the foregut.
PATHOPHYSIOLOGIC MECHANISMS OF DYSPHAGIA SYMPTOMS AND ASPIRATION
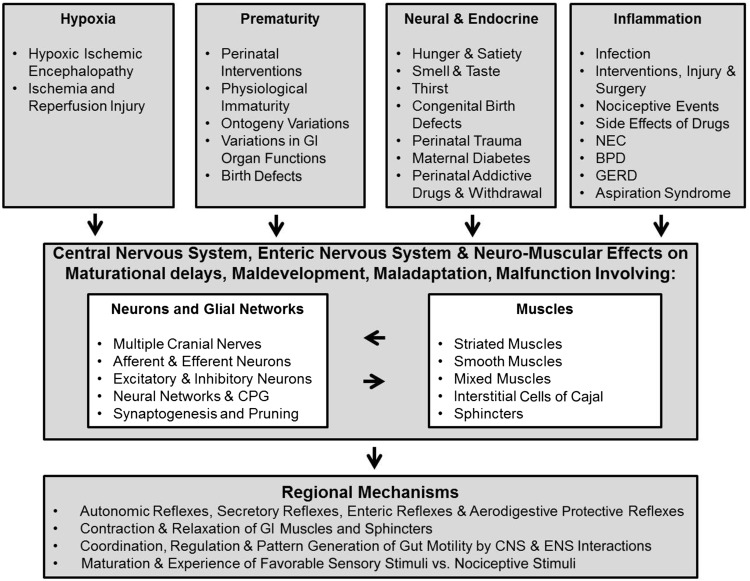
Understanding the physiology, maturation, and regulation of neonatal swallowing and the ontogeny of aerodigestive protection will provide a background into differentiating the physiology from the pathology of neonatal swallowing. The vagal neural pathways are involved in regulating swallowing and aerodigestive functions either directly or indirectly by influencing the supranuclear regulatory centers, basal ganglia, cerebrum, and cerebellum. As summarized in Figure 5, these neurosensory and neuromotor aerodigestive pathways are influenced by pathologies during perinatal events, prematurity, inflammatory states, and coexisting medical and surgical conditions (9, 10, 26, 27).
FIGURE 5 .
Contributory factors and potential central and regional mechanisms associated with neonatal dysphagia. BPD, bronchopulmonary dysplasia; CPG, central pattern generator; CNS, central nervous system; ENS, enteric nervous system; GERD, gastroesophageal reflux disease; GI, gastrointestinal; NEC, necrotizing enterocolitis.
Swallowing also involves integratory functions of multiple tissues, such as neural (involving multiple cranial nerves, afferent and efferent neurons, excitatory and inhibitory neurons, neural networks, central pattern generator and various neuronal synapses), which are directly involved in coordinating the muscle tissues (smooth and striated muscles and sphincters). In addition, other influences on the neural and muscle pathways, such as the neuroendocrine (e.g., hunger and satiety, smell and taste, and thirst) or inflammatory (e.g., infection, injury, nociceptive, and drug action) nature contribute to the functional or dysfunctional mechanisms of swallowing.
Some of the abnormalities (Figure 2) and mechanisms (Figure 5) can be due to oromotor inertia and oral pooling, delayed initiation of the pharyngeal phase, cardiorespiratory events, penetration, aspiration and airway symptoms, GER disease (GERD), and oral aversion. These abnormal manifestations lead to neonatal swallowing problems in the oropharyngeal phase and the pharyngo-esophageal phase. The presenting symptoms and signs observed in the oropharyngeal phase include latching problems, delay in suck, lack of rhythm and lingual movement, poor extraction of bolus, nasopharyngeal regurgitation, delayed initiation of pharyngeal swallow, silent aspiration, peristaltic failure, gagging, arching, and irritability. The symptoms and signs observed in the pharyngo-esophageal phase include pharyngeal pooling, wet gurgly breathing, cough with feedings, stridor, nasopharyngeal regurgitation, delayed pharyngeal phase, pharyngo-UES dyscoordination, laryngeal penetration, laryngeal aspiration, apnea, bradycardia and desaturations, and cardiorespiratory events. The symptoms in both of these phases could cause overt or silent anterograde aspiration and airway/lung disease or GERD and retrograde aspiration and airway/lung disease as well as oral aversion and behavioral feeding problems. Understanding the mechanisms for the genesis of symptoms is critical to the development of innovative therapies for dysphagia.
As an example, the mechanistic basis and management of dysphagia with aspiration diagnosed during fluoroscopy studies are highlighted in the following sections. Aspiration occurs when a bolus passes through the true vocal folds, thus breaching airway protection usually provided by normally functioning true vocal folds. The 3 major types of aspiration are anterograde (characterized by pre-, intra-, and postdeglutitive), retrograde (as in GER events), and silent aspiration (without any symptoms). The incidence of aspiration ranges from 25% to 73% for infants with swallowing dysfunction (5); ∼85% (n = 125) of children exhibiting deep laryngeal penetration eventually aspirated, and aspiration occurred 15 s after laryngeal penetration (28, 29). Video-manometry results in 8 dysphagic children (age 2–28 mo) differed from adult swallowing in terms of epiglottic movement, tongue driving force, amplitude of pharyngeal contraction, and UES pressure. There is, however, comparable pharyngeal shortening and pharyngeal wall movement (30).
Aspiration can occur in small quantities in adults, and if it is cleared by the body’s defense mechanism there is low risk for any serious harm. Fifty percent of normal subjects aspirate small volumes (0.01–0.2 mL) during sleep (31). Approximately 45% of normal patients had detectable aspiration during sleep, and those sleeping more soundly were found to be at greater risk (32). Neurologically normal subjects also aspirate (33). However, the amounts aspirated by neonates and infants are not known.
In a recent study in NICU neonates with abnormal videofluoroscopy (n = 20; gestational age: 30.9 ± 4.9 wk), 30% had nasopharyngeal reflux, 35% experienced pooling, 35% had delayed swallow, 55% had aspiration, and 90% experienced laryngeal penetration; the video fluoroscopy characteristics were similar between patients with feeding success (n = 15) and those with feeding failure (n = 5) (34). Thus, the videofluoroscopic markers of aspiration are not very specific. Hence, we recommend a personalized multidisciplinary pathophysiology-guided approach to improve neonatal feeding outcomes (7).
INVESTIGATIVE APPROACH TO EVALUATE NEONATAL DYSPHAGIA
A detailed history and clinical assessment must be made to define predisposing risk factors and anatomic defects and to recognize alternate diagnoses for feeding problems before attributing them to oro-pharyngo-esophageal pathologies in infants. Such an examination involves careful assessment of the structural integrity of the aerodigestive apparatus, functions of the cranial nerves (V, VII, IX, X, XI, XII), and airway-breathing status. Prenatal and antecedent factors likely involved with dysphagia need to be explored. Predisposing risk factors and alternate diagnoses must be excluded (35). Risk factors for feeding problems, dysphagia, or GERD include anatomic malformations and congenital foregut anomalies, such as craniofacial birth defects, pharyngeal clefts and webs, esophageal atresia or tracheo-esophageal fistula, omphalocele, gastroschisis, duodenal atresia or web, hiatus hernia, diaphragmatic hernia, intestinal malrotation, and hypertrophic pyloric stenosis. Feeding problems of esophageal origin persist despite restoration or modification of the primary structural abnormality. Reasons for swallowing or esophageal problems may include mechanical or functional obstruction, dysmotility, stasis and delayed peristalsis, or GERD.
In addition, nonstructural causes and nonesophageal causes of dysfunction need to be considered. External compression on the esophagus due to pressure from the trachea or left bronchus, left atrial enlargement, or post–cardiothoracic surgery consequences need to be considered on a case-by-case basis. In addition to sepsis and metabolic diseases, structural abnormalities of the brain also need be ruled out in cases of persistent feeding problems of oro-pharyngo-esophageal origin. Of importance, eosinophilic esophagitis is increasingly recognized in older infants and children (36).
Diagnostic methods to evaluate dysphagia or of its mechanisms are not well studied and are not widely available. Swallowing disorders require diverse approaches and strategies in diagnosing and correcting the problems. For example, esophageal pH-multichannel intraluminal impedance monitoring, manometry, upper gastrointestinal fluoroscopy, and videofluoroscopic swallow studies can be helpful in characterizing the pharyngo-esophageal structural and functional pathologies objectively. Each of these tests has its own merits and demerits. The advantages, limitations, and clinical relevance of these techniques are summarized in Table 1.
TABLE 1.
Studies to evaluate neonatal dysphagia mechanisms1
| Test | Advantages | Disadvantages |
| Videofluoroscopic swallow study | Evaluates anatomic abnormalities in the upper aerodigestive tract, a test for detecting bolus movement during swallowing | Involves radiation exposure |
| Variability with diagnosis, interpretation of findings and recommendations | ||
| Upper gastrointestinal fluoroscopy study | Evaluates anatomic abnormalities in the upper gastrointestinal tract | Inadequate to screen for GER events |
| Involves radiation exposure | ||
| Distal esophageal pH monitoring | Smaller probe size Ambulatory | Feedings alter pH; nonacid GER and total GER are not measured |
| Automated analysis available | Cannot detect most proximal extent | |
| User-friendly | ||
| pH-Multichannel intraluminal impedance | Ambulatory, detects differentiates liquid, mixed, and gas GER events | No quantitative data regarding volume or pressure or mechanisms |
| Detects acid and nonacid GER | Analysis cumbersome, semiautomated, and labor intensive | |
| Detects frequency, height, and duration of reflux events, regardless of pH | ||
| Basal and adaptive esophageal manometry | Provides mechanistic sensory-motor evaluation of esophageal peristaltic reflexes in response to provocation | Not commonly available |
| Clinical correlation is needed | ||
| Lacks sensitivity/specificity |
GER, gastroesophageal reflux.
CONCLUSIONS
It is important to understand that there is no single symptom or sign that is specific to esophageal pathologies, and there is no single test to provide a definitive diagnosis of esophageal dysfunction. Therefore, clinicians need to cautiously weigh the benefits and weaknesses of different technologies and methods, the scientific appropriateness of the testing conditions, and clinico-pathologic correlation. When evaluating esophageal functions, it is important to assess structural, functional, and protective functions. Practice of consensus guidelines on GERD can be helpful (37). Coordinated interdisciplinary feeding management strategies are essential because the problems pertinent to the esophageal domain are managed by several disciplines, such as nutrition, speech-language pathology, occupational therapy, neonatology and general pediatrics, pediatric gastroenterology, pediatric surgery, oto-rhino-laryngology, radiology, pediatric pulmonology, and primary care.
Acknowledgments
I thank Dhevamaalini Murugham for assistance with figures and art work and Tanvi Khot for assistance with manuscript editing and the submission process.
The sole author had responsibility for all parts of the manuscript and had no conflicts of interest to disclose.
Footnotes
Abbreviations used: GER, gastroesophageal reflux; GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter; NICU, neonatal intensive care unit; UES, upper esophageal sphincter.
REFERENCES
- 1.Motion S, Northstone K, Emond A. Persistent early feeding difficulties and subsequent growth and developmental outcomes. Ambulatory Child Health 2001;7(3/4):231–7. [Google Scholar]
- 2.Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and co-morbidities on feeding milestones in neonates: a retrospective study. J Perinatol 2010;30:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field D, Garland M, Williams K. Correlates of specific childhood feeding problems. J Paediatr Child Health 2003;39:299–304. [DOI] [PubMed] [Google Scholar]
- 4.Rommel N, De Meyer AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr 2003;37:75–84. [DOI] [PubMed] [Google Scholar]
- 5.Mercado-Deane MG, Burton EM, Harlow SA, Glover AS, Deane DA, Guill MF, Hudson V. Swallowing dysfunction in infants less than 1 year of age. Pediatr Radiol 2001;31:423–8. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics 2008;122:1119–26. [DOI] [PubMed] [Google Scholar]
- 7.Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandex S, Erdman SH, DiLorenzo C. Impact of personalized feeding program in 100 NICU infants: pathophysiology-based approach for better outcomes. J Pediatr Gastroenterol Nutr 2012;54:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piazza CCC-HT. Assessment and treatment of pediatric feeding disorders. Montreal (Canada): Centre of Excellence for Early Childhood Development; 2004. [Google Scholar]
- 9.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil 2005;17:663–70. [DOI] [PubMed] [Google Scholar]
- 10.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 1997;336:924–32. [DOI] [PubMed] [Google Scholar]
- 11.Pena EM, Parks VN, Peng J, Fernandex SA, DiLorenso C, Shaker R, Jadcherla SR. Lower esophageal sphincter relaxation reflex kinetics: effects of peristaltic reflexes and maturation in human premature neonates. Am J Physiol Gastrointest Liver Physiol 2010;299:G1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castell D, Ritcher J. The esophagus. 4th ed. Philadelphia: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 13.Jadcherla SR, Shaker R. Physiology of aerodigestive reflexes in neonates and adultsIn: Johnson L, editor. Physiology of the gastrointestinal tract. 5th ed. London: Elsevier; 2012. [Google Scholar]
- 14.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr 2003;143:31–8. [DOI] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr 2006;149:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Gulati P, Kim W, Fernandez S, Shaker R, Jadcherla SR. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol 2009;104:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemström G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 2001;16:23–8. [DOI] [PubMed] [Google Scholar]
- 18.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med 2001;111(Suppl 8A):95S–105S. [DOI] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol 2007;102:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005;4:CD001071. [DOI] [PubMed] [Google Scholar]
- 21.Rocha AD, Moreira ME, Pimenta HP, Ramos JR, Lucena SL. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birthweight infants. Early Hum Dev 2007;83:385–8. [DOI] [PubMed] [Google Scholar]
- 22.Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. Evidence-based systematic review: effects of oral motor interventions on feeding and swallowing in preterm infants. Am J Speech Lang Pathol 2010;19:321–40. [DOI] [PubMed] [Google Scholar]
- 23.Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. J Pediatr 2002;141:230–6. [DOI] [PubMed] [Google Scholar]
- 24.Barlow SM, Finan DS, Lee J, Chu S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatol 2008;28:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol 2009;104:2572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil 2011;23:e401–8. [DOI] [PubMed] [Google Scholar]
- 27.Omari TI, Miki K, Fraser R, Davidson G, Haslam R, Goldsworthy W, Bakewell M, Kawahara H, Dent J. Esophageal body and lower esophageal sphincter function in healthy premature infants. Gastroenterology 1995;109(6):1757–64. [DOI] [PubMed] [Google Scholar]
- 28.Friedman B, Frazier JB. Deep laryngeal penetration as a predictor of aspiration. Dysphagia 2000;15:153–8. [DOI] [PubMed] [Google Scholar]
- 29.Newman LA, Keckley C, Petersen MC, Hamner A. Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics 2001;108:E106. [DOI] [PubMed] [Google Scholar]
- 30.Rommel N, Dejaeger E, Bellon E, Smet M, Veereman-Wauters G. Videomanometry reveals clinically relevant parameters of swallowing in children. Int J Pediatr Otorhinolaryngol 2006;70:1397–405. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest 1997;111:1266–72. [DOI] [PubMed] [Google Scholar]
- 32.Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med 1978;64:564–8. [DOI] [PubMed] [Google Scholar]
- 33.Weir KA, McMahon S, Taylor S, Chang AB. Oropharyngeal aspiration and silent aspiration in children. Chest 2011;140:589–97. [DOI] [PubMed] [Google Scholar]
- 34.Jadcherla SR, Stoner E, Gupta A, Bates DG, Fernandex S, DiLorenzo C, Linscheid T. Evaluation and management of neonatal dysphagia: impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. J Pediatr Gastroenterol Nutr 2009;48:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolph CD, Mazur LJ, Laptak GS. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society of Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 2001;32:S1–31. [DOI] [PubMed] [Google Scholar]
- 36.Hommel KA, Franciosi JP, Gray WN, Hente EA, Ahrens A, Rothenberg ME. Behavioral functioning and treatment adherence in pediatric eosinophilic gastrointestinal disorders. Pediatr Allergy Immunol 2012;5:494–9. [DOI] [PubMed] [Google Scholar]
- 37.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A, Thomson M, Veereman-Wauters G, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 2009;49:498–547. [DOI] [PubMed] [Google Scholar]