Abstract
Background: Physical inactivity triggers a rapid loss of muscle mass and function in older adults. Middle-aged adults show few phenotypic signs of aging yet may be more susceptible to inactivity than younger adults.
Objective: The aim was to determine whether leucine, a stimulator of translation initiation and skeletal muscle protein synthesis (MPS), can protect skeletal muscle health during bed rest.
Design: We used a randomized, double-blind, placebo-controlled trial to assess changes in skeletal MPS, cellular signaling, body composition, and skeletal muscle function in middle-aged adults (n = 19; age ± SEM: 52 ± 1 y) in response to leucine supplementation (LEU group: 0.06 g ∙ kg−1 ∙ meal−1) or an alanine control (CON group) during 14 d of bed rest.
Results: Bed rest decreased postabsorptive MPS by 30% ± 9% (CON group) and by 10% ± 10% (LEU group) (main effect for time, P < 0.05), but no differences between groups with respect to pre-post changes (group × time interactions) were detected for MPS or cell signaling. Leucine protected knee extensor peak torque (CON compared with LEU group: −15% ± 2% and −7% ± 3%; group × time interaction, P < 0.05) and endurance (CON compared with LEU: −14% ± 3% and −2% ± 4%; group × time interaction, P < 0.05), prevented an increase in body fat percentage (group × time interaction, P < 0.05), and reduced whole-body lean mass loss after 7 d (CON compared with LEU: −1.5 ± 0.3 and −0.8 ± 0.3 kg; group × time interaction, P < 0.05) but not 14 d (CON compared with LEU: −1.5 ± 0.3 and −1.0 ± 0.3 kg) of bed rest. Leucine also maintained muscle quality (peak torque/kg leg lean mass) after 14 d of bed-rest inactivity (CON compared with LEU: −9% ± 2% and +1% ± 3%; group × time interaction, P < 0.05).
Conclusions: Bed rest has a profoundly negative effect on muscle metabolism, mass, and function in middle-aged adults. Leucine supplementation may partially protect muscle health during relatively brief periods of physical inactivity. This trial was registered at clinicaltrials.gov as NCT00968344.
Keywords: skeletal muscle protein synthesis, physical inactivity, atrophy, dietary supplementation, nutrition
INTRODUCTION
The negative consequences of physical inactivity on skeletal muscle health have been well documented (1–4). Although young adults are not immune to an inactivity-induced loss of muscle mass and function, bed rest accelerates the rate of loss in older populations (2, 5, 6). A reduction in postabsorptive and/or postprandial muscle protein synthesis (MPS)9 appears to drive the loss of muscle mass and function in unloading studies (2, 7–10). Although skeletal muscle protein breakdown appears not to be altered by bed rest in young, healthy research volunteers (5, 7, 11), it may be transiently elevated during the first several days of disuse or play an increasing role in aging populations, different models of disuse, and clinical environments or when additional catabolic stimuli are present (12–17).
We proposed that adequate nutritional support represents the prerequisite framework to protect muscle health during periods of physical inactivity (18). Exercise may act synergistically with nutrition to protect muscle health during bed rest or disuse (19–21). However, obstacles such as weakness, fatigue, injury, and disease limit its utility in some circumstances (22).
Dietary interventions that include supplements should not be unduly burdensome, provide excessive energy, or compromise the intake of regular meals and macronutrients (23). In young and older adults, the ingestion of as little as 2–3 g leucine as part of a mixed amino acid bolus, was shown to acutely stimulate MPS via the phosphorylation of mammalian target of rapamycin (mTOR) and its downstream targets, p70 S6 kinase 1 (S6K1) and 4E binding protein 1 (4E-BP1) (24–26). These acute stimulatory effects appear to be maintained for at least 2 wk in healthy ambulatory adults receiving leucine-supplemented mixed meals (27). However, the ability of chronic leucine supplementation to improve skeletal muscle mass and function in ambulatory, well-nourished adults is doubtful (28, 29).
Traditionally, muscle metabolism research has discretely targeted young (18–40 y) and/or older (≥65 y) adults (30–33). Middle-aged adults are a largely unexamined research demographic. Despite maintaining a generally youthful phenotype, middle-aged adults may exhibit subtle behavioral and physiologic changes that preempt the onset of sarcopenia and increase vulnerability to catabolic stressors such as bed rest (18, 34). We hypothesized that leucine supplementation would preserve muscle anabolism and protect common indexes of skeletal muscle health during 14 d of bed rest in healthy middle-aged adults.
METHODS
Subjects
Healthy community-dwelling men and women aged 45–60 y old participated in this randomized, double-blind, placebo-controlled study. Volunteers were recreationally active but athletically untrained (Table 1). All of the study protocols and procedures were conducted in accordance with the Declaration of Helsinki and were reviewed and approved by the University of Texas Medical Branch’s Institutional Review Board. After providing written informed consent, volunteers were screened in the University of Texas Medical Branch’s Institute for Translational Sciences–Clinical Research Center via a rigorous battery of medical tests and interviews (5, 8, 35). Subjects were randomly assigned to an experimental group who received leucine (LEU group: 0.06 g ∙ kg−1 ∙ meal−1) or to the control condition who received an isonitrogenous alanine supplement (CON group: 0.06 g ∙ kg−1 ∙ meal−1). On the basis of our previous bed-rest studies, we calculated that a sample size of n = 9/group would have >0.90 power to detect a post–bed-rest difference in means between groups for our primary metabolic outcome, fractional synthesis rate (FSR) of MPS of 0.025%/h, with an SD of 0.015%/h at the 0.05 level. The general experimental design is depicted in Figure 1.
TABLE 1.
Baseline subject characteristics1
| CON (n = 9) | LEU (n = 10) | |
| Age, y | 52 ± 1 | 51 ± 1 |
| Sex, n/n | 3 F/6 M | 4 F/6 M |
| Body mass, kg | 75.7 ± 3.9 | 73.1 ± 3.7 |
| Height, cm | 175 ± 3 | 173 ± 4 |
| BMI, kg/m2 | 24.7 ± 1.4 | 24.6 ± 0.9 |
Values are means ± SEMs. CON, control group; LEU, leucine-supplemented group.
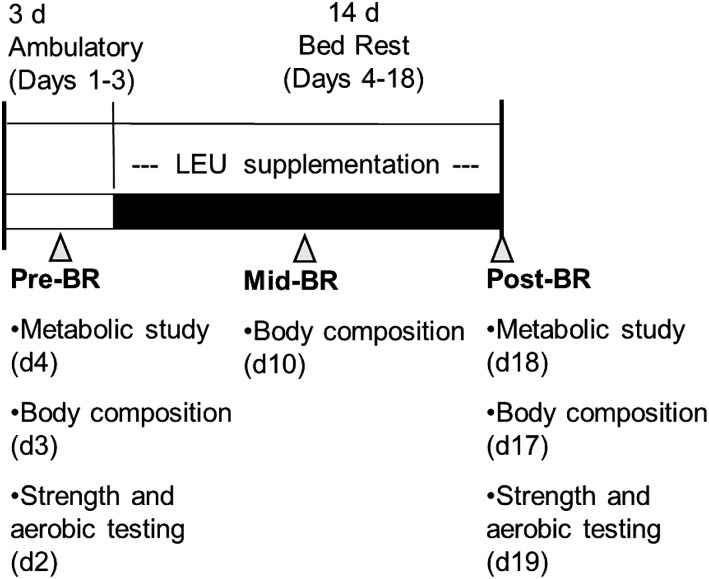
FIGURE 1.
Study timeline. The pre–bed-rest phase consisted of a 3-d inpatient stay during which subjects completed baseline testing of dependent measures and consumed a controlled diet. During the 14-d bed-rest phase, subjects continued to consume the research diet in addition to either a leucine (experimental) or alanine (control) supplement with each of the 3 daily meals. Dependent measures were reassessed post–bed rest; body composition was also measured after 7 d of bed rest. Control group, n = 9; leucine-supplemented group, n = 10. BR, bed rest; LEU, leucine.
Bed rest
The horizontal bed-rest model, 24 h/d subject monitoring, safety, and comfort provisions were consistent with our previous studies (5, 8, 35). All bathing and toiletry activities were performed without bearing weight. To facilitate eating, bed backs were raised to 5° during three 2-h periods each day, which corresponded to daily meals.
Diet and supplementation
Subjects received controlled isoenergetic diets (55% carbohydrate, 30% fat, and 15% protein) with protein intake evenly distributed across 3 daily meals (0800, 1300, and 1800); snacking was not permitted. Daily energy requirements were estimated using the Harris-Benedict equation. Activity factors of 1.6 and 1.3 were used during the ambulatory and bed-rest period, respectively (5, 8, 35). Powdered l-leucine or l-alanine (0.06 g ∙ kg−1 ∙ meal−1; Sigma-Aldrich) was mixed with juice or milk and consumed with each meal during the bed-rest phase of the study. Leucine and alanine supplements were not provided during the initial 3-d ambulatory period or the night before or during metabolic studies. Water was provided ad libitum. Macronutrient intake and plate waste were analyzed by using Nutrition Data System for Research software (version 2006), developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota.
Metabolic studies
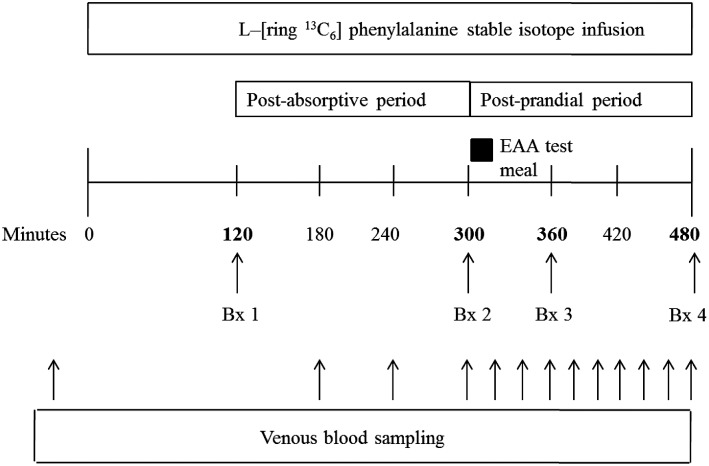
At 0700 on days 4 and 18, after an overnight fast, an 18-gauge polyethylene catheter (Insyte-W; BD Biosciences) was inserted into an antecubital vein. Baseline blood samples were drawn for analysis of phenylalanine enrichment. A second 18-gauge polyethylene catheter was placed in the contralateral antecubital vein and used to maintain a primed (2 μmol/kg), continuous infusion (0.06 μmol ∙ kg−1 ∙ min−1) of l-[ring-13C6]phenylalanine (Cambridge Isotope Laboratories) throughout the study (Figure 2). Muscle biopsy samples were obtained from the vastus lateralis muscle by using a 5-mm Bergstrom biopsy needle and standard technique (36). A standardized essential amino acid “research meal” was consumed in beverage form immediately after the second biopsy. The research meal was not representative of the meals consumed during the general bed-rest period but rather was intended to provide a reproducible anabolic stimulus during the stable isotope studies. The research meal contained 1.2 g histidine, 1.0 g isoleucine, 2.5 g leucine, 2.5 g lysine, 0.8 g methionine, 1.0 g phenylalanine, 1.2 g threonine, and 1.5 g valine and 0.1 g l-[ring-13C6]phenylalanine to maintain plasma phenylalanine enrichment.
FIGURE 2.
Metabolic study timeline. Postabsorptive and postprandial cell signaling were determined from biopsy samples 1 and 3, respectively. Postabsorptive FSR was calculated by using biopsy samples 1 and 2; FSR in the postprandial state was determined by using biopsy samples 2 and 4. Control group, n = 9; leucine-supplemented group, n = 10. Bx, muscle biopsy; EAA, essential amino acid; FSR, fractional synthesis rate.
Cell signaling and immunoblotting
Muscle tissue from biopsy 1 (postabsorptive) and biopsy 3 (1 h postprandial) was used to assess changes in cell signaling, as previously described (37). Briefly, frozen muscle tissue was homogenized and total protein content was assayed. Fifty micrograms of total protein was loaded in duplicate along with an internal loading control and separated on either a 7.5% or 15% polyacrylamide gel by electrophoresis (Criterion; Bio-Rad) at 150 V for 60 min. After separation, proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad) at 50 V for 60 min and then blocked in 5% nonfat dry milk. After an overnight incubation with the primary antibody at 4°C, the membranes (blots) were incubated with secondary antibody for 60 min at room temperature. The primary antibodies used were all purchased from Cell Signaling: total and phospho-mTOR (Ser2448; 1:1000), total and phospho-p70 S6K1 (Thr389; 1:250), and total and phospho-4E-BP1 (Thr37/46; 1:1000). Anti-rabbit IgG horseradish peroxidase–conjugated secondary antibody was purchased from Amersham Bioscience (1:2000). After secondary incubation, the blots were washed and exposed to a chemiluminescence reagent (ECL plus Western Blotting Detection System; Amersham Biosciences). Optical density measurements were made with a ChemiDoc XRS imaging system (Bio-Rad); densitometric analysis was performed by using Quantity One 1-D analysis software (version 4.5.2; Bio-Rad). The activity of each protein was expressed as phosphorylated/total, and fold change was calculated as postprandial activation/postabsorptive activation.
Skeletal MPS
Venous blood samples were immediately mixed and precipitated in tubes containing 1 mL sulfosalicylic acid solution. Samples were centrifuged for 20 min (3000 rpm and 4° C), and the supernatant was removed and frozen (−80°C) until analysis. After thawing, blood amino acids were extracted from 500 μL supernatant by cation exchange chromatography (Dowex AG 50W-8X, 100–200 mesh H+ form; Bio-Rad Laboratories). Phenylalanine enrichments were determined on the tert-butyldimethylsilyl derivative by using gas chromatography–mass spectrometry (HP model 5973; Hewlett-Packard) with electron impact ionization. Ions 336 and 342 were monitored. Muscle biopsy samples were immediately rinsed in ice-cold saline, blotted, and frozen in liquid nitrogen until analysis. Frozen samples were cut on dry ice (∼25 mg) and weighed, and protein was precipitated with 800 μL 10% perchloric acid. Approximately 1.5 mL supernatant was collected after tissue homogenization and centrifugation and processed in the same manner as the supernatant from the blood samples (Dowex AG 50W-8X, 200–400 mesh H+ form). Intracellular phenylalanine enrichments were determined by using the tert-butyldimethylsilyl derivative. The remaining muscle pellet was washed and dried, and the proteins were hydrolyzed in 3 mL of 6 N HCl at 110°C for 24 h. The protein-bound l-[ring-13C6]phenylalanine enrichments were determined by using gas chromatography–mass spectrometry with electron impact ionization. Ions 238 and 240 were monitored for bound protein enrichments; ions 336 and 342 were monitored for intracellular enrichments.
Postabsorptive and postprandial mixed muscle protein FSRs (%/h) were calculated by measuring the direct incorporation of l-[ring-13C6]phenylalanine into protein by using the precursor-product model (5, 8, 35, 38, 39) as follows:
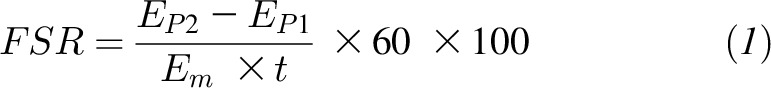 |
where EP1 and EP2 are the bound enrichments of l-[ring-13C6]phenylalanine for 2 muscle biopsies, Em is the mean enrichment of l-[ring-13C6]phenylalanine in the muscle intracellular pool, and t represents the time interval (min) between the 2 biopsies (e.g., 180 min).
Body composition
Whole-body lean mass (WBLM), leg lean mass (LLM), whole-body fat mass, and body fat percentage were determined by dual-energy X-ray absorptiometry on days 3, 10, and 17 (Lunar iDXA; GE Medical Systems). To standardize and minimize the effects of fluid shifts, subjects were required to lie supine for 10 min before scanning.
Muscle function
Unilateral knee and ankle extensor strength [peak torque; Newton-meter (Nm)] and knee muscle endurance (total work) were assessed using isokinetic dynamometry on days 2 and 19 (Biodex System 4; Biodex Medical Systems). Familiarization sessions were conducted on admission (day 1). Peak torque was assessed via 5 maximal repetitions at 60°/s (knee and ankle) and 180°/s (knee only), whereas knee total work/muscular endurance was assessed using 20 repetitions at 180°/s. An estimate of muscle quality was determined by dividing right knee extensor peak torque by right LLM.
Peak aerobic capacity
Peak oxygen uptake (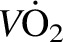 peak) was assessed with a metabolic cart (VMax Encore 29; Care Fusion) using a graded exercise test on a cycle ergometer on days 2 and 19 (Monark Ergomedic 828E; Monark Exercise). Data were expressed in absolute (L/min) and relative terms (mL ∙ kg body mass−1 ∙ min−1 and mL ∙ kg lean mass−1 ∙ min−1) to account for potential changes in body composition during bed rest.
peak) was assessed with a metabolic cart (VMax Encore 29; Care Fusion) using a graded exercise test on a cycle ergometer on days 2 and 19 (Monark Ergomedic 828E; Monark Exercise). Data were expressed in absolute (L/min) and relative terms (mL ∙ kg body mass−1 ∙ min−1 and mL ∙ kg lean mass−1 ∙ min−1) to account for potential changes in body composition during bed rest.
Statistical analyses
All analyses were performed by using Stata 14.0 software (StataCorp LP). Mixed-effects linear regression techniques were used to analyze all dependent variables with Stata’s “xtmixed” command. MPS, body composition, muscle function, and aerobic capacity outcomes were analyzed with group and time fixed effects plus a group × time interaction term; models also included a random intercept term to accommodate the within-subject experimental design. Cell signaling outcomes were analyzed with group, time, and fed state (postabsorptive compared with postprandial) as fixed effects and all resultant interaction terms. Statistical assumptions were tested before interpreting results (e.g., normally distributed residuals, outlier detection). When model residuals were skewed, a natural log transformation was performed to meet the normality assumption of this statistical technique; in some instances, it was necessary to exclude overly influential outlying values to meet model assumptions. The interaction effects (group × time) examining changes relative to pre–bed rest were of primary interest because, pursuant to the hypotheses of the study, they compared changes between groups. Only when a significant interaction effect was detected were individual contrasts performed to evaluate within-group changes between time points; Bonferroni corrections were made to adjust for the inflated type I error risks imposed by these additional comparisons. Data are expressed as means ± SEMs; significance was set a priori at P ≤ 0.05.
RESULTS
Subjects
All of the subjects who passed the medical screening and were admitted to the inpatient, experimental phase of the protocol successfully completed the study (see Supplemental Figure 1). Compliance was also excellent as all subjects rigorously adhered to the diet, supplementation, and bed-rest requirements of the protocol; there were no adverse events related to participation in the study.
Diet and supplementation
Total energy and macronutrient consumption throughout the study was similar in the CON and LEU groups. Meal-specific and 24-h dietary intake data during bed rest are presented in Table 2. Dietary protein intake (unsupplemented) was 0.95 ± 0.02 and 0.98 ± 0.02 g ∙ kg−1 ∙ d−1 for the CON and LEU groups, respectively.
TABLE 2.
Nutritional intake during bed rest1
| Group and meal | Energy, kcal | Carbohydrate, g | Fat, g | Protein, g | Supplement, g |
| CON | |||||
| Breakfast | 656 ± 12 | 84 ± 2 | 26 ± 1 | 25 ± 1 | 4.5 ± 0.2 |
| Lunch | 609 ± 16 | 87 ± 2 | 20 ± 1 | 24 ± 1 | 4.5 ± 0.2 |
| Dinner | 660 ± 13 | 98 ± 2 | 20 ± 1 | 26 ± 1 | 4.5 ± 0.2 |
| Total | 1837 ± 42 | 258 ± 6 | 62 ± 2 | 71 ± 1 | 13.5 ± 0.6 |
| LEU | |||||
| Breakfast | 666 ± 12 | 87 ± 2 | 26 ± 1 | 25 ± 1 | 4.4 ± 0.2 |
| Lunch | 602 ± 16 | 86 ± 2 | 20 ± 1 | 23 ± 1 | 4.4 ± 0.2 |
| Dinner | 656 ± 13 | 97 ± 2 | 20 ± 1 | 26 ± 1 | 4.4 ± 0.2 |
| Total | 1831 ± 42 | 258 ± 6 | 62 ± 2 | 71 ± 2 | 13.2 ± 0.7 |
Values are means ± SEMs; n = 9 (CON) and n = 10 (LEU). CON, control group; LEU, leucine-supplemented group.
Cell signaling
No differences between groups with respect to pre-post changes (group × time interaction effects) were detected for any cell signaling outcomes. Before bed rest, the research meal increased phosphorylation of mTOR (Ser2448), S6K1 (Thr389), and 4E-BP1 (Thr37/46) (main effect for feeding, P < 0.05, compared with postabsorptive; Table 3); these collective feeding responses were maintained after bed rest (main effect for feeding, P < 0.05, compared with postabsorptive; Table 3).
TABLE 3.
Cell signaling and skeletal muscle protein synthesis before and after 14 d of bed rest1
| Pre–bed rest |
Post–bed rest |
|||
| CON | LEU | CON | LEU | |
| mTOR (Ser2448), fold change | 2.2 ± 0.4 | 2.3 ± 0.4 | 2.8 ± 0.5 | 2.2 ± 0.3 |
| S6K1 (Thr389), fold change | 3.5 ± 1.0 | 3.1 ± 0.8 | 2.2 ± 0.6 | 2.6 ± 0.7 |
| 4E-BP1 (Thr37/46), fold change | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 |
| FSR, %/h | ||||
| Postabsorptive | 0.062 ± 0.005 | 0.077 ± 0.004 | 0.042 ± 0.005 | 0.068 ± 0.005 |
| Postprandial | 0.093 ± 0.014 | 0.083 ± 0.013 | 0.090 ± 0.014 | 0.083 ± 0.014 |
Values are means ± SEMs and were assessed on days 4 and 18; n = 9 (CON) and n = 9 (LEU). The activity of each signaling protein was expressed as phosphorylated/total, and fold change was calculated as postprandial activation/postabsorptive activation. Cell signaling data were analyzed with a mixed-effects model in which time, group, and fed state (postabsorptive compared with postprandial) were fixed effects and subject was a random effect; FSR data were analyzed with a mixed-effects model in which time and group were fixed effects and subject was a random effect. No differences between groups with respect to pre-post changes (group × time interaction effects) were detected for any cell signaling or muscle protein synthesis outcome. All cell signaling outcomes showed a main effect of feeding (P < 0.05 compared with postabsorptive) both pre– and post–bed rest. Postabsorptive FSR showed a main effect of time (P < 0.05 compared with pre–bed rest). CON, control group; FSR, fractional synthesis rate of skeletal muscle; LEU, leucine-supplemented group; mTOR, mammalian target of rapamycin; S6K1, p70 S6 kinase 1; 4E-BP1, 4E binding protein 1.
Skeletal MPS
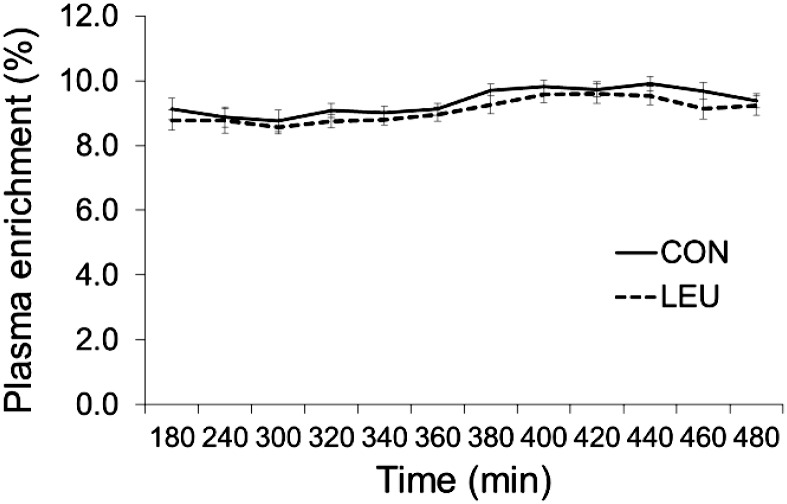
Subjects remained in isotopic steady state throughout the infusion studies (Figure 3). No differences between groups with respect to pre-post changes (group × time interaction effects) after 14 d of bed rest were detected for postabsorptive or postprandial MPS, although the CON group experienced a 30% ± 9% reduction in postabsorptive FSR compared with a 10% ± 10% decrease in the LEU group (main effect for time, P < 0.05, compared with pre–bed rest; Table 3). Bed rest did not alter postprandial FSR (main effect for time, P > 0.05, compared with pre–bed rest).
FIGURE 3.
Plasma enrichment of l-[ring-13C6]phenylalanine (tracer:tracee ratio) during metabolic studies; enrichments for pre– and post–bed-rest metabolic studies were averaged within groups. Values are means ± SEMs; CON, n = 9; LEU, n = 10. CON, control group; LEU, leucine-supplemented group.
Body composition
Body-composition data are presented in Table 4. Bed rest had a rapid and profoundly negative effect on lean tissue mass in middle-aged adults. After 7 d of bed rest, the CON group experienced reductions of 2.9% ± 0.5% and 5.1% ± 0.9% in WBLM and LLM, respectively (both P < 0.05 compared with pre–bed rest), whereas leucine supplementation attenuated these losses (WBLM: −1.7% ± 0.5%, group × time interaction, P < 0.05; LLM: −3.2% ± 0.6%, group × time interaction, P = 0.09). After 14 d of bed rest, no differences between groups with respect to pre-post changes (group × time interaction effects) were detected, and both groups sustained significant losses in muscle mass (WBLM: CON compared with LEU, −2.8% ± 0.6% compared with −2.1% ± 0.7%; LLM: CON compared with LEU, −6.8% ± 0.9% compared with −5.0% ± 0.8%; all P < 0.05 compared with pre–bed rest). During bed rest, the CON group experienced modest, yet significant increases in whole-body fat mass and body fat percentage. Leucine supplementation prevented the accumulation of body fat (Table 4).
TABLE 4.
Body composition (DXA) before and after 7 and 14 d of bed rest1
| Pre–bed rest |
Mid–bed rest, Δ |
Post–bed rest, Δ |
||||
| CON | LEU | CON | LEU | CON | LEU | |
| Body mass, kg | 75.7 ± 3.9 | 73.1 ± 3.7 | −0.9 ± 0.2* | −0.9 ± 0.2* | −1.2 ± 0.2* | −1.0 ± 0.2* |
| WBLM, kg | 51.4 ± 3.1 | 49.5 ± 2.9 | −1.5 ± 0.3* | −0.8 ± 0.3*† | −1.5 ± 0.3* | −1.0 ± 0.3* |
| LLM, kg | 17.3 ± 1.1 | 17.1 ± 1.1 | −0.9 ± 0.1* | −0.5 ± 0.1* | −1.2 ± 0.1* | −0.9 ± 0.1* |
| WBFM, kg | 22.3 ± 2.7 | 21.0 ± 2.5 | 0.4 ± 0.2* | −0.2 ± 0.1† | 0.4 ± 0.2 | 0.1 ± 0.1 |
| Body fat, % | 29.7 ± 3.3 | 29.8 ± 3.1 | 0.9 ± 0.2* | 0.2 ± 0.2† | 0.9 ± 0.2* | 0.5 ± 0.2† |
Values are means ± SEMs and were assessed on days 3, 10, and 17; n = 9 (CON) and n = 10 (LEU). Data were analyzed with a mixed-effects model in which time and group were fixed effects and subject was a random effect. Within group, pre– to post–bed-rest comparisons were Bonferroni-corrected to minimize the potential for type I errors. A trend for a group × time interaction effect was present for LLM (P = 0.09). †Significant interaction effect (group × time compared with pre–bed rest, P < 0.05); *different from pre–bed rest, P < 0.05. CON, control group; DXA, dual-energy X-ray absorptiometry; LEU, leucine-supplemented group; LLM, leg lean mass; WBFM, whole-body fat mass; WBLM, whole-body lean mass; Δ, change.
Muscle function and quality
Bed rest had a negative impact on all indexes of muscle function (Table 5). Leucine supplementation partially or fully protected most outcomes, including knee extensor peak torque at 60°/s (CON compared with LEU: −15% ± 2% compared with −7% ± 3%; group × time interaction, P < 0.05) and 180°/s (CON compared with LEU: −19% ± 3% compared with −6% ± 2%; group × time interaction, P < 0.05) and knee extensor endurance (CON compared with LEU: −14% ± 3% compared with −2% ± 4%; group × time interaction, P < 0.05). Muscle quality (relative strength) was also negatively affected by bed rest (CON: −9% ± 2%; P < 0.05 compared with pre–bed rest) but was preserved by leucine supplementation (LEU: +1% ± 3%; group × time interaction, P < 0.05; Table 5). No differences between groups with respect to pre-post changes (group × time interaction effects) were detected for absolute or relative (mL ∙ kg−1 ∙ min−1) 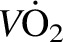 peak (main effects for time, P < 0.05, compared with pre–bed rest); a trend for a group × time effect was present for
peak (main effects for time, P < 0.05, compared with pre–bed rest); a trend for a group × time effect was present for 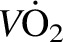 peak relative to lean mass (mL ∙ kg lean mass−1 ∙ min−1; P = 0.09). In the CON group, bed rest reduced
peak relative to lean mass (mL ∙ kg lean mass−1 ∙ min−1; P = 0.09). In the CON group, bed rest reduced 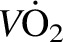 peak by −14% ± 4%, −12% ± 4%, and −12% ± 4% (absolute, relative to body mass, and relative to lean mass, respectively) (Table 6); for the LEU group, these changes were −8% ± 3%, −6% ± 3%, and −6% ± 3%, respectively (Table 6).
peak by −14% ± 4%, −12% ± 4%, and −12% ± 4% (absolute, relative to body mass, and relative to lean mass, respectively) (Table 6); for the LEU group, these changes were −8% ± 3%, −6% ± 3%, and −6% ± 3%, respectively (Table 6).
TABLE 5.
Muscle function before and after 14 d of bed rest1
| Pre–bed rest |
Post–bed rest, Δ |
|||
| CON | LEU | CON | LEU | |
| Knee extensor torque at 60°/s, Nm | 159 ± 13 | 148 ± 12 | −24 ± 4* | −10 ± 4*† |
| Knee extensor torque at 180°/s, Nm | 104 ± 10 | 103 ± 10 | −20 ± 3* | −5 ± 3*† |
| Knee extensor total work at 180°/s, J | 1630 ± 196 | 1685 ± 185 | −248 ± 63* | −20 ± 56† |
| Ankle extensor torque at 60°/s, Nm | 66 ± 6 | 57 ± 5 | −14 ± 4* | −9 ± 3* |
| Muscle quality, Nm ∙ kg right leg lean mass−1 | 18.7 ± 1.0 | 17.2 ± 0.9 | −1.6 ± 1.3* | 0.1 ± 1.3† |
Values are means ± SEMs and were assessed on days 2 and 19; n = 9 (CON) and n = 10 (LEU). Muscle quality was calculated as knee extensor peak torque (at 60°/s)/right leg lean mass (Nm/kg). Data were analyzed with a mixed-effects model in which time and group were fixed effects and subject was a random effect. Within group, pre– to post–bed-rest comparisons were Bonferroni-corrected to minimize the potential for type I errors. †Significant interaction effect (group × time compared with pre–bed rest, P < 0.05); *different from pre–bed rest, P < 0.05. CON, control group; LEU, leucine-supplemented group; Nm, Newton-meter; Δ, change.
TABLE 6.
Aerobic capacity before and after 14 d of bed rest1
| Pre–bed rest |
Post–bed rest, Δ |
|||
| CON | LEU | CON | LEU | |
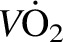 peak peak |
||||
| L/min | 2.05 ± 0.17 | 2.14 ± 0.16 | −0.24 ± 0.07 | −0.15 ± 0.07 |
| mL ∙ kg−1 ∙ min−1 | 27.3 ± 2.0 | 28.9 ± 1.9 | −2.6 ± 0.9 | −0.8 ± 0.9 |
| mL ∙ kg lean mass−1 ∙ min−1 | 41.5 ± 2.2 | 42.4 ± 2.2 | −5.2 ± 1.8 | −0.8 ± 1.8 |
| Peak workload,2 W | 164 ± 16 | 168 ± 15 | −22 ± 7 | −18 ± 3 |
| Peak heart rate,2 beats/min | 168 ± 5 | 169 ± 6 | 1 ± 3 | 6 ± 3 |
Values are means ± SEMs and were assessed on days 2 and 19; n = 8 (CON) and n = 9 (LEU). Data were analyzed with a mixed-effects model in which time and group were fixed effects and subject was a random effect. All 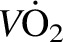 peak outcomes showed a main effect of time (P < 0.05 compared with pre–bed rest); a trend for a group × time interaction effect was present for
peak outcomes showed a main effect of time (P < 0.05 compared with pre–bed rest); a trend for a group × time interaction effect was present for 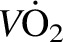 peak relative to lean mass (P = 0.09). CON, control group; LEU, leucine-supplemented group;
peak relative to lean mass (P = 0.09). CON, control group; LEU, leucine-supplemented group;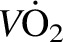 peak, peak oxygen uptake; Δ, change.
peak, peak oxygen uptake; Δ, change.
Peak workload and peak heart rate are presented descriptively and were not subjected to statistical testing.
DISCUSSION
Bed rest has a rapid and profoundly negative impact on skeletal muscle metabolism, lean tissue mass, and function in otherwise healthy middle-aged adults. The rate and magnitude of lean mass loss were substantially greater than in previously studied cohorts of younger adults but were consistent with changes reported in older bed-rest study participants. Leucine supplementation preserved post–bed-rest anabolic cell signaling and skeletal MPS and had a partial protective effect on body composition and muscle function outcomes.
Bed-rest studies provide an opportunity to examine the consequences of physical inactivity in a controlled, standardized environment. The model is also well suited to examine mechanisms of action and translational outcomes in a short period of time. Most early bed-rest investigations were designed as flight analog studies, modeling the effects of microgravity. Enrollment was largely restricted to healthy young men <40 y of age (5, 7, 19–21, 40–43). Later, investigators recognized the potential clinical relevance of the bed-rest model and started to conduct inpatient analog studies in healthy older men and women (>65 y) to reflect the reduced physical activity associated with aging, hospitalization, illness, and injury. Although unloading models that target a single limb (e.g., limb suspension, immobilization) have adversely affected younger adults more than their older counterparts (44, 45), whole-body bed-rest studies appear to have a greater negative effect on muscle mass and function in older populations (1, 2, 4, 5, 46, 47).
We enrolled volunteers with a mean (middle-) age squarely between those of previous bed-rest study cohorts (52 ± 1 y). This “pre-elderly” population shows few negative metabolic or phenotypic consequences of aging but has increasing representation in hospitalized inpatient populations (48) and specialized groups such as the astronaut corps (49). Although direct translation of our results to these populations would be premature, our data do support the need for clinical trials targeting physically inactive/mobility-impaired cohorts. Studies that directly compare specific age groups (e.g., young compared with middle-aged compared with older adults) and/or disuse-model differences (i.e., bed rest compared with limb immobilization) would be particularly valuable.
We hypothesized that middle-aged volunteers would experience a modest reduction in key outcome measures intermediate to the documented losses in younger and older cohorts. However, the loss of LLM in our middle-aged control group (aged 52 ± 1 y; −1.2 ± 0.1 kg over 14 d) was ∼3-fold greater than in studies with younger participants (aged 38 ± 8 y; −0.4 ± 0.1 kg over 28 d) (5, 7) and was consistent with losses reported in older adults (aged 67 ± 5 y; −1.0 ± 0.2 kg over 10 d) (2). A similar pattern was observed for most muscle function outcomes. The relative loss of isokinetic knee extensor peak torque during bed rest in both middle-aged (−15%; pre compared with post: 159 ± 12 compared with 135 ± 11 Nm) and older adults (−16%; pre compared with post: 120 ± 11 compared with 101 ± 9 Nm) (2) was similar, although absolute strength values in older adults were lower, which increases the risk of impaired functional performance (50, 51).
The potential for middle-aged adults to experience such a rapid and substantial loss of muscle mass and function after a relatively short period of bed rest underscores the need for continued education, preventative efforts, and effective treatment options for all age groups. Although any form of physical loading is clearly desirable in most inactivity/bed-rest settings (11), we chose to model a quasi–worst case situation in which nutritional support (leucine supplementation) was the only available exogenous anabolic stimulus.
Our decision to use a single amino acid to protect muscle health during bed rest was supported by the mechanistic and practical attributes of leucine. We hypothesized that a small amount of leucine added to the moderate amount of protein consumed during regular meals (25 g protein/meal) would serve as an anabolic trigger (52) and positively influence cell signaling (mTOR, S6K1, and 4E-BP1) and skeletal MPS (24, 26, 53, 54). We anticipated that this, in turn, would have a protective effect on body composition and muscle function. Previously, we successfully manipulated this translational pathway in a series of bed-rest studies in young (5) and older (50) adults by providing large quantities of all of the essential amino acids (49.5 g/d). Despite showing proof of concept, these studies lacked clinical relevance because of issues such as poor palatability, high cost, and large fluid volume and energy content of the supplement (18). The leucine supplement used in the present study contributed only 18 kcal/meal (54 kcal/d), was easily combined with regular menu items, and was well tolerated by study participants. Similar positive results were also reported by Deutz et al. (47), who used a very small amount (1.5 g twice daily, total of 3 g/d) of the leucine metabolite β-hydroxy-β-methylbutyrate to preserve muscle mass in older adults (60–76 y) during 10 d of bed rest. Although it is possible, or even likely, that additional protein or leucine-rich foods would also have a protective effect (55), continued efforts to develop efficient, mechanistically targeted, yet practical interventions may have broader clinical relevance. Our volunteers consumed ∼1.15 g protein ∙ kg−1 ∙ d−1 (meals and supplement) and lost muscle mass while gaining body fat. Exceeding this moderate quantity of protein may be beneficial in many situations (e.g., flight analog studies, hypermetabolic patient populations) (55, 56). However, it may be challenging or impractical for individuals with lower energy requirements, dietary restrictions, or satiety issues (57). In such instances, promoting muscle anabolism via a lower volume, lower energy alternative would be of considerable practical value.
Leucine supplementation had a positive, protective effect on almost all of our outcome measures. However, it must be stressed that leucine did not fully prevent the loss of muscle mass or function during bed rest. Furthermore, the protective effects may have a limited time course. Leucine appeared to exert its greatest protective effect on lean mass during the initial week of our 14-d protocol. During the final 7 d, the rate of loss in the LEU group was similar to that in the CON group (Table 4). One of the more novel effects of leucine was its ability to protect muscular endurance (knee extensor total work) and to a lesser extent 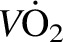 peak during bed rest. Whereas physical activity fully (58, 59) or partially (60) protects aerobic capacity during bed rest, the potential effect of leucine on muscle energetics and mitochondrial function merits further attention (61, 62).
peak during bed rest. Whereas physical activity fully (58, 59) or partially (60) protects aerobic capacity during bed rest, the potential effect of leucine on muscle energetics and mitochondrial function merits further attention (61, 62).
A methodologic limitation of this study was the use of an essential amino acid test meal during the metabolic studies. Although providing a robust and reproducible acute anabolic stimulus, the test meal did not represent the whole-food meals consumed during bed rest and may have hampered our ability to detect anabolic resistance or changes in cell signaling and postprandial MPS (2, 7–10). Similarly, our small sample size may have reduced our statistical power and impaired our ability to identify between-group differences.
In summary, strategies and behaviors to protect muscle health during periods of inactivity should not focus exclusively on older individuals. Bed rest of 14 d has a profoundly negative effect on muscle metabolism, mass, and function in healthy middle-aged adults. Leucine supplementation has the potential to be a simple, minimally invasive dietary strategy to help preserve muscle health during relatively brief periods of physical inactivity.
Acknowledgments
We thank Kate Randolph and Christopher Danesi for technical assistance and Elena Volpi, Randall Urban, and Charles Mathers for medical oversight.
The authors’ responsibilities were as follows—DP-J: designed the research; KLE, JAM, JBE, MMM, JMP, and DP-J: conducted the research; KLE, JAM, EA-L, RP-S, MS-M, and DP-J: analyzed the data; KLE, EA-L, and DP-J: wrote the manuscript; DP-J: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest.
Footnotes
Abbreviations used: CON group, control group; FSR, fractional synthesis rate, LEU group, leucine-supplemented group; LLM, leg lean mass; MPS, muscle protein synthesis; mTOR, mammalian target of rapamycin; Nm, Newton-meter; S6K1, p70 S6 kinase 1; 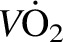 peak, peak oxygen uptake; WBLM, whole-body lean mass; 4E-BP1, 4E binding protein 1.
peak, peak oxygen uptake; WBLM, whole-body lean mass; 4E-BP1, 4E binding protein 1.
REFERENCES
- 1.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–4. [DOI] [PubMed] [Google Scholar]
- 3.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600. Erratum in: JAMA 2014;311(6):625. [DOI] [PubMed] [Google Scholar]
- 4.Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci 2015;70:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 2004;89:4351–8. [DOI] [PubMed] [Google Scholar]
- 6.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care 2014;17:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 1996;270:E627–33. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 2006;91:4836–41. [DOI] [PubMed] [Google Scholar]
- 9.Stuart CA, Shangraw RE, Peters EJ, Wolfe RR. Effect of dietary protein on bed-rest-related changes in whole-body-protein synthesis. Am J Clin Nutr 1990;52:509–14. [DOI] [PubMed] [Google Scholar]
- 10.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 2008;586:6049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol 2009;107:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, et al. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 2009;4:e6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 2010;35:125–33. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson T, Osterlund T, Flanagan JN, von Walden F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol 2010;109:721–7. [DOI] [PubMed] [Google Scholar]
- 15.Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 2008;105:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips SM, McGlory C. CrossTalk proposal: the dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 2014;592:5341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: the dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol 2014;592:5345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 2004;93:294–305. [DOI] [PubMed] [Google Scholar]
- 20.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 2004;97:119–29. [DOI] [PubMed] [Google Scholar]
- 21.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 2007;191:147–59. [DOI] [PubMed] [Google Scholar]
- 22.Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc 2011;59:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiatarone Singh MA, Bernstein MA, Ryan AD, O’Neill EF, Clements KM, Evans WJ. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J Nutr Health Aging 2000;4:5–12. [PubMed] [Google Scholar]
- 24.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 2006;136(1 Suppl):227S–31S. [DOI] [PubMed] [Google Scholar]
- 25.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 2000;130:139–45. [DOI] [PubMed] [Google Scholar]
- 26.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413–9. [DOI] [PubMed] [Google Scholar]
- 27.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr 2012;31:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, Saris WH, van Loon LJ. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr 2011;141:1070–6. [DOI] [PubMed] [Google Scholar]
- 29.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 2009;89:1468–75. [DOI] [PubMed] [Google Scholar]
- 30.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286:E321–8. [DOI] [PubMed] [Google Scholar]
- 31.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 1999;277:E513–20. [DOI] [PubMed] [Google Scholar]
- 33.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 2013;71:195–208. [DOI] [PubMed] [Google Scholar]
- 35.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab 2005;90:1453–9. [DOI] [PubMed] [Google Scholar]
- 36.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975;35:609–16. [PubMed] [Google Scholar]
- 37.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumann PQ, Stirewalt WS, O’Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol 1994;267:E203–9. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken (NJ): Wiley; 2005. [Google Scholar]
- 40.Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 1997;82:807–10. [DOI] [PubMed] [Google Scholar]
- 41.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 1998;84:157–63. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 1992;73:2172–8. [DOI] [PubMed] [Google Scholar]
- 43.Dudley GA, Gollnick PD, Convertino VA, Buchanan P. Changes of muscle function and size with bedrest. Physiologist 1989;32(1 Suppl):S65–6. [PubMed] [Google Scholar]
- 44.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 2009;107:1172–80. [DOI] [PubMed] [Google Scholar]
- 45.Wall BT, Dirks ML, Snijders T, Stephens FB, Senden JM, Verscheijden ML, van Loon LJ. Short-term muscle disuse atrophy is not associated with increased intramuscular lipid deposition or a decline in the maximal activity of key mitochondrial enzymes in young and older males. Exp Gerontol 2015;61:76–83. [DOI] [PubMed] [Google Scholar]
- 46.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 2008;63:1076–81. [DOI] [PubMed] [Google Scholar]
- 47.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr 2013;32:704–12. [DOI] [PubMed] [Google Scholar]
- 48.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;29:1–20, 24. [PubMed] [Google Scholar]
- 49.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol 2009;106:1159–68. [DOI] [PubMed] [Google Scholar]
- 50.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 2010;29:18–23. [DOI] [PubMed] [Google Scholar]
- 51.Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, Goodpaster B, De Rekeneire N, Newman AB, Simonsick EM, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc 2007;55:451–7. [DOI] [PubMed] [Google Scholar]
- 52.Norton LE, Wilson GJ, Layman DK, Moulton CJ, Garlick PJ. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr Metab (Lond) 2012;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care 2008;11:222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein TP, Blanc S. Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit Rev Food Sci Nutr 2011;51:828–34. [DOI] [PubMed] [Google Scholar]
- 56.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism 1997;46:573–8. [DOI] [PubMed] [Google Scholar]
- 57.Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr 2012;51:173–9. [DOI] [PubMed] [Google Scholar]
- 58.Lee SM, Schneider SM, Boda WL, Watenpaugh DE, Macias BR, Meyer RS, Hargens AR. LBNP exercise protects aerobic capacity and sprint speed of female twins during 30 days of bed rest. J Appl Physiol 2009;106:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watenpaugh DE, Ballard RE, Schneider SM, Lee SM, Ertl AC, William JM, Boda WL, Hutchinson KJ, Hargens AR. Supine lower body negative pressure exercise during bed rest maintains upright exercise capacity. J Appl Physiol 2000;89:218–27. [DOI] [PubMed] [Google Scholar]
- 60.Stenger MB, Evans JM, Knapp CF, Lee SM, Phillips TR, Perez SA, Moore AD Jr, Paloski WH, Platts SH. Artificial gravity training reduces bed rest-induced cardiovascular deconditioning. Eur J Appl Physiol 2012;112:605–16. [DOI] [PubMed] [Google Scholar]
- 61.Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. J Nutr Metab 2014;2014:239750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaughan RA, Garcia-Smith R, Gannon NP, Bisoffi M, Trujillo KA, Conn CA. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 2013;45:901–11. [DOI] [PubMed] [Google Scholar]