Abstract
Background
Meningococcal epidemics in Sub-Sahara caused by serogroup A strains are controlled by a group A polysaccharide conjugate vaccine. Strains with serogroups C, W and X continue to cause epidemics. Protein antigens in licensed serogroup B vaccines are shared among serogroup B and non-B strains.
Purpose
Compare serum bactericidal antibody responses elicited by an investigational native outer membrane vesicle vaccine with over-expressed Factor H binding protein (NOMV-FHbp) and a licensed serogroup B vaccine (MenB-4C) against African serogroup A, B, C, W and X strains.
Methods
Human Factor H (FH) transgenic mice were immunized with NOMV-FHbp prepared from a mutant African meningococcal strain containing genetically attenuated endotoxin and a mutant sub-family B FHbp antigen with low FH binding, or with MenB-4C, which contains a recombinant sub-family B FHbp antigen that binds human FH, and three other antigens, NHba, NadA and PorA P1.4, capable of eliciting bactericidal antibody.
Results
The NOMV-FHbp elicited serum bactericidal activity against 12 of 13 serogroup A, B, W or X strains from Africa, and four isogenic serogroup B mutants with subfamily B FHbp sequence variants. There was no activity against a serogroup B mutant with sub-family A FHbp, or two serogroup C isolates from a recent outbreak in Northern Nigeria, which were mismatched for both PorA and sub-family of the FHbp vaccine antigen. For MenB-4C, NHba was expressed by all 16 African isolates tested, FHbp sub-family B in 13, and NadA in five. However, MenB-4C elicited titers ≥1:10 against only one isolate, and against only two of four serogroup B mutant strains with sub-family B FHbp sequence variants.
Conclusions
NOMV-FHbp has greater potential to confer serogroup-independent protection in Africa than the licensed MenB-4C vaccine. However, the NOMV-FHbp vaccine will require inclusion of sub-family A FHbp for coverage against recent serogroup C strains causing outbreaks in Northern Nigeria.
Keywords: Neisseria meningitidis, vaccine, complement, Factor H, Factor H-binding protein, transgenic mouse, OMV, outer membrane vesicle, Bexsero
Introduction
Epidemics of meningococcal disease have occurred in Sub-Saharan Africa for over one hundred years [1]. In 2010, a low cost serogroup A polysaccharide-protein conjugate vaccine (MenAfriVac) was introduced [2–4]. The vaccine conferred protection against invasive disease [5, 6] as well as asymptomatic nasopharyngeal carriage of serogroup A strains [7–9], but failed to curtail disease or carriage by strains with serogroups C, X, or W, which also cause epidemics in the region [10–15]. Meningococcal A,C,Y and W conjugate vaccines, which are available in industrialized countries, can prevent epidemics caused by these strains. The conjugate vaccines, however, do not prevent serogroup X disease; and are not affordable in Sub-Sahara, which is one of the poorest regions in the world.
Two recently licensed serogroup B vaccines target protein antigens [16]. One vaccine (Trumenba®), which is licensed in the United States [16], contains two Factor H binding protein (FHbp) sequence variants [17], and is referred to as “MenB-FHbp” [16]. The second vaccine (Bexsero®, GlaxoSmith Kline), which is licensed in Europe and 24 other countries including the U.S., contains FHbp and three other components capable of eliciting serum bactericidal antibodies [18]. In Europe, this vaccine is referred to as “4CMenB” and, in the U.S. as “MenB-4C” [16]. Both vaccines target antigens shared among serogroup B and non-group B strains. MenB-4C has been reported to elicit serum bactericidal antibody against serogroup X strains from Africa [19]. However, neither serogroup B vaccine is affordable in Sub-Sahara at current prices.
Two laboratories are developing meningococcal native outer membrane vesicle (NOMV) vaccines intended for Africa [20, 21]. These vaccines are prepared from mutant African meningococcal strains with genetically attenuated endotoxin and over-expressed Factor H binding protein (FHbp). In wild-type mice, NOMV-FHbp vaccines, given alone [20, 21], or in combination with a serogroup A conjugate vaccine [20], elicited high serum bactericidal titers against genetically diverse serogroup A, W, X and B strains.
Binding of FH to FHbp is specific for human and some non-human primate FH [22, 23]. The immunogenicity of FHbp-based vaccines is best assessed experimentally in the presence of Factor H that binds to FHbp since data from human FH transgenic mice (reviewed in [24]), and non-human infant primates [25], indicate that binding of FH to FHbp vaccines impairs protective anti-FHbp antibody responses. The purpose of the present study was to compare the breadth of serum bactericidal antibody response elicited by a licensed serogroup B vaccine (MenB-4C), to that of a prototype NOMV-FHbp vaccine being developed for prevention of meningococcal disease in Africa, using a human FH transgenic mouse model.
Methods
Mice
The protocol was approved by the CHORI Animal Care and Use Committee. The human FH transgenic BALB/c mouse (TG) line has been described [26]. For the present study, only TG mice with serum human FH concentrations ≥240 μg/mL (which approximate human serum FH levels [27]) were included. Wildtype BALB/c mice (WT) were purchased from Charles Rivers (Wilmington, MA) and were housed for 3 weeks before beginning the immunization protocol.
Vaccines
A one-fifth human dose (0.1 ml) of MenB-4C, which was used in our mouse studies, contains 10 μg each of the three recombinant proteins, combined with 5 μg of OMV, which were adsorbed with aluminum hydroxide (0.1 mg Al3+). The investigational NOMV-FHbp vaccine [20] was prepared from a serogroup W mutant of strain Sudan 1/06 with genetically attenuated endotoxin (deletion of lpxl1 gene [28, 29]), inactivation of capsular synthesis [20], and over-expression of a mutant R41S Factor H binding protein (FHbp) peptide identification number (ID) 9. This substitution decreased human FH binding >50 fold, compared to the wildtype FHbp antigen [26]. Strain Sudan 1/06 expresses NadA (ID 6 in group 2/3), Neisserial Heparin binding antigen (NHba) ID 96, and PorA with variable regions (VR) sequence types P1.5,2 (Table 1). This PorA VR type is prevalent among epidemic African serogroup W ST-11 strains [30]. The NOMV-FHbp dose contained 5 μg of protein to match the OMV content of the 1/5th human MenB-4C dose. By SDS PAGE, PorA represented ~25% of the total protein content of the NOMV-FHbp vaccine (supplemental Figure S2 of our previous publication [20]), and by quantitative Western blot, FHbp was ~5% [20]. Thus, the 5 μg NOMV-FHbp dose contained approximately 1.25 μg of PorA and 0.25 μg of FHbp. The amount of NadA or NHba has not yet been fully characterized since we are currently developing appropriate methods. By flow cytometry, the mutant vaccine strain used to prepare the vaccine expressed NHba and NadA (data not shown).
Table 1.
African meningococcal strains used to test serum bactericidal activity.
| Strain | Capsular Group | CC [ST]1 | PorA (VR)2 | Strain Expression of MenB-4C Antigens | |||||
|---|---|---|---|---|---|---|---|---|---|
| NadA | FHbp | NHba | |||||||
| Peptide ID (Variant group)3 | Expression4 | Peptide ID (Sub-family)3 | Expression4 | Peptide ID3 | Expression4 | ||||
| Z2491 | A | 4 [4] | 7,13-1 | NA | − | 5 (B) | + | 29 | ++ |
| Z1275 | A | 1 [1] | 5-2,10 | NA | − | 4 (B) | + | 29 | ++ |
| Senegal 1/99 | A | 5 [5] | 20,9 | 8 (2/3) | + | 5 (B) | + | 27 | + |
| LNP20868 | A | 5 [2859] | 20,9 | 8 (2/3) | ++ | 5 (B) | + | 29 | ++ |
| E23/03 | A | 5 [7] | 20,9 | 8 (2/3) | + | 5 (B) | + | 126 | + |
| Kenya 1/06 | X | UA [5403] | 19,26 | NA | − | 74 (B) | ++ | 228 | + |
| BuFa 12/03 | X | 181 [751] | 5-1,10-1 | NA | − | 73 (B) | + | 358 | + |
| Uganda 23/06 | X | UA [5403] | 19,26 | NA | − | 74 (B) | ++ | 228 | + |
| BF 5/97 | X | 181 [751] | 5-1,10-1 | NA | − | 73 (B) | ++ | 358 | + |
| HF 78 | X | 181 [3687] | 7,9 | NA | − | 4 (B) | ++ | 228 | ++ |
| Bufa 2/03 | W | 11 [11] | 5,2 | 6 (2/3) | ++ | 23 (A) | + | 29 | + |
| Su 1/06 | W | 11 [11] | 5,2 | 6 (2/3) | + | 9 (B) | + | 96 | + |
| C6/01 | W | 175 [175] | 5-1,2-2 | NA | − | 350 (B) | ++ | 9 | + |
| 675 | B | 41/44 [8233] | 7-1,1 | NA | − | 347 (B) | ++ | 357 | + |
| NIG 8/13 | C | UA [10217] | 21-15,16 | NA | − | 27 (A) | +/− | 798 | + |
| NIG 21/13 | C | UA [10217] | 21-15,16 | NA | − | 27 (A) | +/− | 798 | + |
Clonal complex and sequence type as defined in http://pubmlst.org/neisseria/ UA, clonal complex unassigned
As defined in http://pubmlst.org/neisseria/PorA/
Peptide IDs for FHbp, NadA and NHba are defined in http://pubmlst.org/neisseria/. Sub-family or variant group for FHbp and NadA, respectively, were also assigned according to the public database. For NHba, the protein variants are not classified into families or variant groups [45].
Antigen expression was measured by flow cytometry using live bacteria and monoclonal or polyclonal mouse sera previously described [41, 46] (See for example, Supplemental Figure S2). For FHbp, ++ is high, + is medium, and +/− is low. For NadA, + is 40 to 100%, and ++, >100%, as compared to strain 5/99, which was tested in parallel and is naturally a high NadA expresser [32]. For NHba, + is 40 to 100%, and ++ is >100% of strain M4407, which was tested in parallel and is naturally a high NHba-expresser [40, 41].
Mouse immunogenicity
Mice, ages 7- to-12 weeks, were immunized with three i.p. injections, each separated by three weeks. Twenty human FH TG animals received MenB-4C, and eight received NOMV-FHbp. We used a larger number of TG mice in the MenB-4C group to provide greater statistical power to detect serum autoantibody to human FH, which was observed previously with MenB-4C in 2 of 11 human FH transgenic mice [31]. As controls to assess immunogenicity in mice in the absence of human FH, we immunized groups of eight WT mice with MenB-4C or NOMV-FHbp. One WT NOMV-FHbp-vaccinated animal died from causes that appeared unrelated to vaccination, leaving 7 animals in that group. Negative control WT and TG mice (N=8 per group) were immunized with aluminum hydroxide without a vaccine antigen (dose of 0.1 mg Al3+ to match the Al3+ content in 1/5 human dose of MenB-4C). Blood samples were obtained three weeks after the third dose. All sera were heated at 56°C for 30 mins to inactivate internal complement before use.
N. meningitidis test strains
The MenB-4C vaccine contains four antigens known to elicit serum bactericidal antibody: FHbp, NHba, NadA, and PorA P1.4 (as part of detergent-treated outer membrane vesicles [OMV]) [18]. To assess MenB-4C antigen-specific bactericidal antibodies, we used three previously described serogroup B strains [31, 32], which were each mismatched for three of the four antigens in MenB-4C. Strain H44/76 is specific for MenB-4C-induced bactericidal antibodies to FHbp, strain 5/99 to NadA [32], and strain SK016 to PorA P1. 4 [31].
We tested serum bactericidal responses against 16 African isolates (Table 1). The isolates were selected from 106 African case isolates to include representatives from different epidemics between 1963 to 2013 [30]. We included five serogroup A isolates, each with different multilocus sequence types (MLST) (i.e., ST-1, -4, -5, -7 and -2859). Because we had fewer serogroup X isolates, we included two ST-751 isolates from outbreaks in Burkina Faso in 1997 and 2003, and two ST-5403 isolates from an outbreak in Uganda and Kenya in 2006, and one ST-3687 isolate from South Africa in the 1970s. Thus, even when there were “replicates” with respect to MLST and major antigens studied, we attempted to have diversity based on differences in year of isolation or geographic area. Similarly for the three serogroup W isolates, two with ST-11 had different FHbp sub-families (A or B) and the third was from clonal complex 175. The only other replicates were two serogroup C isolates from a 2013 epidemic in Northern Nigeria, which represent a new clone spreading in Africa [12]. The percent amino acid identities of the antigens expressed by the test strains and the respective antigens in the vaccines are summarized in Supplemental Table S1.
We also tested serum anti-FHbp bactericidal antibody responses against four previously described mutants of strain H44/76 in which the gene encoding the native FHbp ID 1 had been replaced by either ID 4, 9 or 15 (sub-family B), or ID 22 (sub-family A)[25]. Each of the mutants showed similar relative expression of FHbp as that of the parent strain used to prepare the mutants as measured by flow cytometry [25].
Serum bactericidal antibody activity
The assay was performed as previously described [31]. The complement source was IgG-depleted human serum prepared as previously described [26]. The bactericidal titer was the dilution of serum that resulted in a 50% decrease in colony-forming units (CFU/ml) after 60 minutes of incubation at 37° C, compared to CFU/ml in negative controls wells.
Statistical analyses
For calculation of geometric means, titers were transformed (Log10). We used a nonparametric test (Mann-Whitney) to compare the respective titers of two independent groups of mice. A two-tailed p value ≤0.05 was considered statistically significant.
Results
Human FH impairs serum anti-FHbp bactericidal response elicited by MenB-4C
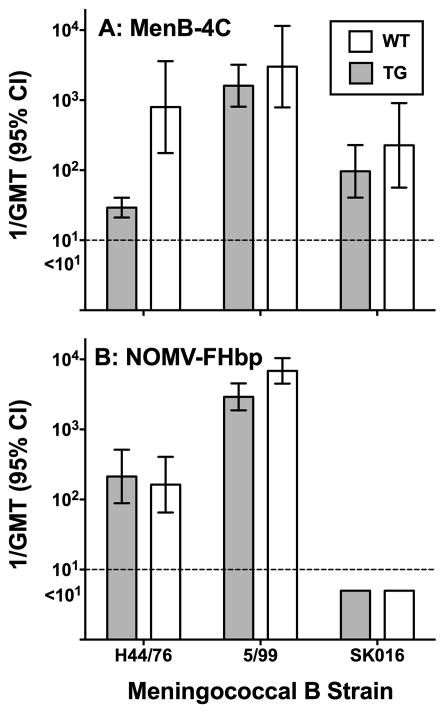
In previous studies there was evidence that binding of human or macaque FH to FHbp in MenB-4C impaired anti-FHbp serum bactericidal responses [25, 31]. We compared serum bactericidal antibody responses of human FH TG mice and WT mice whose mouse FH didn’t bind to FHbp. The TG mice immunized with MenB-4C had lower anti-FHbp bactericidal titers than WT mice when tested against strain H44/76 (P=0.002, Figure 1, Panel A). The WT and TG mice had similar respective bactericidal titers against strains 5/99 and SK106, which are specific for MenB-4C-induced bactericidal activity to NadA and PorA P1.4, respectively; (P>0.2, Figure 1, Panel A). These two antigens are not known to bind human FH.
Figure 1. Serum bactericidal antibody responses of human FH transgenic mice or wildtype mice measured against control serogroup B meningococcal strains.
Each bar represents the GMT of individual serum titers of groups of 7 to 20 mice. Panel A: MenB-4C vaccinated animals. Strain H44/76 is mismatched for each of the antigens in MenB-4C except for FHbp; strain 5/99 is mismatched for each of the antigens except NadA; and strain SK016 is mismatched for each of the antigens except PorA P1.4. Panel B: NOMV-FHbp vaccinated animals. For NOMV-FHbp vaccine, strain H44/76 is matched for FHbp subfamily with the vaccine; strain 5/99 is matched with PorA P1.2,5 and NadA, and strain SK016 is not matched with either PorA, FHbp or NadA. The differences that were statistically significant were Panel A, H44/76 (P=0.002) and Panel B 5/99, P=0.01.
No effect of human FH on anti-FHbp bactericidal response elicited by NOMV-FHbp containing a mutant, low FH-binding FHbp antigen
There were no significant differences between the anti-FHbp bactericidal titers of TG or WT mice immunized with NOMV-FHbp (strain H44/76, P>0.6, Figure 1, Panel B). This result was expected since the mutant R41S FHbp antigen in NOMV-FHbp has impaired binding of Factor H [26, 33]. Note that in WT mice whose mouse FH doesn’t bind to FHbp, the licensed MenB-4C elicited higher serum anti-FHbp bactericidal titers than the investigational NOMV-FHbp (compare data for H44/76, Panels A and B, P=0.02). The most likely reasons are an exact match between FHbp ID 1 in MenB-4C and the target H44/76 strain, whereas the FHbp in NOMV-FHbp was ID 9, and/or introduction of the FHbp R41S mutation in NOMV-FHbp, which may decrease FHbp immunogenicity in the absence of human FH [33].
NOMV-FHbp elicited high serum bactericidal titers in both WT and TG mice against strain 5/99 (Figure 1, Panel B). By ELISA, NOMV-FHbp elicited serum anti-NadA antibodies (see below). However, in contrast to MenB-4C, the bactericidal titers elicited by NOMV-FHbp against strain 5/99 were not specific for NadA since the strain also has PorA P1.5,2, which matched the PorA in the NOMV-FHbp vaccine. Interestingly, although in both mouse lines, the bactericidal titers elicited by NOMV-FHbp against strain 5/99 were >1:5000, the titers in WT mice were higher than in TG mice (P=0.01). PorA or NadA are not known to be FH ligands. The reason for the higher responses of the WT mice is unknown.
The NOMV-FHbp vaccine did not elicit serum bactericidal antibody against strain SK016 (Panel B), which was expected since this strain contains PorA (P1.4) and FHbp from sub-family A, which are both mismatched for the respective antigens in NOMV-FHbp. We also measured bactericidal antibody responses in serum pools from WT mice immunized with NOMV-FHbp (N=2 pools) or MenB-4C (n=2 pools) against 14 case isolates from Africa, and five mutants of serogroup B strain H44/76 with different FHbp sequence variants. For most of the isolates, NOMV-FHbp elicited higher titers than MenB-4C (Supplementary Figures S1 and S2). Note that for strains where the error bar overlaps the lower limit of the assay (titer of 1:10), one serum pool from the respective vaccine group was positive and the other was <1:10.
The NOMV-FHbp vaccine elicits broader bactericidal activity in human FH transgenic mice against epidemic meningococcal isolates from Africa
For the remaining analyses we focused on the antibody responses of TG mice, since for vaccines containing FHbp that bind human FH, these data likely are more relevant to human antibody responses than WT mice [26, 31, 33].
The serum IgG antibody titers measured by ELISA against FHbp, NHba and NadA are summarized in Supplementary Figure S3. For this analysis we measured titers of individual sera from all 8 TG mice immunized with NOMV-FHbp, and 8 representative TG mice immunized with MenB-4C. The negative control was a serum pool from five TG mice immunized with aluminum hydroxide alone. As expected MenB-4C elicited serum antibodies to all three antigens present as recombinant proteins in the vaccine. NOMV-FHbp elicited titers to two of these antigens, FHbp and NadA, but not NHba. The respective geometric mean anti-FHbp titers were similar in the two vaccine groups. However, there was more variability in the titers of the MenB-4C group (difference in variance, P<0.001). The geometric mean anti-NadA titer of the MenB-4C group was 6-fold higher than the NOMV-FHbp group (P<0.001).
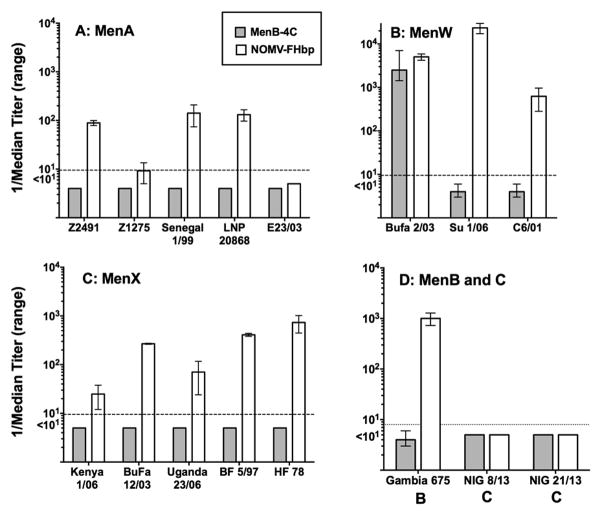
Because of the large number of test strains, we used serum pools from the TG mice to measure bactericidal activity (4 sera per pool, 2 pools in the NOMV-FHbp group and 5 pools in the MenB-4C group). The median bactericidal titers and ranges of titers of the serum pools against each African strain are summarized in Figure 2. TG mice immunized with NOMV-FHbp developed serum bactericidal titers ≥1:10 against 13 of the 16 African isolates: These included four of five serogroup A strains tested; all three serogroup W strains; all five serogroup X strains, and one serogroup B strain from The Gambia (Figure 2). There was no activity against two serogroup C strains from Northern Nigeria, which were mismatched for both PorA and FHbp sub-family in NOMV-FHbp (Table 1). Mice immunized with MenB-4C developed serum bactericidal titers ≥1:10 against only one of the 16 African strains tested. Thus, despite all 16 isolates expressing NHba, five expressing NadA, and 13 having subfamily B FHbp, which matched the sub-family B of the FHbp in MenB-4C (Table 1), only one African isolate, serogroup W strain BuFa 2/03, was killed. The susceptible strain was mismatched for PorA and the FHbp sub-family in MenB-4C, but had moderate expression of NHba and high expression of NadA (Table 1). A second serogroup W strain (Su 1/06) with moderate expression of NHba and only slightly lower NadA expression than in BuFa 2/03 (Supplemental Figure S4), was resistant to antibodies elicited in TG mice by MenB-4C. The reason for the strain disparity in anti-NadA and/or anti-NHba bactericidal activity is not known; the two isolates showed similar respective bactericidal susceptibility to mouse anticapsular or anti-PorA mAbs (data not shown).
Figure 2. Serum bactericidal antibody responses of TG mice.
Panels A, B, and C: Meningococcal serogroup A, W, and X isolates, respectively, from cases of disease during epidemics in Africa. Panel D, a serogroup B isolate from The Gambia (675) and two serogroup C isolates from recent outbreaks in Northern Nigeria [12]. Bars represent the reciprocal median and ranges of the respective titers measured in serum pools (N=5 pools for MenB-4C, and 2 for NOMV-FHbp). Gray bars, TG mice immunized with MenB-4C; white bars, TG mice immunized with the NOMV-FHbp vaccine. All negative control sera from TG mice immunized with aluminum hydroxide without a vaccine antigen had titers <1:10 (data not shown). The two serogroup C isolates were killed by human complement and ≤0.5 μg/ml of a serogroup C anticapsular mAb.
NOMV-FHbp elicits broader serum anti-FHbp bactericidal activity than MenB-4C against mutants of serogroup B strain H44/76 with different FHbp sequence variants
To evaluate the breadth of serum anti-FHbp antibody responses we tested bactericidal activity against isogenic serogroup B mutants of strain H44/76 that expressed different FHbp amino acid sequence variants. As described above, the wildtype H44/76 parental strain is mismatched for each of the antigens in MenB-4C or NOMV-FHbp except for the sub-family B FHbp antigen. NOMV-FHbp elicited serum anti-FHbpmbactericidal activity against all four mutants with sub-family B FHbp sequence variants (ID 1, 4, 9 15), but not against the mutant with sub-family A FHbp (ID 22) (Figure 3). In contrast, MenB-4C only elicited anti-FHbp bactericidal activity against H44/76 with ID 1 (an exact sequence match to the FHbp in MenB-4C) or with ID 15.
Figure 3. Serum bactericidal antibody titers of immunized TG mice against mutants of H44/76 expressing different FHbp sequence variants.
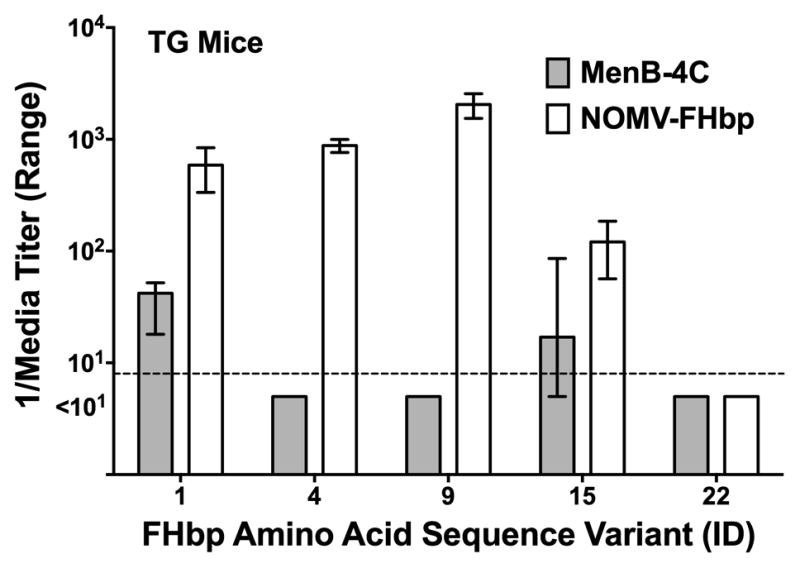
Data are expressed as the median titers and ranges of pooled sera from each group of mice (N=5 pools for MenB-4C, and 2 pools for NOMV-FHbp; see methods). The mutant FHbp ID is shown in the X-axis. Test strains with FHbp ID 1, 4, 9 and 15 are sub-family B, and ID 22 is sub-family A. For all strains but ID 15 and ID 22, the differences between the two groups were statistically significant (P<0.05 by Mann-Whitney test).
Neither vaccine induced auto-antibodies to human FH
In a previous study, 2 of 11 human FH transgenic mice immunized with MenB-4C developed serum IgM reactivity to human FH as measured by ELISA [31]. We therefore measured serum anti-FH antibodies in sera from individual TG mice immunized with the MenB-4C or NOMV-FHbp vaccines in the present study. None of the sera was positive when tested at a dilution of 1:100. A serum from a transgenic mouse from the previous study with IgM anti-FH reactivity served as a control and was positive (O.D. ~1).
Discussion
Meningococcal serogroup A disease is at its lowest level in Sub-Saharan Africa than in recent decades, in large part because of widespread vaccination with a low cost serogroup A polysaccharide-protein conjugate vaccine [6, 15]. The remaining challenge is prevention of serogroup C, W or X disease [12–15, 34] (for unknown reasons, serogroup B disease is rare in the region [30]). One solution would be a low cost pentavalent meningococcal polysaccharide-conjugate vaccine that includes serogroup X [35] since conjugate vaccines are highly effective in preventing disease and also decrease colonization.
Alternatively, a vaccine containing protein antigens, which are shared across strains with different capsular groups, also could potentially elicit broad strain coverage. The proteins could be delivered as a “cocktail” of recombinant proteins with a suitable adjuvant or, as in the present study, a NOMV-FHbp vaccine with over-expressed desirable antigens (such as FHbp) and deleted or attenuated undesirable molecules (such as endotoxin). One disadvantage, however, of targeting protein antigens is that there are only limited data in humans on the ability of protein antigens to elicit bactericidal activity against non-serogroup B strains. A second disadvantage is that protein antigen vaccines may have only a modest effect on decreasing meningococcal colonization [36]. However, as shown in the present study, NOMV-FHbp vaccines have the potential to elicit higher serum bactericidal response than MenB-4C, which also might translate into a greater effect on decreasing colonization. In a human CEACAM1 transgenic mouse model, an investigational NOMV-FHbp vaccine decreased meningococcal colonization [37]. A third possible disadvantage of protein-based vaccines is that antigens might drive immune selection for resistant strains (from antigenic drift, or loss of antigen expression from frameshift mutations or deletion of genes) [38].
In the present study, we first replicated previous findings [31] that MenB-4C elicited lower serum anti-FHbp bactericidal antibody responses in human FH TG mice against a serogroup B strain than in wildtype mice whose mouse FH didn’t bind to FHbp. Binding of FH to the FHbp antigen also limited cross-protection against non-B strains since sera from MenB-4C-vaccinated TG mice failed to activate bactericidal activity against 15 of 16 African meningococcal strains tested. In contrast, sera from TG mice immunized with NOMV-FHbp, which contained a mutant FHbp antigen with low binding to human FH, elicited broadly cross-reactive bactericidal activity against nearly all of the serogroup B and non-B strains. One exception was a group A strain (E23/03) with a sub-family B FHbp that matched the FHbp sub-family in NOMV-FHbp. Based on our previous study, this strain was relatively resistant to anti-FHbp bactericidal activity [20]; the basis of which is not known. The high bactericidal titers elicited by the NOMV-FHbp against the serogroup W strain Bufa 2/03, which expressed NadA and a sub-family A FHbp, were probably a result of antibodies directed at NadA and/or PorA P1.5,2, which matched the PorA in the vaccine. However, the anti-FHbp antibody resistance of the serogroup B mutant with a sub-family A FHbp variant, and two serogroup C strains with sub-family A FHbp from recent epidemics in Northern Nigeria, underscored the need to express a sub-family A FHbp in an NOMV-FHbp vaccine for broad protection in Africa.
The low serum bactericidal antibody responses of the TG mice immunized with MenB-4C against the African isolates was unexpected considering that all 16 had NHba expression by flow cytometry, 13 expressed FHbp sub-family B, which matched the FHbp sub-family in MenB-4C, and 5 had with moderate to high expression of NadA (Table 1). Resistance to anti-FHbp bactericidal antibody is likely explained by binding of human FH to the MenB-4C vaccine antigen, which in a previous study in human FH TG mice greatly impaired anti-FHbp bactericidal responses [31]. The low anti-NHba bactericidal activity also was not entirely unexpected, having been reported with hyperimmune mouse anti-NHba antiserum and human complement in 2003 against serogroup B strains [39] and, more recently, in sera from immunized humans or rhesus macaques [40, 41]. Whether the lack anti-NHba bactericidal activity elicited by MenB-4C against the African strains in the present study represented intrinsic resistance to anti-NHba antibody and/or low serum anti-NHba titers (Supplemental Figure S3), will require further study.
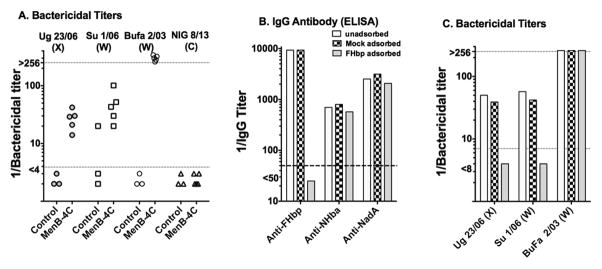
Our findings with MenB-4C and the serogroup X strains in human FH transgenic mice contradict data reported by Hong and co-workers who found that sera from MenB-4C-immunized humans were broadly bactericidal against serogroup X strains from Africa [19]. The most likely explanation is species differences between antibody responses of inbred TG BALB/c mice and humans to MenB-4C. To illustrate this point, we tested sera from a previous study of five MenB-4C-vaccinated rhesus macaques whose macaque FH bound to FHbp [25] for bactericidal activity against four African meningococcal isolates (three resistant to sera from MenB-4C-vaccinated TG mice, and one susceptible). The macaque sera (Figure 4, Panel A) had bactericidal activity against two of the mouse-resistant strains (serogroup X strain Ug 23/03 and W strain Su 1/06), and the susceptible serogroup W strain, BuFa 2/03. Thus, while the TG mouse model is useful for investigating the immunogenicity of antigens that bind specifically to human FH [31, 33, 42], or for comparing the breadth of protection elicited by different vaccine candidates containing FHbp antigens [33, 43, 44], the bactericidal antibody responses of TG BALB/c mice appear to be narrower in breadth than in immunized macaques (or humans). Interestingly, depletion of the anti-FHbp antibodies from the post-immunization macaque serum pools (Figure 4, Panels B and C) removed all of the bactericidal activity against the X or W strains that were resistant to bactericidal activity of sera from the TG mice immunized with MenB-4C (despite moderate expression of NHba in both strains, and moderate expression of NadA in serogroup W strain Su 1/06, Supplemental Figure S4). Thus, despite multiple “matched” antigens with MenB-4c, the macaque bactericidal activity against these two strains required antibodies to FHbp, which was similar to our recent findings with two serogroup B outbreak strains [41]. In contrast, depletion of anti-FHbp antibody from the macaque serum pool did not decrease anti-NadA and/or anti-NHba bactericidal against the second serogroup W, strain BuFa 2/03, which was susceptible to bactericidal activity of the mouse sera (Figure 4, Panel C).
Figure 4. Effect of depletion of anti-FHbp antibodies on serum bactericidal antibody responses of infant macaques immunized with MenB-4C.
Panel A. Serum bactericidal titers of five infant macaques immunized in a previous study with two doses of MenB-4C [25] measured in the present study against four representative African serogroup X, W or C strains. The macaque FH in all five animals bound strongly to FHbp. Control macaques are unvaccinated animals of the same ages as the vaccinated animals. Each symbol represents the serum titer of an individual animal. Strains Ug23/06, SU 1/06 and NIG 8/13 were resistant to serum bactericidal activity elicited in human FH transgenic mice immunized with the MenB-4C vaccine, and strain BuFa 2/03 was susceptible. The resistant serogroup C strain, NIG 8/13, was killed by a positive control anticapsular mAb. Panel B. Adequacy of depletion of anti-FHbp antibodies in previously described [41] adsorbed and unadsorbed serum pools from the five MenB-4C-vaccinated animals as measured by ELISA. Panel C. Effect of depletion of anti-FHbp on bactericidal activity measured in the present study against three of the African strains. The bars represent the titers of the unadsorbed and adsorbed pooled serums. Each of the strains expresses NHba; strains Su 1/06 and BuFa 2/03 also have moderate and high NadA expression, respectively (Supplemental Figure S2). Strains BuFa 2/03 and NIG 8/13 have FHbp sub-family A and strains Ug 23/06 and Su 1/06 have FHbp sub-family B (Table 1).
In the present study we did not observe serum auto-antibodies to FH in TG mice immunized with MenB-4C. The reason for the different result than in our previous study [31] is not known: the vaccine used and the transgenic mouse line were the same in the two studies. However, the lack of auto-antibody in the present study (0/20 TG mice) was not statistically significant from our first study (2/11 TG mice, P=0.11 by Fisher Exact Text). Thus, despite including larger numbers of TG mice allocated to the MenB-4C in the present study, we still may have been underpowered to detect autoantibodies if they occurred at a low frequency. It is noteworthy that in a study of infant rhesus macaques immunized with MenB-4C, two of 14 animals developed serum IgG antibodies to FH after dose 2 [45]. Thus, the question of whether FHbp vaccination elicits autoantibodies to FH in humans and their potential clinical significance, remains unknown.
In summary, we developed a prototype NOMV-FHbp vaccine that elicits broader bactericidal responses in human FH transgenic mice than the currently licensed MenB-4C vaccine. In a previous study, the NOMV-FHbp vaccine when combined with a serogroup A conjugate vaccine, elicited higher bactericidal antibody responses in WT mice than a licensed meningococcal quadrivalent conjugate vaccine [20]. Collectively, the data support investigating a combined meningococcal serogroup A conjugate vaccine/NOMV-FHbp vaccine for prevention of epidemics in Africa irrespective of capsular group, and for the use of the NOMV-FHbp vaccine for prevention of serogroup B disease in industrialized countries (or should serogroup B strains one day emerge in Africa). Whether NOMV-FHbp vaccines can be manufactured at sufficiently low cost to be affordable in Africa is beyond the scope of the present study and will need to be addressed in future studies.
Supplementary Material
Research highlights.
Epidemic serogroup A meningococcal disease in Sub-Sahara Africa is currently controlled by a monovalent serogroup A conjugate vaccine
Epidemics caused by serogroup C, W and X strains continue to occur in the region
Novel protein antigens such as Factor H binding protein (FHbp) used in licensed serogroup B vaccines are shared across capsular groups
One approach for Africa is a native outer membrane vesicle vaccine prepared from a mutant strains with genetically attenuated endotoxin and over-expressed Factor H binding protein (NOMV-FHbp)
In human FH transgenic mice a NOMV-FHbp vaccine elicited broader serum bactericidal antibody responses against serogroup B and non-serogroup B African strains than a licensed serogroup B vaccine.
Acknowledgments
We are grateful to Andrew Fergus, Isabella Costa PhD, and Serena Giuntini, PhD, for their expert technical assistance. Professor Dominique Caugant, Norwegian Institute of Public Health kindly provided the African strains. The work was supported by Public Health Service grants R01 AI 46464 and R01 AI 82263 from the National Institute of Allergy and Infectious Diseases, NIH, and performed in a facility funded by Research Facilities Improvement Program grant number C06 RR 16226 from the National Center for Research Resources, NIH. Eduardo Lujan was supported in part by a San Francisco State University MBRS-RISE fellowship funded by the R25-GM059298 grant from the National Institutes of Health, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi: 10.1016/s0035-9203(99)90106-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee CH, Kuo WC, Beri S, Kapre S, Joshi JS, Bouveret N, et al. Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine. 2009;27:726–32. doi: 10.1016/j.vaccine.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 3.Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac. Hum Vaccin Immunother. 2012;8:715–24. doi: 10.4161/hv.19619. [DOI] [PubMed] [Google Scholar]
- 4.Djingarey MH, Barry R, Bonkoungou M, Tiendrebeogo S, Sebgo R, Kandolo D, et al. Effectively introducing a new meningococcal A conjugate vaccine in Africa: The Burkina Faso experience. Vaccine. 2012;30(Suppl 2):B40–5. doi: 10.1016/j.vaccine.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 5.Novak RT, Kambou JL, Diomande FV, Tarbangdo TF, Ouedraogo-Traore R, Sangare L, et al. Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis. 2012;12:757–64. doi: 10.1016/S1473-3099(12)70168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristiansen PA, Jorgensen HJ, Caugant DA. Serogroup A meningococcal conjugate vaccines in Africa. Expert review of vaccines. 2015:1–18. doi: 10.1586/14760584.2015.1084232. [DOI] [PubMed] [Google Scholar]
- 7.Kristiansen PA, Ba A, Ouedraogo AS, Sanou I, Ouedraogo R, Sangare L, et al. Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis. 2014;14:663. doi: 10.1186/s12879-014-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbe M, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2014;383:40–7. doi: 10.1016/S0140-6736(13)61612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, Ouedraogo R, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56:354–63. doi: 10.1093/cid/cis892. [DOI] [PubMed] [Google Scholar]
- 10.Mueller JE, Borrow R, Gessner BD. Meningococcal serogroup W135 in the African meningitis belt: epidemiology, immunity and vaccines. Expert review of vaccines. 2006;5:319–36. doi: 10.1586/14760584.5.3.319. [DOI] [PubMed] [Google Scholar]
- 11.Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Mainassara HB, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 12.Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013–14. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.b50c2aaf1032b3ccade0fca0b63ee518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacNeil JR, Medah I, Koussoube D, Novak RT, Cohn AC, Diomande FV, et al. Neisseria meningitidis serogroup W, Burkina Faso, 2012. Emerg Infect Dis. 2014;20:394–9. doi: 10.3201/eid2003.131407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hossain MJ, Roca A, Mackenzie GA, Jasseh M, Hossain MI, Muhammad S, et al. Serogroup W135 meningococcal disease, The Gambia, 2012. Emerg Infect Dis. 2013;19:1507–10. doi: 10.3201/eid1909.130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meningococcal disease control in countries of the African meningitis belt, 2014. Wkly Epidemiol Rec. 2015;90:123–31. [PubMed] [Google Scholar]
- 16.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR Centers for Disease C. Use of serogroup B meningococcal vaccines in persons Aged >/=10 years at increased risk for serogroup B meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608–12. [PMC free article] [PubMed] [Google Scholar]
- 17.Zlotnick GW, Jones TR, Liberator P, Hao L, Harris S, McNeil LK, et al. The discovery and development of a novel vaccine to protect against Neisseria meningitidis serogroup B disease. Hum Vaccin Immunother. 2015;11:5–13. doi: 10.4161/hv.34293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong E, Giuliani MM, Deghmane AE, Comanducci M, Brunelli B, Dull P, et al. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31:1113–6. doi: 10.1016/j.vaccine.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Pajon R, Fergus AM, Granoff DM. Mutant native outer membrane vesicles combined with a serogroup A polysaccharide conjugate vaccine for prevention of meningococcal epidemics in Africa. PLoS One. 2013;8:e66536. doi: 10.1371/journal.pone.0066536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, et al. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA) Vaccine. 2014;32:2688–95. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 22.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beernink PT, Shaughnessy J, Stefek H, Ram S, Granoff DM. Heterogeneity in rhesus macaque complement Factor H binding to meningococcal Factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines. Clin Vaccine Immunol. 2014;21:1505–11. doi: 10.1128/CVI.00517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granoff DM, Ram S, Beernink PT. Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses? Clin Vaccine Immunol. 2013;20:1099–107. doi: 10.1128/CVI.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. Binding of complement Factor H (FH) decreases protective anti-FH binding protein antibody responses of infant rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis. 2015;212:784–92. doi: 10.1093/infdis/jiv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011;186:3606–14. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–27. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–90. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprong T, Stikkelbroeck N, van der Ley P, Steeghs L, van Alphen L, Klein N, et al. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J Leukoc Biol. 2001;70:283–8. [PubMed] [Google Scholar]
- 30.Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. Meningococcal factor H binding proteins in epidemic strains from Africa: Implications for vaccine development. PLoS Negl Trop Dis. 2011;5:e1302. doi: 10.1371/journal.pntd.0001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa I, Pajon R, Granoff DM. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio. 2014;5:e01625–14. doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71–9. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 33.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 2012;8:e1002688. doi: 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meningococcal disease control in countries of the African meningitis belt, 2013. Wkly Epidemiol Rec. 2014;89:206–14. [PubMed] [Google Scholar]
- 35.Micoli F, Romano MR, Tontini M, Cappelletti E, Gavini M, Proietti D, et al. Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. Proc Natl Acad Sci U S A. 2013;110:19077–82. doi: 10.1073/pnas.1314476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 37.Pajon R, Buckwalter CM, Johswich KO, Gray-Owen SD, Granoff DM. A native outer membrane vesicle vaccine confers protection against meningococcal colonization in human CEACAM1 transgenic mice. Vaccine. 2015;33:1317–23. doi: 10.1016/j.vaccine.2015.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin Vaccine Immunol. 2011;18:1002–14. doi: 10.1128/CVI.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188:1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 40.Vu DM, Wong TT, Granoff DM. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and Neisserial heparin binding antigen. Vaccine. 2011;29:1968–73. doi: 10.1016/j.vaccine.2010.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi R, Beernink PT, Giuntini S, Granoff DM. Susceptibility of meningococcal strains responsible for two serogroup B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C vaccine. Clin Vaccine Immunol. 2015;22:1227–34. doi: 10.1128/CVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lujan E, Pajon R, Granoff DM. Impaired immunogenicity of meningococcal Neisserial Surface Protein A in human xomplement Factor H transgenic mice. Infect Immun. 2015;84 doi: 10.1128/IAI.01267-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi R, Granoff DM, Beernink PT. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine. 2013;31:5451–7. doi: 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konar M, Rossi R, Walter H, Pajon R, Beernink PT. A mutant library approach to identify improved meningococcal Factor H binding protein vaccine antigens. PLoS One. 2015;10:e0128185. doi: 10.1371/journal.pone.0128185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giuntini S, Beernink PT, Granoff DM. Effect of complement Factor H on anti-FHbp serum bactericidal antibody responses of infant rhesus macaques boosted with a licensed meningococcal serogroup B vaccine. Vaccine. 2015;33:7168–75. doi: 10.1016/j.vaccine.2015.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun. 2011;79:3751–9. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.