Abstract
We investigated the effect of reward expectation on the processing of emotional words in two experiments using event-related potentials (ERPs). A cue indicating the reward condition of each trial (incentive vs non-incentive) was followed by the presentation of a negative or neutral word, the target. Participants were asked to discriminate the emotional content of the target word in Experiment 1 and to discriminate the color of the target word in Experiment 2, rendering the emotionality of the target word task-relevant in Experiment 1, but task-irrelevant in Experiment 2. The negative bias effect, in terms of the amplitude difference between ERPs for negative and neutral targets, was modulated by the task-set. In Experiment 1, P31 and early posterior negativity revealed a larger negative bias effect in the incentive condition than that in the non-incentive condition. However, in Experiment 2, P31 revealed a diminished negative bias effect in the incentive condition compared with that in the non-incentive condition. These results indicate that reward expectation improves top-down attentional concentration to task-relevant information, with enhanced sensitivity to the emotional content of target words when emotionality is task-relevant, but with reduced differential brain responses to emotional words when their content is task-irrelevant.
Keywords: reward expectation; emotional Stroop; negative bias; event-related potential
Introduction
A growing body of evidence indicates that attention and motivation interact with one another. Selective attention allocates limited processing resources in a space- or feature-based manner to stimuli that are central to current behavioral goals (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002; Egner and Hirsch, 2005; Polk et al., 2008), while the motivational system encodes incentive value, which defines and weights task goals to energize behavior (Robbins and Everitt, 1996; Schultz, 2000; Pessoa, 2009; Pessoa and Engelmann, 2010; Chiew and Braver, 2011; Chelazzi et al., 2013).
Although the mechanisms involved in the interaction between attention and motivation are still not clear, it was highlighted recently that motivational factors may exert a strong influence on task performance by enhancing executive function and task concentration (Huguet et al., 2004; Pessoa, 2009; Savine and Braver, 2010; Veling and Aarts, 2010; Chiew and Braver, 2011; Padmala and Pessoa, 2011; Chelazzi et al., 2013). For example, following a monetary incentive or non-incentive cue, Padmala and Pessoa (2011) asked participants to perform a response conflict task, in which a target picture of a house or building was presented together with a task-irrelevant congruent, incongruent, or neutral word. The results revealed that participants exhibited a reduced conflict effect during the reward vs no-reward condition, and that cue-related responses in the frontoparietal attentional control regions were predictive of reduced conflict-related signals in the medial prefrontal cortex and the anterior cingulate cortex regions during the upcoming target phase. This study demonstrated that monetary incentives may fine-tune top-down attentional settings to better select task-relevant information and to inhibit task-irrelevant information. Recent electrophysiological evidence has demonstrated that better attentional preparation, indexed by larger contingent negative variation, predicts shorter response times (RTs) and smaller congruency effects to subsequent Stroop stimuli (van den Berg et al., 2014).
Although recent behavioral and neurocognitive studies have demonstrated the effects of monetary incentive on attentional selection across time (Bijleveld et al., 2011), across space (Small et al., 2005; Baines et al., 2011; Theeuwes and Belopolsky, 2012) and across features (Locke and Braver, 2008; Kiss et al., 2009; Hickey et al., 2010; Krebs et al., 2010; Padmala and Pessoa, 2011; Wang et al., 2013; van den Berg et al., 2014), little is known about the effect of motivational cues on the processing of emotional stimuli (Kaltwasser et al., 2013; Wei and Kang, 2014; Wei et al., 2014; Kang et al., 2015). We asked participants in a recent study to identify the emotional content of a target face, following a monetary incentive or a non-incentive cue (Wei et al., 2014). We observed that the amplitude of N300 revealed an interaction between reward expectation and emotional valence, providing that the negative bias effect (i.e. the amplitude differences between negative and neutral faces) was larger in the incentive condition than in the non-incentive condition. However, a recent study by Kaltwasser et al. (2013), using a similar incentive cuing paradigm in which participants were asked to identify the concreteness of positive, negative or neutral target words, found that emotion-related and reward-related effects occurred in different time windows, did not interact statistically, and revealed different topographies. Kaltwasser et al. concluded that reward expectancy and the processing of emotional word content were independent. When comparing these two studies, one should note that the emotionality of the target (either a face or a word) was task-relevant in the Wei et al. (2014) study, but was task-irrelevant in the study by Kaltwasser et al. (2013). It is possible, therefore, that the interaction between reward expectation and emotion is regulated by the task-relevance of the emotional content of the target (Wei and Kang, 2014). The primary aims of the current study were to determine whether reward-related motivational biases influenced the processing of the task-relevant and the task-irrelevant emotional information, and to chart the relationship between the effects of motivational bias and attentional selection in modulating the processing of emotional stimuli by using electrophysiological techniques.
The reward value and the emotional content of the target words were manipulated on a trial-by-trial basis in a cue-target paradigm using factorial designs in two electrophysiological experiments. A cue indicating the reward condition of each trial (incentive vs non-incentive) was followed by the presentation of an emotionally negative word or neutral word, the target. Participants were asked to discriminate the emotional content of the target word in Experiment 1 and to discriminate the color of the target word in Experiment 2, i.e. to perform an emotional Stroop task (Gotlib and McCann, 1984; Dalgleish and Watts, 1990). Hence, the emotional content of the target word was task-relevant in Experiment 1 but task-irrelevant in Experiment 2. Event-related potentials (ERPs) components of interest were the N1, P2, early posterior negativity (EPN), N400, and late positive complex/potential (LPC/LPP). The effects of emotional valence on ERPs have been reported to be evident from 100 ms after word onset in reading and lexical decision tasks (LDT; Hofmann et al., 2009; Scott et al., 2009; Rellecke et al., 2011; Bayer et al., 2012), in emotional categorization tasks (Herbert et al., 2006; González-Villar et al., 2014) and the emotional Stroop task (Thomas et al., 2007; González-Villar et al., 2014). Larger amplitudes of sensory components (N1, P2) to negative words have been hypothesized to reflect the rapid detection of relevant emotional information prior to full processing on a semantic level (Bernat et al., 2001; Thomas et al., 2007).
The EPN is a negativity at temporo-occipital electrodes around 200–320 ms, which increases in amplitude to emotional pictures or words compared with neutral stimuli (Junghöfer et al., 2001; Schupp et al., 2003, 2004; Franken et al., 2009; Schacht and Sommer, 2009a, 2009b), and it is thought to index enhanced sensory encoding, resulting from reflex-like visual attention to emotional stimuli. Some studies suggested that the EPN reflects an automatic, implicit processing of emotion, which was associated with effortless initial stages of attention orientation during access to emotional information, and was not affected by the depth of processing (Kissler et al., 2009; Schacht and Sommer, 2009b). For example, Kissler et al. (2009) found enhanced EPN for emotionally arousing words (pleasant and unpleasant), compared with neutral words, during both a reading task and a task in which words belonging to a particular word-class were counted, regardless of whether the word belonged to a target or a non-target category. However, later studies have observed that the EPN amplitudes were sensitive to the emotional content only when sufficient attention was allocated to the target stimuli in tasks requiring deep processing. For instance, the EPN has been observed in LDT (Scott et al., 2009; Hinojosa et al., 2010; Rellecke et al., 2011; Bayer et al., 2012) and emotional categorization tasks (Frühholz et al., 2011; González-Villar et al., 2014), but not in reading tasks (Bayer et al., 2012) or the emotional Stroop task (Frühholz et al., 2011). The experimental manipulations in the current study allow us to further compare ERP amplitudes elicited by emotional words under different levels of processing requirements and, importantly, under different motivational states. The results can provide further evidence about the extent of automatic processing of emotional words at this stage and whether this stage of processing is affected by top-down motivational significance.
The LPC, a positivity belonging to the P300 family, typically develops around 300 ms after stimulus onset and lasts for several 100 ms, including P31 and P32 (or P3a and P3b: Comerchero and Polich, 1999; Polich, 2007). Augmented LPC amplitudes to emotional stimuli, as compared with neutral stimuli, are supposed to reflect elaborate processing and stimulus evaluation (Cuthbert et al., 2000; Polich, 2007; Schacht and Sommer, 2009b), and are found to be modulated by different task demands (e.g. González-Villar et al., 2014; Schacht and Sommer, 2009b; but see Frühholz et al., 2011). Moreover, enlarged LPC amplitudes were observed recently to stimuli following an incentive cue compared with that following a non-incentive cue, indicating enhanced allocation of attention to rewarding stimuli (Baines et al., 2011; Krebs et al., 2013; Schevernels et al., 2014; van den Berg et al., 2014; but see Kaltwasser et al., 2013).
The N400 component, a centro-parietal negativity arising around 400 ms after stimulus onset, traditionally has been considered the most prominent ERP component indicating postlexical semantic processing (Kutas and Federmeier, 2000), and has been reported to be modulated by the emotional content of the processed words (Kissler et al., 2006; Citron, 2012).
In accordance with previous studies, we expected emotional words to elicit larger amplitudes of the EPN and the LPC components. Earlier emotional effects on ERPs responses were expected in Experiment 1, in which the emotional content of the target words was task-relevant, but not in Experiment 2. Moreover, we assumed that the reward expectation would lead to the allocation of neural resources, as reflected in the enhancement of attention-related components (e.g. N1, P2, N400 and the LPC), and lead to improved behavioral performance compared with the non-incentive condition. Most importantly, if the effects of motivational bias are mediated through better biased attentional control toward task-relevant information, we would expect to observe interactions in the modulation of certain potentials by reward values and emotional content, but with reversed patterns. Enhanced top-down attentional tuning under the monetary incentive condition might amplify the potential differences between negative and neutral stimuli when emotionality is task-relevant, but it might reduce the interference effect caused by processing the task-irrelevant emotional information when emotionality is task-irrelevant.
Materials and methods
Participants
Two groups of 18 undergraduate and graduate students participated in Experiments 1 and 2. Data from two participants in Experiment 1 and one participant in Experiment 2 were discarded due to excessive error rates (>20%). Another participant’s data in Experiment 2 were discarded due to difficulty of concentrating on the task and erratic brain waves. All remaining participants (8 females, 19–25 years of age in Experiment 1; 8 females, 20–25 years of age in Experiment 2) were right-handed with normal or corrected-to-normal vision and had no known cognitive or neurological disorders. This study was approved by the Ethics Committee of the Department of Psychology at Capital Normal University, and all participants gave informed consent prior to the experiments, in accordance with the Declaration of Helsinki.
Design and materials
A 2 × 2 within-participant factorial design was used for both experiments, with the first factor being the reward condition (incentive vs non-incentive), and the second factor being the emotional content of the target words (negative vs neutral).
A total of 96 negative words and 96 neutral words selected from the Affective Norms for Chinese Words (Wang et al., 2008) and matched according to their word frequency (M ± s.d.: Neutral = 5.3 ± 0.56; Negative = 4.5 ± 0.57) and word complexity in writing (M ± s.d.: Neutral = 16.8 ± 4.2; Negative = 17.5 ± 4.6). The normative valence ratings (M ± s.d.: Neutral = 5.4 ± 0.57; Negative = 2.8 ± 0.35, P < 0.001) and arousal values (M ± s.d.: Neutral = 3.9 ± 0.32; Negative = 6.2 ± 0.49, P < 0.001) of the words differed significantly between the two word categories. The words used in this study are listed in the Supplementary material in Chinese, along with English translations.
Procedures
The presentation of stimuli and recording of RTs and error rates were controlled by Presentation software (http://nbs.neuro-bs.com/). Participants were seated in a dimly lit and sound-attenuated room. At the start of each trial (Figure 1), a white fixation cross measuring 0.4° × 0.4° in visual angle appeared at the center of a black screen for 500 ms, followed by a cue (* or #) measuring 2.3° × 2.3° in visual angle for 1000 ms. For half of the participants, the ‘*’ cue indicated an incentive trial and the ‘#’ cue indicated a non-incentive trial, and vice versa for the other half of the participants. After a variable cue-target interval of 600–1000 ms, the target word (visual angle, 2.9° × 1.3°), colored white in Experiment 1, and colored red or green (RGB: 255, 0, 0 and 7, 168, 7, respectively) in Experiment 2, was presented in the center of the screen for 300 ms. Participants were instructed to respond to the emotionality of the target word in Experiment 1 and to respond to the color of the target word in Experiment 2, as quickly and accurately as possible upon the presentation of the target word using two response buttons (the left and right buttons on the computer mouse) under the right index finger and the right middle finger. The assignments of the response buttons to the target emotions (negative vs neutral) in Experiment 1 and the response buttons to the target colors (red vs green) in Experiment 2 were counterbalanced across participants within each experiment.
Fig. 1.
Example of the trial sequence in Experiments 1 and 2. The task in Experiment 1 was to discriminate the emotional content of the target word, while the task in Experiment 2 was to discriminate the color of target word, which was colored in red or in green in the actual display.
After the target word was displayed, the fixation point was shown again for 1400–1800 ms, followed by the presentation of a feedback stimulus for 500 ms. For non-incentive trials, a filled gray circle was the feedback stimulus indicating a correct response and an empty gray circle was the feedback stimulus indicating an incorrect response. For the incentive trials, a picture of one Chinese Yuan coin was presented following responses that were correct and faster than the baseline RT (determined in the practice session, see later), a filled gray circle was presented following responses that were correct but slower than the baseline RT, and an empty gray circle was presented following incorrect responses, as in the non-incentive trials. The fixation point was then presented during the intertrial interval (1100–1600 ms).
The experimental series had 384 trials in total, with each experimental condition having 96 trials. The experimental trials were divided into eight sessions, with each session consisting of 48 trials (and each condition having 12 trials) in pseudo-randomized order.
Participants received 32 practice trials before the experiment. During the practice phase, participants were informed that the cue pictures were task-irrelevant and they were instructed to ignore them. They were required to respond as quickly and accurately as possible by following the corresponding task instructions. There was only correct and false feedback (no coin feedback) during the practice session. The filled or empty gray circle was presented to indicate a right or wrong response, respectively. The averaged RT for each participant during the practice phase was used as that participant’s baseline RT.
After the practice session, participants were informed of the meaning of the cue picture and the presentation rule of the coin feedback in the formal experiment. They were informed that they would gain an additional 20 Chinese Yuan as a reward if they managed to get the coin feedback in a certain amount of trials (>75% of the total incentive trials in Experiment 1 and >60% in Experiment 2).
ERP recordings and analyses
ERP recordings were obtained from 62 scalp sites using Ag/AgCI electrodes embedded in an elastic cap at locations from the extended International 10–20 System (NeuroScan; Compumedics, EI Paso, TX, USA). These electrodes were referenced to the right mastoid during recording and rereferenced to the average of the right and left mastoid potentials offline. Two additional channels were used for monitoring horizontal and vertical electro-oculographic (EOG) recordings. Impedance was reduced below 5 kΩ, and electroencephalograph signals were filtered with a band-pass of 0.05–40 Hz and sampled at a rate of 500 Hz. Each averaging epoch lasted 1000 ms, with an additional 100 ms recorded prior to stimulus onset to allow for baseline correction. Erroneous trials were excluded from the analyses. Trials with a voltage, relative to the 100 ms baseline, exceeding ±75 µV at any electrode were excluded from the analysis, as were trials with artifacts in the EOG channels. The average percentages of excluded trials in the incentive negative, incentive neutral, non-incentive negative and non-incentive neutral conditions were 3.5, 3.5, 3.5 and 3.6%, respectively, in Experiment 1, with no significant difference between conditions, F < 1. In Experiment 2, the average percentages of excluded trials for the earlier conditions were 4.7, 3.5, 4.1 and 4.4%, respectively. Although the percentage of excluded trials in the incentive negative condition was larger than that in the incentive neutral condition, t(15) = 3.0, P < 0.01, they were both <5%. The total number of excluded trials, including erroneous trials and artifact trials, was <8% in each condition of each experiment, resulting in over 80 valid trials per condition in each experiment.
Based on visual inspection of the effects and findings from previous ERP studies on reward and emotional words processing, we calculated posttarget responses over the frontocentral and parietal electrodes (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4, P3, Pz and P4), indexing the N1, P2, P31, N400 and P32 components (time windows: 50–150, 150–250, 300–380, 380–450 and 500–700 ms) in both experiments. Average amplitudes for each condition during each time window were analyzed by analyses of variance (ANOVAs) with three within-participant factors: reward (incentive vs non-incentive), emotional content of the target word (negative vs neutral) and electrodes.
In addition, responses over the lateral temporo-occipital electrodes (P3, P5, PO5, P4, P6, PO6) indexing EPN component (220–320 ms) were calculated for both experiments. Average amplitudes over the left (P3, P5, PO5) and the right electrodes (P4, P6, PO6) were analyzed by ANOVAs with three within-participant factors: reward (incentive vs non-incentive), emotional content of the target word (negative vs neutral) and electrode topography (left vs right). The effect of reward incentive was computed for each emotional condition, or for each topographical location, as the difference in mean amplitude between the incentive trial and the non-incentive trial, and the effect of negative bias was computed for each reward condition, or for each topographical location, as the difference between the mean amplitude for the negative target and the neutral target. The resultant values were subjected to planned pairwise comparisons.
Moreover, comparisons across experiments were performed for certain components by including the experiment as a between-participant factor and the reward condition, the emotional content and the electrodes as within-participant factors.
All the ANOVAs had a level of significance set to 0.05, and were supplemented with Bonferroni pairwise comparisons or simple main-effects comparisons, when appropriate. Greenhouse-Geisser corrections were used with all effects having two or more degrees of freedom in the numerator. All repeated-measures ANOVAs are reported with uncorrected degrees of freedom but corrected P values.
Results
Behavioral results
Incorrect responses were excluded and inverse efficiencies (IEs) were calculated to control for possible speed-accuracy trade-offs. IEs were calculated as the mean correct RT divided by accuracy rate, separately for each participant and each condition (Townsend and Ashby, 1983; Kiss et al., 2009; Lee and Shomstein, 2013). The mean RTs, response error rates and IEs in each experimental condition of the two experiments are reported in Table 1.
Table 1.
Mean RTs (ms), error rates (%) and IEs (ms) with SEs in parentheses in terms of the experimental conditions in both experiments
| Incentive |
Non-incentive |
||||
|---|---|---|---|---|---|
| Negative | Neutral | Negative | Neutral | ||
| Exp 1 | RTs (SE) | 543 (20) | 575 (20) | 630 (21) | 660 (22) |
| Error rates (SE) | 5.0 (0.8) | 5.9 (1.1) | 3.9 (0.7) | 3.8 (0.8) | |
| IEs (SE) | 577 (20) | 617 (24) | 664 (24) | 696 (25) | |
| Exp 2 | RTs (SE) | 420 (18) | 422 (18) | 496 (26) | 500 (27) |
| Error rates (SE) | 2.3 (0.6) | 2.5 (0.6) | 2.9 (0.6) | 4.0 (1.0) | |
| IEs (SE) | 435 (17) | 439 (18) | 518 (27) | 524 (25) | |
An ANOVA was conducted on the IEs in both experiments, with reward (incentive vs non-incentive) and the target’s emotional valence (negative vs neutral) as within-participant factors. The results from Experiment 1 revealed both a main effect of reward, F(1, 15) = 50.13, P < 0.001, and a main effect of emotionality, F(1, 15) = 7.03, P < 0.05, with faster IEs in the incentive condition than in the non-incentive condition (597 vs 680 ms), and with faster IEs to the negative words than to the neutral words (620 vs 657 ms). The interaction effect was not significant, F(1, 15) < 1. The results from Experiment 2 revealed only a main effect of reward, F(1, 15) = 42.87, P < 0.001, with faster IEs in the incentive trials (437 ms) than in the non-incentive trials (521 ms). There was no significant effect of the target emotional content, nor a significant interaction of reward × target emotional content, F(1, 15) = 1.30, P > 0.1, and F(1, 15) < 1, respectively. The ANOVA on the RTs revealed the same pattern as the ANOVA on the IEs, with a significant incentive effect, F(1, 15) = 47.61, P < 0.001, and emotional effect, F(1, 15) = 15.64, P < 0.005, in Experiment 1, and a significant incentive effect in Experiment 2, F(1, 15) = 42.79, P < 0.001.
The same ANOVA was used to analyze error rates in both experiments. The results of Experiment 1 revealed a main effect of reward, F(1, 15) = 6.47, P < 0.05, with more errors committed in the incentive condition (5.4%) than in the non-incentive condition (3.9%). No other effects reached statistical significance. The results of Experiment 2 also revealed a main effect of reward, F(1, 15) = 4.71, P < 0.05, with fewer errors committed in the incentive condition than in the non-incentive condition (2.4 vs 3.5%). Neither a main effect of emotion nor an interaction between incentive conditions and emotion was found with both F’s < 1.
ERP results
Experiment 1
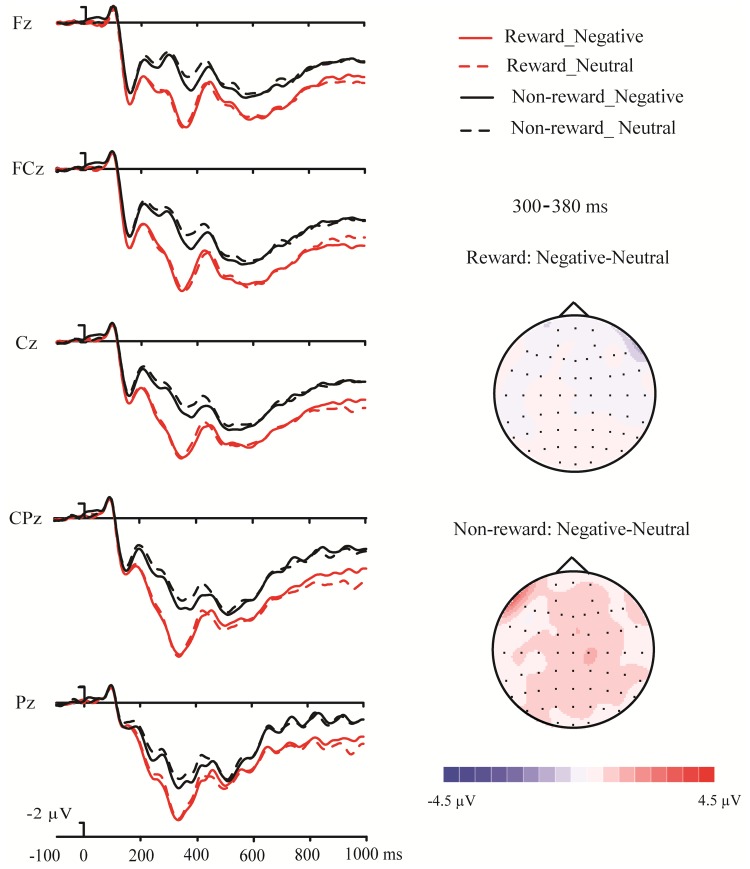
ERP responses time-locked to the target onset from selected example electrodes are depicted in Figures 2 and 4 (left panel). Compared with the non-incentive condition, the target words in the incentive condition elicited more positive-going ERP responses. Moreover, the differences between the ERP responses for negative and neutral words in the incentive condition were larger than that in the non-incentive condition. The results of the ANOVAs on the mean amplitudes of the N1, P2, EPN, P31, N400 and P32 components are reported in the upper panel of Table 2.
Fig. 2.
Grand average waveforms at the midline electrodes showing the potentials produced in response to the presentation of the target stimulus in Experiment 1. The topographies of the P31 potential are shown on the right. Positive voltage is plotted downwards. For all waveforms, rewarded trials are represented in red lines, and non-rewarded trials are in black lines. Negative conditions are plotted in solid lines, and neutral conditions are plotted in dotted lines.
Fig. 4.
Grand average waveforms at the P4 electrode showing the EPN component in response to the target in Experiment 1 (the left one) and in Experiment 2 (the right one). Positive voltage is plotted downwards.
Table 2.
Results of the ANOVAs on mean amplitudes for N1, P2, EPN, P31, N400 and P32 components in both experiments
| N1 (50–150 ms) | P2 (150–250 ms) | EPN (220–320 ms) | P31 (300–380 ms) | N400 (380–450 ms) | P32 (500–700 ms) | |||
|---|---|---|---|---|---|---|---|---|
| Exp 1 | Reward | F | 5.69 | 8.82 | 7.00 | 26.50 | 26.94 | 26.22 |
| P | * | ** | * | *** | *** | *** | ||
| Emotion | F | 37.99 | 44.23 | 39.02 | 11.04 | 4.02 | ||
| P | *** | *** | *** | ** | 0.06 | |||
| Reward × Emotion | F | 4.54 | 5.04 | |||||
| P | * | * | ||||||
| Reward × Electrodes | F | 2.40 | 7.80 | 22.98 | 13.27 | 10.69 | ||
| P | ** | *** | *** | *** | *** | |||
| Emotion × Electrodes | F | 3.68 | 2.99 | |||||
| P | * | * | ||||||
| Reward × Emotion × Electrodes | F | |||||||
| P | ||||||||
| Exp 2 | Reward | F | 6.21 | 35.33 | 19.62 | 117.42 | 55.39 | 21.55 |
| P | * | *** | *** | *** | *** | *** | ||
| Emotion | F | 4.84 | 4.01 | |||||
| P | * | 0.06 | ||||||
| Reward × Emotion | F | 3.98 | ||||||
| P | 0.06 | |||||||
| Reward × Electrodes | F | 8.12 | 8.52 | 9.49 | 16.28 | 6.37 | 9.31 | |
| P | *** | *** | ** | *** | ** | *** | ||
| Emotion × Electrodes | F | |||||||
| P | ||||||||
| Reward × Emotion × Electrodes | F | 5.74 | ||||||
| P | * | |||||||
For EPN, all df = (1, 15). For the other components, for Reward, Emotion and Reward × Emotion, df = (1, 15); for Electrodes, Reward × Electrodes, Emotion × Electrodes and Reward × Emotion × Electrodes, df = (14, 210).
*P < 0.05, **P < 0.01, ***P < 0.001.
Because the analyses on the EPN and the P31 components revealed significant interactions between reward and emotionality, subsequent pairwise comparisons were performed, respectively, for these two components. For the EPN component, the difference in mean amplitude between the negative and the neutral words in the incentive condition (mean difference = 1.45 µV) was larger than that in the non-incentive condition (mean difference = 0.78 µV), t(15) = 2.13, P < 0.05. The same pattern was observed for the P31 component, with significantly larger difference between the mean amplitude for negative and neutral words in the incentive condition (mean difference = 3.22 µV) than in the non-incentive condition (mean difference = 1.93 µV), t(15) = 2.25, P < 0.05.
Experiment 2
ERP responses time-locked to the target onset are shown in Figures 3 and 4 (right panel). Compared with the non-incentive condition, the target words in the incentive condition elicited more positive-going ERP responses. Moreover, while the neutral and negative words elicited differential ERP responses around 400 ms after target onset in the non-incentive condition, this difference was not observed in the incentive condition during the same time window. The results of the ANOVAs on the mean amplitudes of the N1, P2, P31, N400 and P32 components are reported in the lower panel of Table 2.
Fig. 3.
Grand average waveforms at the midline electrodes showing the potentials produced in response to the presentation of the target stimulus in Experiment 2. The topographies of the P31 potential are shown on the right. Positive voltage is plotted downwards.
The analysis on the EPN component revealed a significant Reward × Emotion × Electrodes interaction, F(1, 15) = 5.74, P < 0.05. Separate ANOVAs with reward and the emotional content of the target word as within-participant factors were performed for the left, and the right electrodes, separately. Data from left electrodes revealed only a significant main effect of reward, F(1, 15) = 44.35, P < 0.001, while data from right electrodes revealed both the main effect of reward, F(1, 15) = 24.33, P < 0.001, and the main effect of target emotion, F(1, 15) = 5.23, P < 0.05. No other effects were statistically significant.
Analysis on the P31 component revealed a marginally significant interaction between reward and emotionality, F(1, 15) = 3.98, P = 0.06. Subsequent pairwise comparisons revealed that the difference between the mean amplitude for negative and neutral words was significantly smaller in the incentive condition (mean difference = 0.04 µV) than in the non-incentive condition (mean difference = 0.77 µV), t(15) = 2.00, P = 0.06.
Overall analysis across Experiments 1 and 2
A cross-experiment ANOVA on the EPN component revealed a main effect of reward, F(1, 30) = 35.03, P < 0.001, a main effect of emotion, F(1, 30) = 35.25, P < 0.001, and a main effect of electrode topography, F(1, 30) = 11.98, P < 0.005, with larger amplitudes for the incentive conditions, the negative targets and the right electrodes. Moreover, the emotion factor significantly interacted with the experiment factor, F(1, 30) = 6.50, P < 0.05, with a larger difference in amplitude between the negative and neutral targets in Experiment 1 than in Experiment 2 (1.1 vs 0.5 µV). Importantly, the interaction between reward and emotion also interacted with the experiment factor, F(1, 30) = 4.79, P < 0.05, statistically confirming the differential patterns of Reward × Emotion interaction across the two experiments.
ANOVA on the P31 component revealed both a main effect of reward, F(1, 30) = 92.35, P < 0.001, and a main effect of emotion, F(1, 30) = 32.81, P < 0.001. The experiment factor significantly interacted with emotion, F(1, 30) = 17.38, P < 0.001, with a larger difference in amplitude between negative and neutral targets in Experiment 1 than in Experiment 2 (2.6 vs 0.4 µV). Importantly, although the overall interaction between reward and emotion was not significant, F(1, 30) < 1, this interaction was significantly modulated by the experiment factor, F(1, 30) = 8.79, P < 0.01, confirming that the differential patterns of Reward × Emotion interaction between experiments, as reported earlier, were reliable.
Discussion
By using electrophysiological techniques and by asking participants to perform tasks with the emotionality of the target word as task-relevant or task-irrelevant information under monetary incentive or non-incentive conditions, this study demonstrated that reward generally facilitates task performance and that reward modulates brain responses to negative and neutral stimuli according to the current task goal. ERP responses to target words were reliably modulated by reward from 50 ms after the target onset until later time windows in both experiments, with more positive-going ERPs in the incentive conditions than in the non-incentive conditions. The emotional effects were modulated by the task-relevance of the target’s emotionality, such that ERP amplitude differences between the negative and the neutral targets were observed from 150 ms after the target onset in Experiment 1, but they were observed only for the N400 and the EPN components in Experiment 2. Importantly, P31 (300–380 ms posttarget onset over the frontocentral and parietal electrodes) and EPN (220–320 ms posttarget onset over the bilateral temporo-occipital electrodes) components in Experiment 1 and P31 in Experiment 2 revealed an interaction between reward and emotion, and the interaction pattern was modulated by the task-relevance of the target’s emotionality. Specifically, when the emotionality of the target word was task-relevant in Experiment 1, P31 and EPN revealed greater amplitude differences between the negative and the neutral words under the incentive condition, as compared with the non-incentive condition. However, when the emotionality of the target was task-irrelevant in Experiment 2, P31 exhibited a reverse interaction pattern between reward expectation and emotionality, such that the amplitudes for the negative and the neutral words differed from each other under the non-incentive condition, but did not differ under the incentive condition. These results indicate that reward expectation improves top-down attentional concentration to task-relevant information, with enhanced sensitivity to the emotional content of the target words when emotionality was task-relevant, but with reduced processing of the emotional content when it was task-irrelevant.
In both of our experiments, the main effect of reward was consistently observed in the behavioral data and ERPs, with faster RTs and more positive-going brain responses observed in the incentive condition relative to the non-incentive condition, replicating the effect of monetary reward to facilitate task performance (Small et al., 2005; Navalpakkam et al., 2009; Krebs et al., 2010; Baines et al., 2011; Padmala and Pessoa, 2011; Schevernels et al., 2014; van den Berg et al., 2014). These results are consistent with the notion that motivational incentive improves cognitive control and biases the focus of selective attention toward goal-directed aspects of task stimuli (Pochon et al., 2002; Locke and Braver, 2008; Savine and Braver, 2010; Veling and Aarts, 2010; Chelazzi et al., 2013; Wei and Kang, 2014). The motivational cue affected target processing across a wide range of time windows in both experiments, suggesting that motivational incentive affects successive stages of processing, including stimulus encoding, perception, lexical and semantic identification and response execution. The reward effect in early time window has been observed in previous studies in which the reward was directly related to the physical identity of the critical stimulus. For example, by using an additional singleton paradigm in which participants were asked to discriminate the line orientation in a shape singleton while an irrelevant color singleton was presented in the search display, Hickey et al. (2010) found that the attentional selection of the target stimuli (the P1 and N2pc components) had significantly larger amplitudes in target feature-repetition trials (i.e. the target color was the same as in the previous trial) following high rewards. Similarly, Schacht et al. (2012) asked German participants to learn to associate previously unknown Chinese words with monetary gain, loss, or neither, and later to distinguish the learned stimuli from the novel distracters. An enhanced early (around 150 ms) and a later (550–700 ms) emotional effect were observed for stimuli associated with monetary gain. These results indicate that the perceptual salience of stimulus features that have just been associated with reward may directly affect attentional allocation. Given that these early-stage effects rely on the previous association between a certain stimulus and reward (see also Krebs et al., 2013), they do not reflect preparatory or strategic effects of incentive cues on the processing of the following target (Schevernels et al., 2014; van den Berg et al., 2014).
Recent studies using a modified cue-target paradigm have found the effects of reward expectation on target-elicited brain responses at relatively later time windows compared with the current results (Baines et al., 2011; Schevernels et al., 2014; van den Berg et al., 2014). For example, Baines et al. (2011) manipulated the cue presenting in the center of the screen, which not only indicated the reward value of a given trial but also the probable spatial location of a subsequent target stimulus that would appear in the left or the right side of the periphery. The results found that reward expectation modulated the amplitude of the target-elicited P31 (300–350 ms post stimulus) and P32 (450–550 ms post stimulus) potentials. In the van den Berg et al. (2014) study, Stroop target stimuli following incentive and non-incentive cues elicited no differences in ERP responses until the occipital N2/frontal P2 complex (150–200 ms posttarget onset). Schevernels et al. (2014), who manipulated the cue so that it predicted not only the prospect of reward but also the attentional demand on the upcoming target, found that the effect of reward expectation on the target-elicited ERP responses began to occur also from the P2 component (200–250 ms posttarget onset). However, ERPs responses to target words were modulated by reward expectation from 50 ms after the target onset in the current two experiments. In addition, Kaltwasser et al. (2013) reported larger amplitudes for words with an expected gain cue compared with words with expected loss cue, or words with a zero outcomes cue at 0–100, 100–200 and 200–300 ms after word onset. Although we have no specific hypothesis, we speculate that the earlier effects of a reward prospect on the processing of target stimuli in the current study and the Kaltwasser et al. (2013) study might have occurred because we used emotional stimuli as the targets (half of the targets in the current experiments and two-thirds of the targets in Kaltwasser et al., 2013), instead of non-emotional Stroop words or shapes, as were used in the studies mentioned earlier (Baines et al., 2011; Schevernels et al., 2014; van den Berg et al., 2014). An incentive cue may increase perceptual sensitivity to emotional stimuli because of the rapid communication between the reward and emotion circuits in the brain (e.g. Baxter and Murray, 2002; Beaver et al., 2008), producing prompt reward effects on the target-elicited brain responses.
Although the effects of emotionality in the behavioral data and the ERPs were pronounced in Experiment 1, in which the emotional content of the target word was task-relevant, the effects were only observed in the N400 and EPN components of ERPs in Experiment 2, in which the emotional content was task-irrelevant. This finding is consistent with previous studies showing the importance of task-relevance in determining whether emotional stimuli capture attention or not (Eimer and Holmes, 2007; Thomas et al., 2007; Frühholz et al., 2011; Lichtenstein-Vidne et al., 2012; Vogt et al., 2013; González-Villar et al., 2014). On the one hand, the processing of task-relevant or task-irrelevant emotional information depends strongly on the stimulus domain. Compared with emotional faces or pictures, words are believed to be less evolutionarily prepared and have lower priority for consuming limited attentional resources (Schacht and Sommer, 2009a; Rellecke et al., 2011). In our previous studies using a similar design, but an emotional face as the target (Wei and Kang, 2014; Wei et al., 2014), the emotional information produced stronger effects on both RTs and brain responses compared with the current results. Wei and Kang (2014) reported a main effect of emotion and an interaction between reward and emotion when the emotional content of the target face was task-relevant, and a main effect of emotion when the emotional content was task-irrelevant (the gender discrimination task), which suggests the prioritized processing of emotional facial expressions. On the other hand, the processing of emotional information has been found to depend on the required task (Eimer and Holmes, 2007; Frühholz et al., 2011; Lichtenstein-Vidne et al., 2012; Vogt et al., 2013; González-Villar et al., 2014). For example, although the emotionality of the target word was task-irrelevant in both Kaltwasser et al. (2013) and the current Experiment 2, the emotional effect was observed in the former but not in the latter study. In Kaltwasser et al. (2013), the task was to discriminate the concreteness of the target word, which may required analyzing the meaning of the word and, hence, the processing of the emotional content, resulting in behavioral interference with or facilitation of the main task. In our Experiment 2, the task requirement of discriminating the color of the target word was relatively superficial, without any need to analyze the meaning of the word. Indeed, a number of studies that used this emotional Stroop paradigm with non-clinical participants did not observe the emotional effect at the behavioral level (e.g. Becker et al., 2001; Franken et al., 2009; Kampman et al., 2002; Thomas et al., 2007; Yovel and Mineka, 2004; but see González-Villar et al., 2014).
The timing of the emotional effect on ERP responses in this study is consistent with recent studies using explicit emotion categorization tasks and the implicit emotional Stroop task (Thomas et al., 2007; Franken et al., 2009; Frühholz et al., 2011; González-Villar et al., 2014), but at variance with studies that have reported emotional effects in earlier time windows using other tasks (Bernat et al., 2001; Scott et al., 2009; Rellecke et al., 2011; Bayer et al., 2012). For example, Rellecke et al. (2011) asked participants to perform an easy and superficial face-word discrimination task, in which the emotional content was task-irrelevant, and found emotional effects for both words and facial expressions between 50 and 100 ms after stimulus onset. Scott et al. (2009) used emotionally positive, negative and neutral words with high or low frequency in a LDT and found that high frequency negative words elicited larger N1 and P1 amplitudes than low frequency negative words. Such very early emotion-dependent modulations of words are assumed to occur before full semantic access. However, recent studies comparing an emotion categorization task and the emotional Stroop task usually have found no emotional effect in the P1-N1 time period across the two tasks (Thomas et al., 2007; Franken et al., 2009; Frühholz et al., 2011; González-Villar et al., 2014). These two lines of evidence support the notion that the early processing of emotional words can be regulated by task requirements.
On comparing the explicit and implicit processing of emotional words, the most consistent effects are observed in the P2, N2/EPN and LPC components (Thomas et al., 2007; Franken et al., 2009; Frühholz et al., 2011; González-Villar et al., 2014), which suggests sustained attention to task-relevant or task-irrelevant emotional words. However, no consensus has been reached as to whether the early or late potentials are regulated by the level of processing required for the target words. For example, Thomas et al. (2007) observed that threatening words elicited larger P2 amplitudes (preferentially in the right hemisphere) in the implicit task and a larger P3 in the explicit task, as compared with neutral words. However, a study by González-Villar et al. (2014) using middle-aged women as participants reported that P2 was enhanced for negative nouns across implicit and explicit tasks, but the N2 and the LPP was enhanced for emotional relative to neutral words only in the emotion categorization task. In the current study, the P2 and P3 components were modulated by emotional content in Experiment 1 but not in Experiment 2, supporting the notion that these brain modulations by emotion are dependent upon the degree of attention directed to the word content.
The striking finding here was that the interactions between reward expectation and the emotionality of the target in the brain responses were modulated by motivational significance. EPN and P31 revealed greater amplitude differences between the negative and the neutral words in the incentive condition than in the non-incentive condition in Experiment 1 but P31 exhibited a reverse interaction pattern between reward expectation and emotionality in Experiment 2. First of all, it is important to note that the current EPN, though occurring at the similar time window and the similar brain regions reported in previous studies (Schupp et al., 2004, 2006, 2007; Kissler et al., 2007, 2009; Schacht and Sommer, 2009a, 2009b; Hinojosa et al., 2010; Frühholz et al., 2011; Rellecke et al., 2011), exhibited more positive-going responses for the negative targets than for the neutral targets in both experiments.
Inconsistent results have been reported as to whether the EPN is affected by different levels of processing requirements (Hinojosa et al., 2010; Frühholz et al., 2011; Rellecke et al., 2011; Bayer et al., 2012) or not (Kissler et al., 2009; Schacht and Sommer, 2009b). However, previous studies typically have found that processing negative or positive words relative to neutral words was associated with a negative-going potential over the temporo-occipital regions at ∼150–300 ms post stimulus onset (i.e. the EPN), followed by a positive-going potential (the LPC) over the centro-parietal regions. Yet, we observed more positive-going ERPs for the negative targets than for the neutral targets not only at the frontocentral and parietal electrodes (as shown in Figures 2 and 3) but also at the temporo-occipital electrodes (as shown in Figure 4). Although most studies have been conducted using Western languages as experimental materials, such as German, English or Spanish, some studies have reported that negative Chinese words can elicit a typical EPN in LDT (Schacht et al., 2012). Thus, it is unlikely that our observed effect is specific to Chinese words. However, the early posterior component observed here may differ from the genuine EPN reported in previous research for the following possible reasons.
On the one hand, a previous study of ours that used a similar design but an emotional face as the target, also observed more negative-going ERPs for neutral faces than for negative faces over the temporo-occipital electrodes (though the positive faces elicited the most negative responses) (Wei et al., 2014). The augmented EPN has been suggested to reflect the capturing of attention by emotionally salient stimuli because the scalp distribution and latency of the EPN resemble the ERP components elicited by attentional selection of task-relevant stimuli at this early stage (Potts and Tucker, 2001; Schupp et al., 2007). It is possible that in the current experimental setting (as well as in Wei et al., 2014), in which a cue was presented first, participants exerted a certain degree of top-down expectation of the upcoming target, whether it was an incentive or non-incentive cue. This could have produced a different preparation state of the brain from that of participants in previous studies who did not have a preceding cue. In Experiment 1, if the later more positive amplitude for negative targets relative to neutral targets, as reflected in the P2, N400 and P3 components, represents more elaborate processing of the negative targets, the more positive amplitude for negative targets at the EPN time window might be an earlier manifestation of this elaborate processing at the temporo-occipital regions, resulting from the stronger top-down expectation. Neuroimaging studies have reported enhanced spatial selective signals in the visual cortex when a greater reward magnitude was expected in a spatial cuing paradigm, which suggests that monetary incentives play a role in regulating early visual processing of task-relevant stimuli (Small et al., 2005; Tosoni et al., 2013). Moreover, as employing a different strategy (e.g. does not prepare for the upcoming target, or respond slowly or incorrectly to the target) in the non-incentive trials would not facilitate fast correct responses in the incentive condition, the non-incentive condition revealed a similar pattern of response differences between the negative and neutral targets, although to a lesser degree. In Experiment 2, attentional capture by task-irrelevant negative information was not favored, since it would interfere with the color discrimination task, and thus, this early reflex-like response to negative words might be suppressed by the aforementioned top-down influences. As mentioned earlier, the most relevant paper by Kaltwasser et al. (2013), in which participants were asked to discriminate the concreteness of the emotional target word after incentive or non-incentive cues (i.e. the emotional content was task-irrelevant), reported that the EPN was not regulated by either target valence or reward expectation. The authors explained that the absence of EPN may have resulted from the demanding task requirements that may have consumed cognitive resources and competed with the involuntary attention capturing of the emotional target. Indeed, it seems that the occurrence and, perhaps, even the polarity of the EPN can be affected by top-down task requirements and, possibly, by motivational expectations. To the authors’ knowledge, there is not much evidence about the processing of emotional words under cued motivational paradigms, except for the aforementioned studies. We acknowledge that our study cannot provide direct explanations as to why ERP responses at the temporo-occipital electrodes at this time window revealed more positive amplitudes for negative than for neutral words. Future studies are needed to investigate the ERP responses to emotional stimuli under different monetary incentive conditions, and to what extent the EPN is sensitive to different motivational expectations.
On the other hand, studies have shown that EPN can be affected by non-emotional aspects of target stimuli, such as composition in the picture domain (Bradley et al., 2007; Van Strien et al., 2009; Wiens et al., 2011), and frequency in the word domain (Scott et al., 2009). Although rarely reported, we found several studies that observed more positive-going EPN for negative or high-arousal stimuli relative to neutral stimuli (Scott et al., 2009; Van Strien et al., 2009; Wei et al., 2014). For example, Scott et al. (2009) used positive, negative and neutral words with high or low frequency in a LDT and found an interaction between emotion and frequency for the EPN time window. High frequency negative and positive words elicited more negative voltages than neutral words, as usually observed for the EPN, but low frequency negative words elicited numerically more positive voltages than did neutral words. Words with high frequency may have an advantage in capturing attention at a relatively early stage of processing. In the current study, although we matched word frequency so there was no statistically significant difference between the two categories, the neutral targets had a numerically higher word frequency than the negative words did. There is a small possibility that the slightly higher frequency of neutral target words relative to angry targets affected this early posterior component observed here. However, high frequency words elicited larger P3 amplitude than low frequency words in Scott et al. (2009), suggesting at least, that the larger P2 and P3 amplitudes for negative than for neutral words in the current experiments were not driven by the frequency, per se.
Nevertheless, the currently observed patterns of this early negativity across the two experiments provide evidence that this early posterior component is influenced by the depth of required processing and top-down motivational biases. First, the patterns of this negativity were different in Experiments 1 and 2, as it revealed the main effects of reward and emotion and the interaction between them in Experiment 1, but it only revealed the two main effects in Experiment 2. This suggests that the pattern of this early negativity was actually modulated by the depth of processing of the emotional content. Second, in Experiment 1, this early negativity revealed larger amplitude differences between the negative and the neutral targets in the incentive condition than in the non-incentive condition, indicating that the reward expectation affected the initial ‘automatic’ processing of the emotional stimuli, suggesting that top-down motivational bias may alter the preparedness of the temporo-occipital cortex to better select the task-relevant emotional information to get the expected reward.
Moreover, reward expectation and emotion interacted in the P31 time window in both experiments but with reversed patterns. When the emotionality of the target word was task-relevant, as in Experiment 1, the amplitude of the late positivity component P31 was largest in the rewarded negative condition. This suggests a combined boosting of activity resulted from both the reward and the emotional systems on rewarded negative trials (Baines et al., 2011; Kaltwasser et al., 2013; Schevernels et al., 2014; van den Berg et al., 2014). The potentiated P31 may reflect the engagement of a capacity-limited processing system associated with objective coding, conscious recognition and better preparation and organization of behavioral responses to get the expected reward (Yeung and Sanfey, 2004; Schupp et al., 2007). Indeed, the behavioral data confirmed this suggestion as the shortest RTs occurred for the negative target under the incentive condition. However, when emotionality was task-irrelevant, as in Experiment 2, although the amplitudes were more positive-going for the negative than for the neutral target under the non-incentive condition, they were equally large in the rewarded negative and rewarded neutral conditions. This suggests that reward expectation may reduce the intrinsic significance of the negative target under the incentive condition. Focusing the processing capacity or the attentional endeavor to the task-relevant aspect of the target (i.e. the color), but not the emotional content, was the indemnification for getting the expected reward.
The enlarged P3 component to emotional stimuli, when they are the focus of attentional selection, is typically observed at later time windows (starting at ∼400 ms poststimulus onset), which corresponds well with the current P32 results. Enlarged LPC amplitudes to emotional stimuli at this time window have been suggested to reflect stronger stimulus consolidation related to the construction of representations, conscious recognition and elaborate processing of significant stimuli (Schupp et al., 2000, 2003, 2004, 2006, 2007; Schacht and Sommer, 2009b; Schevernels et al., 2014; van den Berg et al., 2014). As mentioned earlier, it is unclear whether the emotional effect on the LPC component is regulated by task demands. Although there are studies reporting a task-independent emotional effect on the LPC across both implicit and explicit tasks on words (Frühholz et al., 2011), this current study supports the contrary notion that: (i) the emotional content of written stimuli boosts the processing at later stages when attention is explicitly directed to this content and (ii) reduces the processing during implicit tasks when attention is oriented toward non-emotional stimulus features (Thomas et al., 2007; Schacht and Sommer, 2009b; Hinojosa et al., 2010; González-Villar et al., 2014). Moreover, consistent with the notion that an enlarged LPC represents elaborate processing of significant stimuli, the robust modulations of reward expectation on both the P31 and the P32 components in the two experiments indicates reward-related boosting of attentional resources and more controlled response-selection to improve the processing of the target at later stages.
To conclude, by asking participants to perform tasks with the emotionality of the target words being task-relevant or task-irrelevant under monetary incentive or non-incentive conditions, the current findings suggest that reward expectation improves top-down attentional concentration to task-relevant information, with enhanced sensitivity to the emotional content of target words when emotionality is task-relevant, but with reduced differential brain responses to emotional words when their content is task-irrelevant.
Supplementary Material
Acknowledgements
The authors thank the two anonymous reviewers for their constructive comments on the earlier version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (31000502, 31470979).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared
References
- Baines S., Ruz M., Rao A., Denison R., Nobre A.C. (2011). Modulation of neural activity by motivational and spatial biases. Neuropsychologia, 49, 2489–97. [DOI] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. (2002). The amygdala and reward. Nature Review Neuroscience, 3, 563–73. [DOI] [PubMed] [Google Scholar]
- Bayer M., Sommer W., Schacht A. (2012). P1 and beyond: functional separation of multiple emotion effects in word recognition. Psychophysiology, 49, 959–69. [DOI] [PubMed] [Google Scholar]
- Beaver J.D., Lawrence A.D., Passamonti L., Calder A.J. (2008). Appetitive motivation predicts the neural response to facial signals of aggression. Journal of Neuroscience, 28, 2719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E.S., Rinck M., Margraf J., Roth W.T. (2001). The emotional Stroop effect in anxiety disorders: general emotionality or disorder specificity? Journal of Anxiety Disorders, 15, 147–59. [DOI] [PubMed] [Google Scholar]
- Bernat E., Bunce S., Shevrin H. (2001). Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology, 42, 11–34. [DOI] [PubMed] [Google Scholar]
- Bijleveld E., Custers R., Aarts H. (2011). Once the money is in sight: distinctive effects of conscious and unconscious rewards on task performance. Journal of Experimental Social Psychology, 47, 865–9. [Google Scholar]
- Bradley M.M., Hamby S., Low A., Lang P.J. (2007). Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology, 44, 364–73. [DOI] [PubMed] [Google Scholar]
- Chelazzi L., Perlato A., Santandrea E., Della Libera C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. [DOI] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. (2011). Positive affect versus reward: emotional and motivational influences on cognitive control. Frontiers in Psychology, 2, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron F.M.M. (2012). Neural correlates of written emotion word processing: a review of recent electrophysiological and hemodynamic neuroimaging studies. Brain and Language, 122, 211–26. [DOI] [PubMed] [Google Scholar]
- Comerchero M.D., Polich J. (1999). P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology 110, 24–30. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Natute Reviews Neuroscice, 3, 215–29. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. [DOI] [PubMed] [Google Scholar]
- Dalgleish T., Watts F.N. (1990). Biases of attention and memory in disorders of anxiety and depression. Clinical Psychology Review, 10, 589–604. [Google Scholar]
- Egner T., Hirsch J. (2005). Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience, 8, 1784–90. [DOI] [PubMed] [Google Scholar]
- Eimer M., Holmes A. (2007). Event-related brain potential correlates of emotional face processing. Neuropsychologia, 45, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I.H. A., Gootjes L., van Strien J.W. (2009). Automatic processing of emotional words during an emotional Stroop task. NeuroReport, 20, 776–81. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Jellinghaus A., Herrmann M. (2011). Time course of implicit processing and explicit processing of emotional faces and emotional words. Biological Psychology, 87, 265–74. [DOI] [PubMed] [Google Scholar]
- González-Villar A.J., Triñanes Y., Zurrón M., Carrillo-de-la-Peña M.T. (2014). Brain processing of task-relevant and task-irrelevant emotional words: an ERP study. Cognitive, Affective, & Behavioral Neuroscience, 14, 939–50. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., McCann C.D. (1984). Construct accessibility and depression: an examination of cognitive and affective factors. Journal of Personality and Social Psychology, 47, 427–39. [DOI] [PubMed] [Google Scholar]
- Herbert C., Kissler J., Junghöfer M., Peyk P., Rockstroh B. (2006). Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology, 43, 197–206. [DOI] [PubMed] [Google Scholar]
- Hickey C., Chelazzi L., Theeuwes J. (2010). Reward changes salience in human vision via the anterior cingulate. Journal of Neuroscience, 30, 11096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa J.A., Méndez-Bértolo C., Pozo M.A. (2010). Looking at emotional words is not the same as reading emotional words: behavioral and neural correlates. Psychophysiology, 47, 748–57. [DOI] [PubMed] [Google Scholar]
- Hofmann M.J., Kuchinke L., Tamm S., Võ M.L.H., Jacobs A.M. (2009). Affective processing within 1/10th of a second: high arousal is necessary for early facilitative processing of negative but not positive words. Cognitive, Affective, & Behavioral Neuroscience, 9, 389–97. [DOI] [PubMed] [Google Scholar]
- Huguet P., Dumas F., Monteil J. (2004). Competing for a desired reward in the Stroop task: when attentional control is unconscious but effective versus conscious but ineffective. Canadian Journal of Experimental Psychology, 58, 153–67. [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Bradley M.M., Elbert T.R., Lang P.J. (2001). Fleeting images: a new look at early emotion discrimination. Psychophysiology, 38, 175–8. [PubMed] [Google Scholar]
- Kaltwasser L., Ries S., Sommer W., Knight R.T., Willems R.M. (2013). Independence of valence and reward in emotional word processing: electrophysiological evidence. Frontiers in Psychology, 4, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman M., Keijsers G.P.J., Verbraak M., Naring G., Hoogduin C.A.L. (2002). The emotional Stroop: a comparison of panic disorder patients, obsessive–compulsive patients, and normal controls, in two experiments. Journal of Anxiety Disorders, 16, 425–41. [DOI] [PubMed] [Google Scholar]
- Kang G., Zhou X., Wei P. (2015). Independent effects of reward expectation and spatial orienting on the processing of emotional facial expressions. Experimental Brain Research, 233(9), 2571–80. [DOI] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–41. [DOI] [PubMed] [Google Scholar]
- Kiss M., Driver J., Eimer M. (2009). Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science, 20, 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler J., Assadollahi R., Herbert C. (2006). Emotional and semantic networks in visual word processing: insights from ERP studies. Progress in Brain Research, 156, 147–83. [DOI] [PubMed] [Google Scholar]
- Kissler J., Herbert C., Peyk P., Junghöfer M. (2007). Buzzwords—early cortical responses to emotional words during reading. Psychological Science, 18, 475–80. [DOI] [PubMed] [Google Scholar]
- Kissler J., Herbert C., Winkler I., Junghöfer M. (2009). Emotion and attention in visual word processing: an ERP study. Biological Psychology, 80, 75–83. [DOI] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Appelbaum L.G., Woldorff M.G. (2013). Reward associations reduce behavioral interference by changing the temporal dynamics of conflict processing. Plos One, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Woldorff M.G. (2010). The influence of reward associations on conflict processing in the Stroop task. Cognition, 117, 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. (2000). Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Science, 4, 463–70. [DOI] [PubMed] [Google Scholar]
- Lee J., Shomstein S. (2013). The differential effects of reward on space- and object-based attentional allocation. Journal of Neuroscience, 33, 10625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein-Vidne L., Henik A., Safadi Z. (2012). Task relevance modulates processing of distracting emotional stimuli. Cognition and Emotion, 26, 42–52. [DOI] [PubMed] [Google Scholar]
- Locke H.S., Braver T.S. (2008). Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience, 8, 99–112. [DOI] [PubMed] [Google Scholar]
- Navalpakkam V., Koch C., Perona P. (2009). Homo economicus in visual search. Journal of Vision, 9, 31. [DOI] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. (2011). Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience, 23, 3419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13, 160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J.B., Levy R., Fossati P., Lehericy S., Poline J.B., Pillon B., et al. (2002). The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America, 99, 5669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. (2007). Updating p300: an integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk T., Drake R., Jonides J., Smith M., Smith E. (2008). Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the Stroop task. Journal of Neuroscience, 28, 13786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts G.F., Tucker D.M. (2001). Frontal evaluation and posterior representation in target detection. Cognitive Brain Research, 11, 147–56. [DOI] [PubMed] [Google Scholar]
- Rellecke J., Palazova M., Sommer W., Schacht A. (2011). On the automaticity of emotion processing in words and faces: event-related brain potentials evidence from a superficial task. Brain and Cognition, 77, 23–32. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. (1996). Neurobehavioral mechanisms of reward and motivation. Current Opinion in Neurobiology, 6, 228–36. [DOI] [PubMed] [Google Scholar]
- Savine A.C., Braver T.S. (2010). Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. Journal of Neuroscience, 30, 10294–305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schacht A., Adler N., Chen P., Guo T., Sommer W. (2012). Association with positive outcome induces early effects in event-related brain potentials. Biological Psychology, 89, 130–6. [DOI] [PubMed] [Google Scholar]
- Schacht A., Sommer W. (2009a). Emotions in word and face processing: early and late cortical responses. Brain and Cognition, 69, 538–50. [DOI] [PubMed] [Google Scholar]
- Schacht A., Sommer W. (2009b). Time course and task dependence of emotion effects in word processing. Cognitive, Affective, & Behavioural Neuroscience, 9, 28–43. [DOI] [PubMed] [Google Scholar]
- Schevernels H., Krebs R.M., Santens P., Woldorff M.G. (2014). Task preparation processes related to reward prediction precede those related to task-difficulty expectation. NeuroImage, 84, 639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1, 199–207. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.M., Cacioppo J.T., Ito T., Lang P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37, 257–61. [PubMed] [Google Scholar]
- Schupp H.T., Flaisch T., Stockburger J., Junghöfer M. (2006). Emotion and attention: event-related brain potential studies. Progress in Brain Research, 156, 31–51. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Hamm A.O., Weike A.I. (2003). Emotional facilitation of sensory processing in the visual cortex. Psychological Science, 14, 7–13. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Junghöfer M., Weike A.I., Hamm A.O. (2004). The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology, 41, 441–9. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Stockburger J., Codispoti M., Junghöfer M., Weike A.I., Hamm A.O. (2007). Selective visual attention to emotion. Journal of Neuroscience, 27, 1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.G., O’Donnell P.J., Leuthold H., Sereno S.C. (2009). Early emotion word processing: evidence from event-related potentials. Biological Psychology, 80, 95–104. [DOI] [PubMed] [Google Scholar]
- Small D.M., Gitelman D., Simmons K., Bolise S.M., Parrish T., Mesulam M.M. (2005). Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex, 15, 1855–65. [DOI] [PubMed] [Google Scholar]
- Theeuwes J., Belopolsky A.V. (2012). Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Research, 74, 80–5. [DOI] [PubMed] [Google Scholar]
- Thomas S.J., Johnstone S.J., Gonsalvez C.J. (2007). Event-related potentials during an emotional Stroop task. International Journal of Psychophysiology, 63, 221–31. [DOI] [PubMed] [Google Scholar]
- Tosoni A., Shulman G.L., Pope A.L., McAvoy M.P., Corbetta M. (2013). Distinct representations for shifts of spatial attention and changes of reward contingencies in the human brain. Cortex, 49, 1733–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J.T., Ashby F.G., editors. (1983). Stochastic modelling of elementary psychological processes. New York: Cambridge UP. [Google Scholar]
- van den Berg B., Krebs R.M., Lorist M.M., Woldorff M.G. (2014). Utilization of reward-prospect enhances preparatory attention and reduces stimulus conflict. Cognitive, Affective, & Behavioral Neuroscience, 14, 561–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien J.W., Franken I.H.A., Huijding J. (2009). Phobic spider fear is associated with enhanced attentional capture by spider pictures: a rapid serial presentation event-related potential study. Neuroreport, 20, 445–9. [DOI] [PubMed] [Google Scholar]
- Veling H., Aarts H. (2010). Cueing task goals and earning money: relatively high monetary rewards reduce failures to act on goals in a Stroop task. Motivation and Emotion, 34, 184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J., De Houwer J., Crombez G., Van Damme S. (2013). Competing for attentional priority: temporary goals versus threats. Emotion, 13, 587–98. [DOI] [PubMed] [Google Scholar]
- Wang L., Yu H., Zhou X. (2013). Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision, 13, 5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou L., Luo Y. (2008). The pilot establishment and evaluation of Chinese affective words system. Chinese Mental Health Journal, 22, 608–12. [Google Scholar]
- Wei P., Kang G. (2014). Task-relevance regulates the interaction between reward expectation and emotion. Experimental Brain Research, 232, 1783–91. [DOI] [PubMed] [Google Scholar]
- Wei P., Kang G., Ding J., Guo C. (2014). Monetary incentives modulate the processing of emotional facial expressions: an ERP study. Acta Psychologica Sinica, 46, 437–49. [Google Scholar]
- Wiens S., Sand A., Olofsson J.K. (2011). Nonemotional features suppress early and enhance late emotional electrocortical responses to negative pictures. Biological Psychology, 86, 83–9. [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A.G. (2004). Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience, 24, 6258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel I., Mineka S. (2004). Hierarchical models of emotional disorders and emotion-congruent cognitive biases. Personality and Individual Differences, 36, 679–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.